Introduction
HCC causes ~1 million mortalities worldwide annually
and half of these mortalities occur in China (1). The areas of high HCC occurrence in China
are concentrated in the southeast coastal areas, including Fujian
(2). The complicated signal
transduction pathways and underlying molecular pathogenesis
involved in HCC are not fully understood. Several molecular markers
have been proposed that may identify the metastatic potential and
risk of recurrence of HCC; however, none have been approved for
routine clinical use (3). Although
pre-operative serum α-fetoprotein (AFP) is a commonly used
diagnostic and prognostic marker for HCC, not all patients exhibit
elevated AFP levels at the pre-operative stage (4). Therefore, other biomarkers or similar
indicators are required that can be combined with AFP for
prognostic and diagnostic use in HCC. Biological indicators
associated with the recurrence of liver cancer subsequent to
surgery are particularly significant in clinical practice (5). A number of tumor suppressors and
oncogenes have been demonstrated to promote the transformed
phenotype of cancer cells (6), along
with splicing factors that have been previously confirmed as
important participants in cancer development (7). Heterogeneous ribonucleoproteins (hnRNPs)
area nuclear protein class that bind to nascent RNA transcripts,
with a variety of roles in telomere biogenesis, DNA repair, cell
signaling and the regulation of gene expression at the
transcriptional and translational levels (8). hnRNPA1 belongs to a family of hnRNPs
that function as splicing factors, and is involved in telomere
biogenesis; telomeres of an hnRNPA1-deficient cell line were
previously revealed to be shorter than those of a associated
hnRNPA1-expressing cell line (9). In
recent years, hnRNPA1 overexpression has been observed in various
malignancies, and has been identified as a potential biomarker for
early diagnosis, prognosis and monitoring during treatment of
colorectal cancer (10–13). However, a previous study reported its
prognostic value for surgically resected HCC (RHCC) (14), particularly in areas of high HCC
occurrence in China. Studies have also demonstrated lymphatic
infiltration caused by chronic inflammatory responses in the tumor
and its surrounding tissues as a possible underlying cause of
tumorigenesis (15); since the
neutrophil to lymphocyte ratio (NLR) and the platelet to lymphocyte
ratio (PLR) are the markers of immune status, they also reflect the
inflammatory state and are associated with survival prognosis
(16,17). The correlation between inflammation
indicators and hnRNPA1 in HCC is not yet clear. To address this,
the present study investigated the significance of hnRNPA1 in two
cohorts of patients with RHCC in Fujian, where HCC is common the
clinicopathological data of all patients were collected, and
hnRNPA1 expression was determined with reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
western blotting and immunohistochemistry (IHC). The correlations
between hnRNPA1 expression and pre-operative serum AFP levels, NLR
and PLR were also determined.
Materials and methods
Patients and follow-up
Two independent cohorts of patients with HCC were
enrolled for the study. This due to the different specimens we
collected. Between June 2014 and February 2015, fresh liver cancer
tissue samples were collected, which were used for RT-qPCR and
western blot analyses. However, prior to March 2012, only collect
paraffin-embedded liver cancer specimens, that were used for IHC
were available. To determine the expression of hnRNPA1 in HCC
tissues, 54 cancerous and pericancerous liver tissues (PCLT) were
collected for RT-qPCR and western blot analyses (cohort 1). The
samples were collected from patients with HCC who underwent
curative resection at the Department of Hepatobiliary Surgery,
Fuzhou General Hospital (Fujian, China), between June 2014 and
February 2015. These patients were monitored following surgery
until August 15, 2017. All specimens were collected immediately
after resection, transported in liquid nitrogen and stored at
−80°C. To evaluate the prognostic role of hnRNPA1 in HCC, tumor
specimens for IHC analyses were obtained from 426 consecutive
patients with HCC who underwent curative resection between January
2002 and March 2012 (cohort 2). These patients were evaluated from
the day of surgical resection to the last day of follow-up (August
15, 2017), with a median follow-up time of 59 months (range,
2.0–128.0 months). Liver function and tumor differentiation were
assessed by Child-Pugh classification (18) and the Edmondson grading system
(18), respectively. Tumor stages
were assessed according to the 2010 International Union Against
Cancer Tumor-Node-Metastasis (TNM) classification system (19), and curative resection was defined as
previously described (14). Overall
survival (OS) was defined as the interval between surgery and
mortality or between surgery and the last observation point. For
surviving patients, the data were censored at the last follow-up.
Relapse-free survival (RFS) was defined as the interval between the
date of surgery and the date of diagnosis of any type of relapse
(intra-hepatic recurrence or extrahepatic metastasis). The
inclusion criteria included the following: ECOG score (18) of 0–1 prior to surgery, absence of
ascites, hepatic encephalopathy and any distant metastasis, only
one tumor lesion and Child-Pugh class A. Clinicopathological data
of the patients with HCC (Table I)
were collected from the hospital database medical records. Survival
information was obtained from medical records, telephone interviews
and the Social Security Death Index (18), and all pathological data were
pathological characteristics originally assessed by
pathologists.
 | Table I.Clinicopathological characteristics
of patients with hepatocellular carcinoma in two cohorts. |
Table I.
Clinicopathological characteristics
of patients with hepatocellular carcinoma in two cohorts.
