Introduction
Thyroid cancer is the most common endocrine
malignancy in the world, with a rapid increasing incidence
(1); it has been classified into
several subtypes, including papillary thyroid cancer (PTC),
follicular thyroid cancer, medullary thyroid cancer and anaplastic
thyroid cancer (2). PTC is the most
common type and is derived from follicular thyroid cells and
accounts for ~80% of thyroid cancer cases (3). Currently, the majority of PTC patients
that undergo surgical resection in combination with radioiodine and
levothyroxine have a good prognosis; however, recurrence is
observed in ~30% of patients (4).
Therefore, understanding the molecular mechanism underlying the
development and progression of PTC is necessary to elucidate more
effective therapeutic strategies.
MicroRNAs (miRNAs/miRs) are a group of small
endogenous non-coding RNAs with a length of 19–22 nucleotides,
which are derived from double-stranded RNA (5). miRs are capable of regulating many
biological processes, including cell differentiation, apoptosis and
development through the complimentary binding of the
3′-untranslated region (3′-UTR) of target genes, resulting in the
degradation or silencing of the target mRNA (6,7). Evidence
indicates that the alteration of miRNAs was frequently associated
with cancer progression, the miRNAs acting as tumor suppressors or
oncogenes depending on the cellular function of their targets
(8,9).
A number of miRNAs have been identified to contribute to PTC
development and progression, including miR-199a-3p (10), miR-101 (11) and miR-34a (12). Previously, miR-409-3p has been
reported to be a potential tumor suppressor in variety of cancer
types, including colorectal (13),
prostate (14) and breast (15) cancer. However, to the best of our
knowledge, no evidence of miR-409-3p function in PTC has been
documented.
In this study, the expression of miR-409-3p in human
PTC tissues and cells was determined. In vitro experiments
were performed to investigate the function role of miR-409-3p in
PTC cells, as well as the underlying mechanisms. The findings of
the present study suggest that miR-409-3p may be a potential target
for PTC treatment and serve an important role in development and
progression of PTC.
Materials and methods
Clinical tissue specimens and cell
lines
Samples of 20 pairs of primary PTC tissues and
adjacent non-tumor tissues (≥3 cm away from the primary site) were
obtained from patients with thyroid cancer, who had undergone
surgical resection at the Fourth Hospital of Hebei Medical
University (Hebei, China). Specimens were collected, immediately
snap-frozen in liquid nitrogen and stored at −80°C until RNA was
extracted. In the present study, each patient voluntarily signed
written informed consent and the collection and use of patient
samples was approved by the Ethics Committee of the Fourth Hospital
of Hebei Medical University.
Human PTC B-CPAP, TPC-1 and GLAG-66 cell lines and
the human thyroid epithelial Nthy-ori3-1 cell line were purchased
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). All cell lines were cultured in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Sigma-Aldrich, Merck,
KGaA, Darmstadt, Germany) in a humidified incubator of 5%
CO2 at 37°C.
Cell transfection
The synthesized oligos for miR-409-3p mimics
(5′-GAAUGUUGCUCGGUGAACCCCU-3′) or negative control (NC:
5′-ACTACTGAGTGACAGTAGA-3′) were purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China). For cyclin D2 overexpression, the coding
sequence of human cyclin D2 was cloned into a pcDNA3.1 vector
(performed by Thermo Fisher Scientific, Inc.). The empty pcDNA3.1
was used as a negative control. Next, TPC-1 and GLAG-66 cells were
seeded into 24-well plates at a density of 4×105 cells
per well and allowed to attach prior to transfection to ensure
60–70% cell confluence and transfected with the aforementioned
vectors using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The miRNA mimics were used at a final
concentration of 50 nM. Cell samples were collected at 48 h after
transfection for further analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was isolated from tissues and PTC cells with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and RNA templates were reverse transcribed into cDNA using a
One Step PrimeScript cDNA Synthesis kit (Takara Bio, Inc., Otsu,
Japan) according to the manufacturer's protocol. miR-409-3p and
CCND2 expression levels were quantified using a ABI 7500
FAST real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using SYBR Green PCR Master Mix (Takara Bio,
Inc.). The thermocycling conditions of the PCR reaction were as
follows: Initial denaturation for 1 min at 95°C, denaturation for 5
sec at 95°C, and annealing for 40 cycles at 60°C. The expression of
U6 and GAPDH were used to normalize miR-409-3p and CCND2
expression levels in each group, respectively, using the
2−ΔΔCq method (16). The
primer sequences were as follows: miR-409-3p forward,
GAATGTTGCTCGGTGAACCCCT and reverse, GAAUGUUGCUCGGUGAACCCCU;
CCND2 forward, TGCAACCGACGATTCTTCTACTCAA and reverse,
CAAGCAGTGATGTATCTGATAAACAAGG; U6 forward, CTCGCTTCGGCAGCACA and
reverse, AACGCTTCACGAATTTGCGT; and GAPDH forward,
AGAGGCAGGGATGATGTTCTG and reverse, GACTCATGACCACAGTCCATGC.