|
| Cohort 1
(n=54) | Cohort 2
(n=426) |
|---|
|
|
|
|
|---|
|
Characteristics | n | % | n | % |
|---|
| Age, years |
|
|
|
|
|
≤55 | 29 | 53.7 | 174 | 40.8 |
|
>55 | 25 | 46.3 | 252 | 59.2 |
| Sex |
|
|
|
|
|
Male | 45 | 83.3 | 378 | 88.7 |
|
Female | 9 | 16.7 | 48 | 11.3 |
| Tumor location |
|
|
|
|
|
Left | 15 | 27.8 | 101 | 23.7 |
|
Right | 39 | 72.2 | 325 | 76.3 |
| TNM stage |
|
|
|
|
|
I/II | 37 | 68.5 | 292 | 68.5 |
|
IIIa | 17 | 31.5 | 134 | 31.5 |
| Tumor size, cm |
|
|
|
|
| ≤5 | 28 | 51.9 | 219 | 51.4 |
|
>5 | 26 | 48.1 | 207 | 48.6 |
| Vascular
invasion |
|
|
|
|
|
Yes | 29 | 53.7 | 287 | 67.4 |
| No | 25 | 46.3 | 139 | 32.6 |
| Edmonson grade |
|
|
|
|
| I | 43 | 79.6 | 343 | 80.5 |
|
II–IV | 11 | 20.4 | 83 | 19.5 |
| AFP, µg/l |
|
|
|
|
|
≤400 | 32 | 59.3 | 267 | 62.7 |
|
>400 | 22 | 40.7 | 159 | 37.3 |
| HBsAg |
|
|
|
|
|
Negative | 35 | 64.8 | 275 | 64.6 |
|
Positive | 19 | 35.2 | 151 | 35.4 |
| HBV DNA load,
IU/ml |
|
|
|
|
|
≤104 | 30 | 55.6 | 224 | 52.6 |
|
>104 | 24 | 44.4 | 202 | 47.4 |
| NLR |
|
|
|
|
|
≤5.0 | 18 | 33.3 | 125 | 29.3 |
|
>5.0 | 36 | 66.7 | 301 | 70.7 |
| PLR |
|
|
|
|
|
≤91 | 33 | 61.1 | 281 | 66.0 |
|
>91 | 21 | 38.9 | 145 | 34.0 |
| Survival |
|
|
|
|
|
Yes | NA | NA | 236 | 55.4 |
| No | NA | NA | 190 | 44.6 |
| Recurrence |
|
|
|
|
|
Yes | 32 | 59.3 | 205 | 48.1 |
| No | 22 | 40.7 | 221 | 51.9 |
RT-qPCR
Total RNA was extracted from HCC tissues using
TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocols. cDNA
was synthesized by HiScript reverse transcription kit (Vazyme
Biotech Co., Ltd, Nanjing, China) and Real-time PCR SYBR Green kit
(Vazyme Biotech Co., Ltd.) was used to prepare the protein
according to the manufacturer's protocol. The negative control was
used to replace the template with double distilled water and
β-actin as control. The gene expression of hnRNPA1 and β-actin in
from patient tissues was measured using One-Step RT-PCR Master mix
(Thermo Fisher Scientific, Inc.). The primers used were: hnRNPA1
forward, 5′-TTTGACGACCATGACTCCGT-3′ and reverse,
5′-CACGACCACCACCAAAGTTT-3; and β-actin forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse, 5′-GGGCACGAAGGCTCATCATT-3′.
The reaction conditions were set to pre-denaturation at 95°C for 30
sec, PCR reaction at 94°C for 4 min, 94°C for 30 sec, 56°C for 30
sec, 72°C for 25 sec, dissolution curve conditions: 50°C 2 min,
95°C 10 min; 95°C 30 sec, 60°C 30 sec, 40 cycles. The expression of
hnRNPA1 was measured relative to that of the β-actin standard and
expressed as the 2−∆∆Cq value (20).
Western blotting
The newly resected HCC and PCLT tissues were weighed
and protein was extracted by protein lysis buffer (RIPA; Beyotime
Institute of Biotechnology Co., Ltd. Shanghai, China). The protein
concentrations were measured by the bicinchoninic acid method and
40 µg protein was loaded into each lane on a 12% SDS-PAGE gel.
Following electrophoresis, the protein bands were transferred to
polyvinylidene fluoride (PVDF) membranes and blocked for 2 h at
room temperature with PBS-0.05% Tween 20 containing 5% nonfat dry
milk. The membranes were then incubated with the primary
antibodies, rabbit anti-hnRNPA1 (ab177152; 1:4,000; Abcam,
Cambridge, UK) and mouse anti-β-actin (BM0627; 1:200; Wuhan Boster
Biological Technology, Ltd., Wuhan, China), overnight at 4°C. The
membranes were washed with Tris-buffered saline with Tween-20 (1×
PBS, 0.1% Tween-20) 3 times, and then incubated in HRP-conjugated
goat anti-mouse (BA1051; 1:50,000; Wuhan Boster Biological
Technology, Ltd, Wuhan, China) or goat anti-rabbit (BA1054;
1:50,000; Wuhan Boster Biological Engineering Co., Ltd, Wuhan,
China) secondary antibodies in 1% nonfat dry milk in TBST for 1 h
at room temperature. Membranes were washed 3 times in TBST and
bound antibodies were detected using SuperSignal Chemiluminescent
substrates (Thermo Fisher Scientific, Inc.) and chemiluminescence
was detected on CL-XPosure Film (Thermo Fisher Scientific, Inc.).
These data were analyzed via densitometry using Image-Pro plus v6.0
software (National Institutes of Health, Bethesda, MD, USA) and
normalized to the expression of the internal control (β-actin).
IHC
IHC methods published by Liu et al for a Her2
assay (21) were used to quantify
hnRNPA1expression in 426 HCC tissue samples, with one modification:
Anti-hnRNPA1 antibody (ab177152, 1:100; Abcam) replaced anti-HER-2
antibody. Pathological specimens from 426 HCC tissue samples were
fixed in 4% neutral formaldehyde solution at room temperature for
24 h, embedded in paraffin and sliced into 4-mm sections. Paraffin
was removed with xylene (15 min; Sinopharm Group Chemical Reagent
Co., Ltd., Shanghai, China) and the sections were rehydrated
through a graded alcohol series (anhydrous ethanol I, 5 min;
anhydrous ethanol II, 5 min; 95% ethanol, 3 min; 90% ethanol, 3
min; 80% ethanol, 2 min; and 70% ethanol, 2 min) (Sinopharm Group
Chemical Reagent Co., Ltd.). Antigen retrieval was performed by the
microwave method. The sections were placed in a glass box filled
with 10 mM citrate buffer (pH 6.0; Shanghai Yanjing Reagent
Technology Co., Ltd., Shanghai, China), and the glass box was
placed in a microwave oven (700 W; Midea KJ23B-AN; Midea Group Co.,
Foshan, China) and heated at 100°C for 10–15 min. After the repair
solution is cooled to room temperature, the sections were removed
and washed 3 times with PBS (pH 7.4) for 3 min each time. The
sections were incubated overnight with the primary antibody
anti-hnRNPA1 or TBS as negative control at 4°C for 30 min. This was
followed by incubation with a horseradish peroxidase
(HRP)-conjugated secondary antibody (KIT-5010; anti-rabbit/mouse
IgG; 1:50,000; Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) at
37°C for 30 min. Chromagen detection was performed with
3,3′-diaminobenzidine and a substrate-chromogen (EnVision system;
DAKO; Agilent Technologies, Inc., Santa Clara, CA, USA), followed
by counter-staining with hematoxylin at room temperature for 1 min.
In every section, five randomly selected visual fields of view were
assessed by light microscopy at ×200 magnification (Olympus
Corporation, Tokyo, Japan) to evaluate the expression on the
expression of hnRNPA1. Positively stained cells were evaluated by
two pathologists blinded to the patient data. Positive hnRNPA1
expression was localized to the nucleus of RHCC cells and was
graded based on the number of stained cells. When <10% cells
were stained, the assessment was negative (−), while ≥10% staining
was graded as positive (+). Among the positive samples, those with
10–25% positively stained cells were graded as +, those with 26–50%
positive cells were graded as ++ and those with 51–75% positive
cells were graded as +++. A grade of ++++ indicted >75%
positively stained cancer cells. RHCC tissue samples with -, + or
++ grading were classified as low hnRNPA1 expression, whereas those
with +++ and ++++ grading were classified as high hnRNPA1
expression.
Routine blood test
Blood samples were collected into EDTA tubes prior
to surgical resection and routine blood tests were conducted to
measure the pre-operative cell counts (total leukocytes,
neutrophils, lymphocytes, platelets and erythrocytes). Hemoglobin
counts were obtained from the patients' medical records.
Pre-operative NLR (22) and PLR
(23) were measured, and their median
values were found to be 5 and 91, respectively. The PLR and NLR
values were used as a grouping threshold (high and low).
Statistical analysis
All statistical analyses were performed using SPSS
statistical software version 21.0 (IBM Corp., Armonk, NY, USA) and
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Student's
unpaired t-test was used to compare the continuous variables data
between groups. Associations between hnRNPA1 expression and patient
clinicopathological characteristics were evaluated with the
χ2 test. The correlation between hnRNPA1 expression and
NLR/PLR was analyzed using Spearman's rank correlation test. The
survival curves were plotted using Kaplan-Meier method and the
prognostic significance of hnRNPA1 was analyzed using the log-rank
test. Associations between hnRNPA1 expression, clinicopathological
data and mortality risk were assessed using univariate regression
analysis, and Cox multivariate regression analysis was used to
determine independent prognostic factors. All statistical analyses
were two-sided. P<0.05 was considered to indicate a
statistically significant difference.
Results
hnRNPA1 is upregulated in HCC
tissues
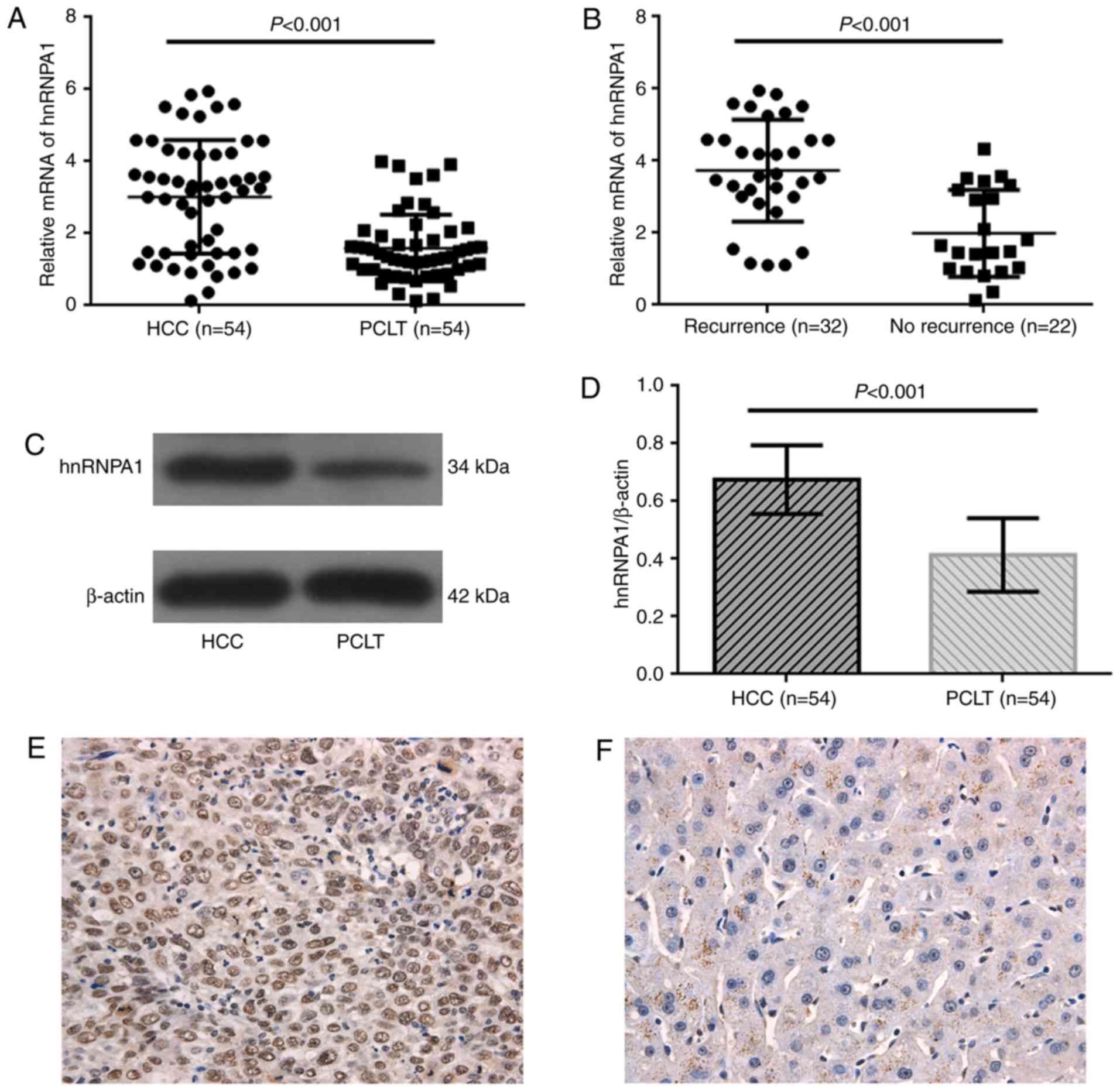
hnRNPA1 mRNA levels were measured in 54 paired HCC
samples from cohort 1 by RT-qPCR. hnRNPA1 was revealed to be
significantly overexpressed in tumor tissues compared with that in
corresponding PCLT samples (P<0.001; Fig. 1A). Samples from patients with tumor
recurrences (32/54) also revealed significantly higher levels of
hnRNPA1mRNA than those from patients without recurrences (22/54;
P<0.001; Fig. 1B). The expression
of hnRNPA1in 54 HCC cases from cohort 1wasalso assessed at the
protein level by western blotting, and significant overexpression
of hnRNPA1 protein was confirmed in tumors compared with that in
corresponding PCLTs (P<0.001; Fig. 1C
and D).