Cell proliferation assay
For cell proliferation assay, approximately
4×103 TPC-1 and GLAG-66 cells following transfection
were seeded in a 96-well plate. After removing the medium, Cell
Counting Kit-8 solution (CCK-8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) was added to cells and incubated at 37°C for
an additional 2 h. The absorbance of the solution at a wavelength
of 450 nm was measured with a MRX II absorbance reader (Dynex
Technologies, Worthing, UK).
Colony formation assay
After 48 h transfection, TPC-1 and GLAG-66 cells at
a density of 500 cells per well were seeded into 6-well plates and
incubated for 7 days at 37°C. Subsequently, the cells were washed
twice with PBS, fixed in 70% ethanol and stained with 1% crystal
violet solution for 30 min at room temperature. Colonies containing
>50 cells were photographed and counted using a light microscope
(×200 magnification).
Cell cycle analysis
Cell cycle distribution was determined using flow
cytometry with propidium iodide (PI) staining (Sigma-Aldrich, Merck
KGaA Darmstadt Germany). Briefly, cells were plated in 6-cm dishes
at a density of 1.0×105 cells per dish after
transfection for 48 h. Next, cells were washed with PBS and fixed
in 70% ethanol overnight at 4°C. The following day, the cells were
stained with a PI/RNase Staining Solution (Beyotime Institute of
Biotechnology) at 4°C for 25 min. Cellular DNA was analyzed on
fluorescence-activated cells sorting (FACS) Calibur flow cytometer
(Gallios, Beckman Coulter, Inc., Brea, CA, USA).
Dual-luciferase reporter assays
The potential target genes of miR-409-3p were
predicted using TargetScan database (http://www.targetscan.org/vert_71/). The 3′-UTR of
CCND2 containing predicted miR-409-3p binding sites
(5′-UGACAUUCCCAUCACAACAUUC-3′) was amplified from human cDNA and
cloned into the psiCHECK-2 dual-luciferase expression vector by
Promega Corporation (Madison, WI, USA). The mutation of the
predicted 3′-UTR was obtained using PCR site-directed mutagenesis
by Promega Corporation (Madison, WI, USA). 293T cells were seeded
into 48-well plates, then co-transfected with miR-409-3p mimics or
NC and luciferase reporter plasmids (50 ng) containing the
psiCHECK-cyclin D2-3′-UTR wild type or mutant
(5′-UGACAUUCCCAUCAGTTGTAAC-3′) using Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.) for 24 h at 37 °C. Firefly and
Renilla luciferase activities were measured at 48 h after
transfection using a dual-luciferase reporter assay system (Promega
Corporation). As per the previous experiment an empty vector
control was usedto validate that there was no-specific effects from
vector integration into the hosts genome during transfection.
Results were presented as the ratio of Renilla luciferase
activity to firefly luciferase activity.