Association between patient clinicopathological
features and hnRNPA1 expression. Patient clinicopathological data
(cohort 1 and 2; Table I) and the
associations with differential hnRNPA1 expression (cohort 2;
Table II) were assessed. Positive
hnRNPA1 expression was confirmed in cancer cell nuclei with IHC in
both high and low hnRNPA1 expression groups in cohort 2 (Fig. 1E and F). High hnRNPA1 expression was
significantly associated with elevated pre-operative blood AFP
(P<0.001), TNM stage (P=0.024), tumor size (>5 cm)
(P<0.001), vascular invasion (P<0.001), Edmonson grade
(P<0.001), NLR (P=0.005), PLR (P=0.002), survival (P<0.001)
and recurrence (P<0.001). Age, sex, tumor location, hepatitis B
virus (HBV) surface antigen and HBV DNA load (>104 IU/ml) were
not associated with hnRNPA1 expression (Table II).
 | Table II.Association between hnRNPA1
expression and clinicopathological characteristics in HCC (n=426;
cohort 2). |
Table II.
Association between hnRNPA1
expression and clinicopathological characteristics in HCC (n=426;
cohort 2).
|
|
| hnRNPA1 expression
level |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | n | Low, n | High, n | P-value |
|---|
| Age, years |
|
|
|
|
|
≤55 | 174 | 92 | 160 | 0.170 |
|
>55 | 252 | 75 | 99 |
|
| Sex |
|
|
|
|
|
Male | 378 | 149 | 229 | 0.867 |
|
Female | 48 | 18 | 30 |
|
| Tumor location |
|
|
|
|
|
Left | 101 | 41 | 60 | 0.743 |
|
Right | 325 | 126 | 199 |
|
| TNM stage |
|
|
|
|
|
I/II | 292 | 125 | 167 | 0.024 |
|
IIIa | 134 | 42 | 92 |
|
| Tumor size, cm |
|
|
|
|
| ≤5 | 219 | 97 | 122 | 0.027 |
|
>5 | 207 | 70 | 137 |
|
| Vascular
invasion |
|
|
|
|
|
Yes | 287 | 94 | 193 | <0.001 |
| No | 139 | 73 | 66 |
|
| Edmonson grade |
|
|
|
|
| I | 343 | 114 | 229 | <0.001 |
|
II–IV | 83 | 53 | 30 |
|
| AFP, µg/l |
|
|
|
|
|
≤400 | 267 | 126 | 141 | <0.001 |
|
>400 | 159 | 41 | 118 |
|
| HBsAg |
|
|
|
|
|
Negative | 275 | 108 | 167 | 0.968 |
|
Positive | 151 | 59 | 92 |
|
| HBV DNA load,
IU/ml |
|
|
|
|
|
≤104 | 224 | 86 | 138 | 0.719 |
|
>104 | 202 | 81 | 121 |
|
| NLR |
|
|
|
|
|
≤5.0 | 125 | 62 | 63 | 0.005 |
|
>5.0 | 301 | 105 | 196 |
|
| PLR |
|
|
|
|
|
≤91 | 281 | 125 | 156 | 0.002 |
|
>91 | 145 | 42 | 103 |
|
| Survival |
|
|
|
|
|
Yes | 236 | 127 | 109 | <0.001 |
| No | 190 | 40 | 150 |
|
| Recurrence |
|
|
|
|
|
Yes | 205 | 57 | 148 | <0.001 |
| No | 221 | 110 | 111 |
Analysis of hnRNPA1 expression during
the observation period and RFS
Elevated hnRNPA1 expression (P<0.001), age
(P=0.006), TNM stage (P<0.001), larger tumor size (P<0.001),
vascular invasion (P<0.001), Edmonson grade (P=0.003), higher
pre-operative serum AFP (P<0.001) and advanced NLR (P=0.031)
were associated with recurrence in patients with RHCC during the
observation period, as determined by Kaplan-Meier survival and
log-rank analyses (Table III).
Elevated hnRNPA1 expression was also significantly associated with
shorter RFS during the same observation period; Fig. 2A demonstrates the different RFS times
(in months) in the high and low hnRNPA1 expression groups
(P<0.001). Univariate analyses confirmed that age (HR, 1.476;
95% CI, 1.107–1.968; P=0.008), TNM stage (HR, 1.978; 95% CI,
1.491–2.624; P<0.001), tumor size (HR, 1.898; 95% CI,
1.438–2.506; P<0.001), vascular invasion (HR, 6.850; 95% CI,
4.352–10.780; P<0.001), Edmonson grade (HR, 0.563; 95% CI,
0.381–0.831; P=0.004), pre-operative blood AFP (HR, 1.742; 95% CI,
1.320–2.300; P=0.001), NLR (HR, 1.394; 95% CI, 1.023–1.899;
P=0.035) and hnRNPA1 expression (HR, 2.295; 95% CI, 1.688–3.120;
P<0.001) were associated with RFS (Table IV). Multivariate analysis confirmed
that age (HR, 0.654; 95% CI, 0.488–0.877; P=0.005), vascular
invasion (HR, 6.125; 95% CI, 3.835–9.870; P<0.001) and hnRNPA1
expression (HR, 0.685; 95% CI, 0.506–0.928; P=0.015) were
independent prognostic factors of RFS (Table IV).
 | Table III.Association between
recurrence/survival and patient characteristics. |
Table III.
Association between
recurrence/survival and patient characteristics.
|
| Survival | Recurrence |
|---|
|
|
|
|
|---|
|
Characteristics | Dead, n | Alive, n | P-value | Yes, n | No, n | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
≤55 | 115 | 137 | 0.613 | 134 | 118 | 0.006 |
|
>55 | 75 | 99 |
| 71 | 103 |
|
| Sex |
|
|
|
|
|
|
|
Male | 174 | 204 | 0.135 | 185 | 193 | 0.292 |
|
Female | 16 | 32 |
| 20 | 28 |
|
| Tumor location |
|
|
|
|
|
|
|
Left | 34 | 67 | 0.053 | 46 | 55 | 0.483 |
|
Right | 156 | 169 |
| 159 | 166 |
|
| TNM stage |
|
|
|
|
|
|
|
I/II | 104 | 188 | <0.001 | 125 | 167 | <0.001 |
|
IIIa | 86 | 48 |
| 80 | 54 |
|
| Tumor size, cm |
|
|
|
|
|
|
| ≤5 | 70 | 149 | <0.001 | 88 | 131 | <0.001 |
|
>5 | 120 | 87 |
| 117 | 90 |
|
| Vascular
invasion |
|
|
|
|
|
|
|
Yes | 166 | 121 | <0.001 | 181 | 106 | <0.001 |
| No | 24 | 115 |
| 24 | 115 |
|
| Edmonson grade |
|
|
|
|
|
|
| I | 172 | 171 | <0.001 | 175 | 168 | 0.003 |
|
II–IV | 18 | 65 |
| 30 | 53 |
|
| AFP, µg/l |
|
|
|
|
|
|
|
≤400 | 99 | 168 | <0.001 | 115 | 152 | <0.001 |
|
>400 | 91 | 68 |
| 90 | 69 |
|
| HBsAg |
|
|
|
|
|
|
|
Negative | 126 | 149 | 0.495 | 136 | 139 | 0.458 |
|
Positive | 64 | 87 |
| 69 | 82 |
|
| HBV DNA load,
IU/ml |
|
|
|
|
|
|
|
≤104 | 90 | 134 | 0.053 | 109 | 105 | 0.243 |
|
>104 | 100 | 102 |
| 96 | 116 |
|
| NLR |
|
|
|
|
|
|
|
≤5.0 | 31 | 94 | <0.001 | 55 | 70 | 0.031 |
|
>5.0 | 159 | 142 |
| 150 | 151 |
|
| PLR |
|
|
|
|
|
|
|
≤91 | 103 | 178 | <0.001 | 134 | 147 | 0.095 |
|
>91 | 87 | 58 |
| 71 | 74 |
|
| hnRNPA1 |
|
|
|
|
|
|
|
Low | 40 | 127 | <0.001 | 57 | 110 | <0.001 |
|
High | 109 | 150 |
| 148 | 111 |
 | Table IV.Univariate and multivariate Cox
regression analysis of RFS and OS. |
Table IV.
Univariate and multivariate Cox
regression analysis of RFS and OS.
|
| RFS | OS |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
|
Univariate
analysisa |
|
|
|
|
| Age,
years (≤55 vs. >55) | 1.476
(1.107–1.968) | 0.008 | 0.928
(0.694–1.242) | 0.615 |
| Sex
(male vs female) | 0.785
(0.495–1.245) | 0.303 | 0.680
(0.407–1.134) | 0.140 |
| Tumor
location (left vs. right) | 1.122
(0.808–1.558) | 0.491 | 1.437
(0.991–2.084) | 0.056 |
| TNM
stage (I/II vs. IIIa) | 1.978
(1.491–2.624) | <0.001 | 2.357
(1.770–3.140) | <0.001 |
| Tumor
size, cm (≤5 vs. >5) | 1.898
(1.438–2.506) | <0.001 | 2.270
(1.690–3.050) | <0.001 |
|
Vascular invasion (yes vs.
no) | 6.850
(4.352–10.780) | <0.001 | 7.542
(4.77–11.908) | <0.001 |
|
Edmonson grade (I vs.
II–IV) | 0.563
(0.381–0.831) | 0.004 | 0.296
(0.181–0.485) | <0.001 |
| AFP,
µg/l (≤400 vs. >400) | 1.742
(1.320–2.300) | <0.001 | 2.122
(1.594–2.827) | <0.001 |
| HBsAg
(negative vs positive) | 0.875
(0.654–1.170) | 0.367 | 0.853
(0.631–1.154) | 0.303 |
| HBV DNA
load, IU/ml (≤104 vs. >104) | 0.946
(0.719–1.244) | 0.699 | 1.231
(0.926–1.637) | 0.153 |
| NLR
(≤5.0 vs. >5.0) | 1.394
(1.023–1.899) | 0.035 | 2.609
(1.775–3.835) | <0.001 |
| PLR
(≤91 vs. >91) | 1.271
(0.952–1.695) | 0.103 | 2.208
(1.658–2.941) | <0.001 |
| hnRNPA1
(low vs high) | 2.295
(1.688–3.120) | <0.001 | 3.184
(2.244–4.518) | <0.001 |
| Multivariate
analysisa |
|
|
|
|
| Age,
years (≤55 vs. >55) | 0.654
(0.488–0.877) | 0.005 |
|
|
| TNM
stage (I/II vs. IIIa) | 1.411
(0.950–2.094) | 0.088 | 1.218
(0.815–1.819) | 0.337 |
| Tumor
size, cm (≤5 vs. >5) | 1.003
(0.681–1.478) | 0.988 | 1.161
(0.763–1.766) | 0.487 |
|
Vascular invasion (yes vs.
no) | 6.125
(3.835–9.870) | <0.001 | 6.118
(3.729–10.040) | <0.001 |
|
Edmonson grade (I vs.
II–IV) | 1.047
(0.688–1.592) | 0.831 | 0.643
(0.380–1.086) | 0.099 |
| AFP,
µg/l (≤400 vs. >400) | 1.350
(1.006–1.811) | 0.045 | 1.564
(1.151–2.126) | 0.004 |
| NLR
(≤5.0 vs. >5.0) | 1.020
(0.734–1.417) | 0.907 | 1.758
(1.161–2.661) | 0.008 |
| PLR
(≤91 vs. >91) |
|
| 1.343
(0.978–1.846) | 0.069 |
| hnRNPA1
(low vs. high) | 0.685
(0.506–0.928) | 0.015 | 0.629
(0.454–0.871) | 0.005 |
Analysis of hnRNPA1 expression and OS
during the observation period
Elevated hnRNPA1 expression (P<0.001), Edmonson
grade (P<0.001), advanced TNM stage (P<0.001), tumor size
>5 cm (P<0.001), vascular invasion (P<0.001), NLR
(P<0.001), PLR (P<0.001) and higher pre-operative serum AFP
(P<0.001) were associated with worse OS for patients with RHCC
(Table III). Univariate analysis
confirmed that TNM stage (HR, 2.357; 95% CI, 1.770–3.140;
P<0.001), tumor size (HR, 2.270; 95% CI, 1.690–3.050;
P<0.001), vascular invasion (HR, 7.542; 95% CI, 4.77–11.908;
P<0.001), Edmonson grade (HR, 0.296; 95% CI, 0.181–0.485;
P<0.001), pre-operative blood AFP (HR, 2.122; 95% CI,
1.594–2.827; P<0.001), NLR (HR, 2.609; 95% CI, 1.775–3.835;
P<0.001), PLR (HR, 2.208; 95% CI, 1.658–2.941; P<0.001) and
hnRNPA1 expression (HR, 3.184; 95% CI, 2.244–4.518; P<0.001)
were associated with OS (Table IV).
Multivariate analysis confirmed that vascular invasion (HR, 6.118;
95% CI, 3.729–10.040; P<0.001), pre-operative blood AFP (HR,
1.564; 95% CI, 1.151–2.126; P=0.004), NLR (HR, 1.758; 95% CI,
1.161–2.661; P=0.008) and hnRNPA1 expression (HR, 0.629; 95% CI,
0.454–0.871; P=0.005) were independent prognostic factors of OS
(Table IV). High hnRNPA1 expression
was significantly associated with increased risk of mortality
(Table IV).
Association between OS and hnRNPA1,
NLR and PLR in patients with HCC during the observation period
following curative resection
By the final follow-up, 48.1% (205/426) of the
patients had suffered from recurrence and 44.6% (190/426) had
succumbed in cohort 2. The 1-, 3- and 5-year OS rates in the whole
cohort were 86.2, 66.7 and 58.9, respectively, and the 1-, 3- and
5-year recurrence rates were 29.6, 45.8 and 47.7%, respectively.
Furthermore, it was revealed that patients with HCC with high
hnRNPA1 expression had the poorest prognosis at 1, 3 and 5 years,
with higher recurrence rates than in patients with low hnRNPA1
expression (51.4 vs. 20.4, 71.4 vs. 54.5 and 90.7 vs. 76.1%,
respectively; P<0.001; Fig. 2A).
Similarly, the 1-, 3- and 5-year overall survival rates of the
patients with low expression of hnRNPA1 were significantly higher
than the survival rates of the hnRNPA1 high group (94.6 vs. 80.7,
62.2 vs. 46.7 and 30.5 vs. 15.4%, respectively; P<0.001;
Fig. 2B).
It was also revealed that patients with HCC with
pre-operative NLR >5 had the poorest prognosis at 1, 3 and 5
years, with higher cumulative recurrence rates than the patients
with pre-operative NLR ≤5 (81.4 vs. 96.8, 48.5 vs. 62.4 and 19.9
vs. 24.8%, respectively; P<0.001; Fig.
2C). Similarly, the 1-, 3- and 5-year survival rates of the
patients with pre-operative PLR >91 were significantly lower
than the survival rates of patients with pre-operative PLR ≤91
(73.8 vs. 92.5, 38.6 vs. 60.1 and 13.1 vs. 25.6%, respectively;
P<0.001; Fig. 2D). The expression
of hnRNPA1 was also positively correlated with NLR (Spearman's
correlation; r=0.122, P=0.012) and PLR (Spearman's correlation;
r=0.140, P=0.004).
Discussion
HCC is a highly malignant type of cancer with poor
survival rates. There are few HCC-specific biomarkers that are used
in clinical practice, but the present study has revealed several
potential prognostic indicators. In an attempt to identify a
prognostic marker for HCC, hnRNPA1 expression was studied in tissue
samples from patients with RHCC. hnRNPA1 is a nucleic acid binding
protein that is distributed in various human tissues and organs.
The selective splicing of hnRNPA1 is regulated throughout the human
transcriptome and uses several mechanisms in conjunction with
several splicing factors (24). It
has been reported that knocking down hnRNPA1 and its downstream
target pyruvate kinase muscle isozyme M2 (PKM2) can inhibit the
growth of cancer cells (25). PKM2
promotes glucose metabolism and cancer cell growth through the
let-7a/c-Myc/hnRNPA1 feedback loop mechanism (26). In addition, hnRNPA1 may prevent tumor
cells from entering senescence by binding to telomeric DNA
sequences (27).
The present study investigated the expression of
hnRNPA1 in two cohorts of clinical HCC samples. In the 54 tumors
samples from cohort 1, hnRNPA1 mRNA was highly expressed in the HCC
tissues compared with that in the corresponding PCLTs. Samples from
patients with recurrent HCC exhibited higher levels of hnRNPA1 than
those without recurrence. In another independent cohort (cohort 2),
similar results were obtained at the protein level. Another finding
from the present study was the correlation between hnRNPA1
expression and a poor prognosis for HCC patients; overexpression of
hnRNPA1 in HCC patients predicted lower OS rates and higher
recurrence rates. Elevated hnRNPA1 expression was significantly
associated with pre-operative blood AFP levels, TNM stage, tumor
size, vascular invasion, Edmonson grade, NLR, PLR, survival and
recurrence, but not with patient clinicopathological
characteristics. These findings indicate an important role of
hnRNPA1 gene in the recurrence of liver cancer. It is known that
hnRNPA1 can activate the epidermal growth factor
receptor/mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (ERK) pathway in HCC cells to regulate the
splicing of the insulin receptor transcript, as well as the
Ras/MAPK/ERK pathway to regulate functional A-Raf gene splicing to
stimulate tumor proliferation. Another study revealed that
hnRNPA1/A2 overexpression can induce malignant transformation of
immortalized hepatocytes and inhibit the apoptosis of HCC cells. If
the inhibition of hnRNPA1/A2 expression can reduce in vitro
growth and in vivo tumorigenesis of HCC cells, it is highly
likely that it is required for HCC growth and development (28).
The present study also analyzed the effect of
hnRNPA1 expression on tumor recurrence and metastasis in HCC
patients, and revealed that the recurrence rate in the
hnRNPA1-positive group was significantly higher, and that the
survival rate was significantly lower compared with that in the
hnRNPA1-negative group (P<0.001), indicating that hnRNPA1
overexpression may present a potential risk of recurrence and
metastasis. Multivariate analyses validated hnRNPA1 as a
significant independent predictor for RFS (HR, 0.685; 95% CI,
0.506–0.928; P=0.015) and OS (HR, 0.629; 95% CI, 0.454–0.871;
P=0.005). These observations were similar to those of previous
reports on HCC (14) and other
malignancies (10–13). Another study (29) reported a major role of hnRNPA1 in the
majority of tumors, along with its association with metastasis in
different cancer types. Furthermore, overexpression of hnRNPA1
promoted tumor invasion through regulating CD44v6, which indicated
that hnRNPA1 may act as a marker for tumor aggressiveness and as a
prognostic predictor in HCC (14).
Currently, the association between hnRNPA1 and the
clinicopathological characteristics of HCC remains controversial.
AFP is a well-known HCC biomarker used for monitoring treatment,
and patients with elevated serum AFP frequently have a worse
prognosis for OS (30). The risk of
mortality increased significantly in patients with higher
pre-operative serum AFP in the present study. Multivariate analyses
validated that a pre-operative blood AFP level >400 µg/l was an
independent prognostic factor for RFS (HR, 1.350; 95% CI,
1.006–1.811; P=0.045) and OS (HR, 1.564; 95% CI, 1.151–2.126;
P=0.004). The results of the present study also revealed a
significant association between elevated hnRNPA1 expression and
elevated pre-operative blood AFP. Therefore, the combination of
hnRNPA1 expression and serum AFP presents a good biomarker for this
specific population.
Elevated pre-operative AFP, tumor diameter >5 cm
and vascular invasion are three widely recognized factors affecting
the prognosis of liver cancer patients, and have been incorporated
into the guidelines for liver cancer diagnosis and treatment
(31). Inflammation serves an
important role in tumor formation, progression and invasion
(32); the inflammatory cytokines
released by immune cells can trigger oxidative damage and malignant
transformation, and aggravate the inflammatory state, which
enhances tumor growth, tissue invasion and metastasis (15,33).
Inflammatory factors released by tumor cells also stimulate
tumorigenesis, tumor angiogenesis, invasion and metastasis
(34). Neutrophils, an important
mediator of inflammatory reactions, can further promote tumor
growth and invasion by remodeling the extracellular matrix, which
significantly inhibits lymphocyte-mediated tumor cell death.
Platelet-reactive protein and other factors released by the
platelets also encourage the proliferation of malignant tumor cells
(35). Therefore, when the NLR and/or
PLR increases, the immune system is weakened and cannot clear the
tumor cells. Studies have shown that pre-operative elevated NLR and
PLR are associated with poor prognosis in patients with liver
cancer (36,37). As biological indicators of systemic
inflammation, NLR and PLR reflect the host immunity level. In the
present study, pre-operative PLR level was not an independent risk
factor for long-term survival, but patients with PLR >91 had
significantly lower long-term OS compared with patients with PLR
≤91. This is in agreement with a previous study, which associated
pre-operative PLR ≥125 with higher tumor staging and aggressive
tumor behavior, and showed it to be an independent prognostic
factor for liver cancer recurrence following liver transplantation
(38). Nevertheless, the
heterogeneity among the selected subjects and PLR truncation
methods may explain the differences between the aforementioned
studies regarding the use of PLR as an independent risk factor for
liver cancer patients. The mortality risk increased significantly
in patients with higher pre-operative NLR, and multivariate
analysis confirmed that higher pre-operative NLR had an unfavorable
impact on OS (HR, 1.758; 95% CI, 1.161–2.661; P=0.008). hnRNPA1
expression level was correlated with NLR (r=0.122, P=0.012) and PLR
(r=0.140, P=0.004). Taken together, the results of the present
study point towards an association between hnRNPA1 expression and
other biological markers of systemic inflammation, although the
specific mechanism remains unclear.
Potential limitations of the present study include
its retrospective nature, single-center sampling and the small
sample size (480 patients in two independent cohorts). Therefore,
to further validate these findings, a large-scale prospective study
should be performed.
In conclusion, elevated hnRNPA1 may be an
independent prognostic factor of increased mortality and recurrence
risk. Clinical detection of hnRNPA1 levels could therefore be
beneficial for the treatment and prognostic evaluation of HCC
patients. In the present study, hnRNPA1 protein expression in HCC
tissue was associated with AFP, NLR and PLR, and the combination of
high hnRNPA1 and pre-operative serum AFP levels was a good
biomarker for this specific population. There is also a possibility
of a correlation between hnRNPA1 expression and biological markers
of systemic inflammation. Future studies should focus on
cytological and molecular investigations that may aid further
understanding of the mechanism of hnRNPA1-mediated
tumorigenesis/progression and unearth novel targets for the
treatment of HCC.
Acknowledgements
Not applicable.
Funding
This study was supported by The Key Project of
Natural Science Foundation of Fujian Province (grant nos.
2015J01406, 2015y0026 and 2016J01585), The Army's Logistics Medical
Research Major Projects Fund Grants (grant nos. CNJ15J002 and
12ZX22) and Startup Fund for Scientific Research, Fujian Medical
University (grant no. 2017×Q2048).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KZ and YJ designed the research. RK, LL, JL and XZ
conducted the experiments. RK, LL and FY analyzed the data. The
manuscript was drafted by RK and LL. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Research
Ethics Committee of Fuzhou General Hospital (Fuzhou, China) and
written informed consent was obtained from all participants.
Patient consent for publication
All participants provided written informed consent
for publication of the data and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yan X and Qiu Y: Impact of current staging
systems on treatment strategy for HBV-related hepatocellular
carcinoma. Cancer Lett. 379:220–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H
and Zou X: Report of cancer incidence and mortality in China, 2010.
Ann Transl Med. 2:612014.PubMed/NCBI
|
|
3
|
Olsen SK, Brown RS and Siegel AB:
Hepatocellular carcinoma: Review of current treatment with a focus
on targeted molecular therapies. Therap Adv Gastroenterol. 3:55–66.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang CH, Wey KC, Mo LR, Chang KK, Lin RC
and Kuo JJ: Current trends and recent advances in diagnosis,
therapy, and prevention of hepatocellular carcinoma. Asian Pac J
Cancer Prev. 16:3595–3604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jonas S, Al-Abadi H, Benckert C, Thelen A,
Hippler-Benscheid M, Saribeyoglu K, Radtke B, Pratschke J and
Neuhaus P: Prognostic significance of the DNA-index in liver
transplantation for hepatocellular carcinoma in cirrhosis. Ann
Surg. 250:1008–1013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim E, Goren A and Ast G: Insights into
the connection between cancer and alternative splicing. Trends
Genet. 24:7–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papaemmanuil E, Cazzola M, Boultwood J,
Malcovati L, Vyas P, Bowen D, Pellagatti A, Wainscoat JS,
Hellstrom-Lindberg E, Gambacorti-Passerini C, et al: Somatic SF3B1
mutation in myelodysplasia with ring sideroblasts. N Engl J Med.
365:1384–1395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han SP, Tang YH and Smith R: Functional
diversity of the hnRNPs: Past, present and perspectives. Biochem J.
430:379–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LaBranche H, Dupuis S, Ben-David Y, Bani
MR, Wellinger RJ and Chabot B: Telomere elongation by hnRNP A1 and
a derivative that interacts with telomeric repeats and telomerase.
Nat Genet. 19:199–202. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cammas A, Lacroix-Triki M, Pierredon S, Le
Bras M, Iacovoni JS, Teulade-Fichou MP, Favre G, Roché H, Filleron
T, Millevoi S and Vagner S: hnRNP A1-mediated translational
regulation of the G quadruplex-containing RON receptor tyrosine
kinase mRNA linked to tumor progression. Oncotarget. 7:16793–16805.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
David CJ, Chen M, Assanah M, Canoll P and
Manley JL: HnRNP proteins controlled by c-Myc deregulate pyruvate
kinase mRNA splicing in cancer. Nature. 463:364–368. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma YL, Peng JY, Zhang P, Huang L, Liu WJ,
Shen TY, Chen HQ, Zhou YK, Zhang M, Chu ZX and Qin HL:
Heterogeneous nuclear ribonucleoprotein A1 is identified as a
potential biomarker for colorectal cancer based on differential
proteomics technology. J Proteome Res. 8:4525–4535. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Golan-Gerstl R, Cohen M, Shilo A, Suh SS,
Bakàcs A, Coppola L and Karni R: Splicing factor hnRNP A2/B1
regulates tumor suppressor gene splicing and is an oncogenic driver
in glioblastoma. Cancer Res. 71:4464–4472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou ZJ, Dai Z, Zhou SL, Fu XT, Zhao YM,
Shi YH, Zhou J and Fan J: Overexpression of HnRNP A1 promotes tumor
invasion through regulating CD44v6 and indicates poor prognosis for
hepatocellular carcinoma. Int J Cancer. 132:1080–1089. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tazzyman S, Barry ST, Ashton S, Wood P,
Blakey D, Lewis CE and Murdoch C: Inhibition of neutrophil
infiltration into A549 lung tumors in vitro and in vivo
using a CXCR2-specific antagonist is associated with reduced tumor
growth. Int J Cancer. 129:847–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki K, Kachala SS, Kadota K, Shen R, Mo
Q, Beer DG, Rusch VW, Travis WD and Adusumilli PS: Prognostic
immune markers in non-small cell lung cancer. Clin Cancer Res.
17:5247–5256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei K, Wang M, Zhang W, Mu H and Song TQ:
Neutrophil-lymphocyte ratio as a predictor of outcomes for patients
with hepatocellular carcinoma undergoing TAE combined with
Sorafenib. Med Oncol. 31:9692014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao Y, Ke R, Wang S, Zhu X, Chen J, Huang
C, Jiang Y and Lv L: DNA topoisomerase IIalpha and Ki67 are
prognostic factors in patients with hepatocellular carcinoma. Oncol
Lett. 13:4109–4116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kee KM, Wang JH, Lee CM, Chen CL,
Changchien CS, Hu TH, Cheng YF, Hsu HC, Wang CC, Chen TY, et al:
Validation of clinical AJCC/UICC TNM staging system for
hepatocellular carcinoma: analysis of 5,613 cases from a medical
center in southern Taiwan. Int J Cancer. 120:2650–2655. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Liu H, Liu J, Haley KN, Treadway JA,
Larson JP, Ge N, Peale F and Bruchez MP: Immunofluorescent labeling
of cancer marker Her2 and other cellular targets with semiconductor
quantum dots. Nat Biotechnol. 21:41–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Fushiya N, Koike K, Nishino H and Tajiri H: Comparison of
the prognostic value of inflammation-based prognostic scores in
patients with hepatocellular carcinoma. Br J Cancer. 107:988–993.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong L, Bai K, Cao Y, Huang Q, Lv L and
Jiang Y: Prognostic value of pre-operative platelet to lymphocyte
ratio in patients with resected primary hepatocellular carcinoma.
Clin Lab. 62:2191–2196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jean-Philippe J, Paz S and Caputi M: hnRNP
A1: The Swiss army knife of gene expression. Int J Mol Sci.
14:18999–19024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Wei JS, Li SQ, Badgett TC, Song
YK, Agarwal S, Coarfa C, Tolman C, Hurd L, Liao H, et al: MYCN
controls an alternative RNA splicing program in high-risk
metastatic neuroblastoma. Cancer Lett. 371:214–224. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luan W, Wang Y, Chen X, Shi Y, Wang J,
Zhang J, Qian J, Li R, Tao T, Wei W, et al: PKM2 promotes glucose
metabolism and cell growth in gliomas through a mechanism involving
a let-7a/c-Myc/hnRNPA1 feedback loop. Oncotarget. 6:13006–13018.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ford LP, Wright WE and Shay JW: A model
for heterogeneous nuclear ribonucleoproteins in telomere and
telomerase regulation. Oncogene. 21:580–583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chettouh H, Fartoux L, Aoudjehane L,
Wendum D, Clapéron A, Chrétien Y, Rey C, Scatton O, Soubrane O,
Conti F, et al: Mitogenic insulin receptor-A is overexpressed in
human hepatocellular carcinoma due to EGFR-mediated dysregulation
of RNA splicing factors. Cancer Res. 73:3974–3986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Zhou Y, Lou Y and Zhong H:
Knockdown of HNRNPA1 inhibits lung adenocarcinoma cell
proliferation through cell cycle arrest at G0/G1 phase. Gene.
576:791–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhen L, Shijie N and Shuijun Z: Tumor PHD2
expression is correlated with clinical features and prognosis of
patients with HCC receiving liver resection. Medicine. 93:e1792014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vitale A, Morales RR, Zanus G, Farinati F,
Burra P, Angeli P, Frigo AC, Del Poggio P, Rapaccini G, Di Nolfo
MA, et al: Barcelona clinic liver cancer staging and transplant
survival benefit for patients with hepatocellular carcinoma: A
multicentre, cohort study. Lancet Oncol. 12:654–662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pichler M, Hutterer GC, Stoeckigt C,
Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A,
Mannweiler S, Pummer K and Zigeuner R: Validation of the
pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in
a large European cohort of renal cell carcinoma patients. Br J
Cancer. 108:901–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aliustaoglu M, Bilici A, Ustaalioglu BB,
Konya V, Gucun M, Seker M and Gumus M: The effect of peripheral
blood values on prognosis of patients with locally advanced gastric
cancer before treatment. Med Oncol. 27:1060–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gomez D, Farid S, Malik HZ, Young AL,
Toogood GJ, Lodge JP and Prasad KR: Preoperative
neutrophil-to-lymphocyte ratio as a prognostic predictor after
curative resection for hepatocellular carcinoma. World J Surg.
32:1757–1762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song W, Wang K, Zhong FP, Fan YW, Peng L
and Zou SB: Clinicopathological and prognostic significance of
platelet-to-lymphocyte ratio in patients with hepatocellular
carcinoma. Oncotarget. 7:81830–81838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qi X, Li J, Deng H, Li H, Su C and Guo X:
Neutrophil-to-lymphocyte ratio for the prognostic assessment of
hepatocellular carcinoma: A systematic review and meta-analysis of
observational studies. Oncotarget. 7:45283–45301. 2016. View Article : Google Scholar : PubMed/NCBI
|