Western blot analysis
At 48 h after transfection, cells were harvested,
lysed using RIPA lysis buffer (Beyotime Institute of Biotechnology)
and the protein concentration in samples was quantified with the
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology). Equivalent quantities (30 µg per lane) of protein
were separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Membranes were blocked for 1 h with 5% non-fat milk at room
temperature and then incubated overnight at 4°C with primary
antibodies, including anti-cyclin D2 (1:1,000, ab81359, Abcam,
Cambridge, UK) and anti-GAPDH (1:10,000, 10494-1-AP, ProteinTech
Group, Inc., Chicago, IL, USA). Next, the membranes were washed
three times in TBST containing 0.1% Tween-20 and incubated with the
corresponding horseradish peroxidase-conjugated goat anti-rabbit
(1:5,000; sc-2054, Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
at room temperature for 1 h. The blots were visualized using an
enhanced chemiluminescence system (BeyoECLPlus; Beyotime Institute
of Biotechnology). Band density was measured by Image-Pro Plus
version 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
All experiments were repeated in triplicate and the
results were expressed as the mean ± standard deviation. All
analyses were performed using GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA). A two-tailed Student's t-test
was applied for comparisons between the two groups. Multiple
comparisons were performed using a one-way analysis of variance
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-409-3p is
downregulated in PTC tissues and cell lines
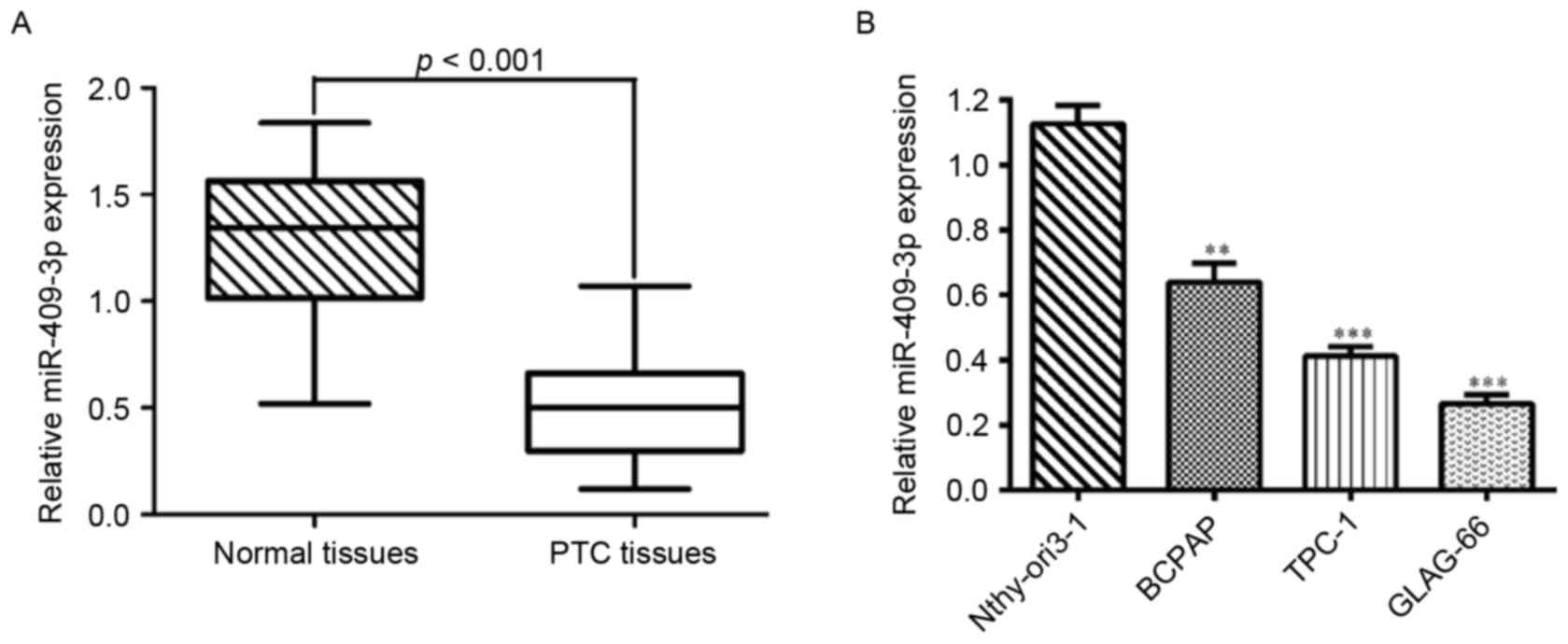
The expression levels of miR-409-3p were first
determined by RT-qPCR in 20 pairs of human PTC tissues and adjacent
normal tissues. As shown in Fig. 1A,
the expression level of miR-409-3p was significantly lower in tumor
tissues compared with matched non-tumor tissues (P<0.001). Also,
the expression levels of miR-409-3p were measured in three PTC cell
lines (B-CPAP, TPC-1 and GLAG-66) and a normal human thyroid
epithelial cell line (Nthy-ori3-1). miR-409-3p was significantly
downregulated in all PTC cell lines compared with Nthy-ori3-1
(Fig. 1B; P<0.001). Thus, it was
concluded that a decrease in miR-409-3p expression may serve a role
in PTC development.
Overexpression of miR-409-3p induces
cell growth inhibition and G0/G1 phase arrest
in PTC cells
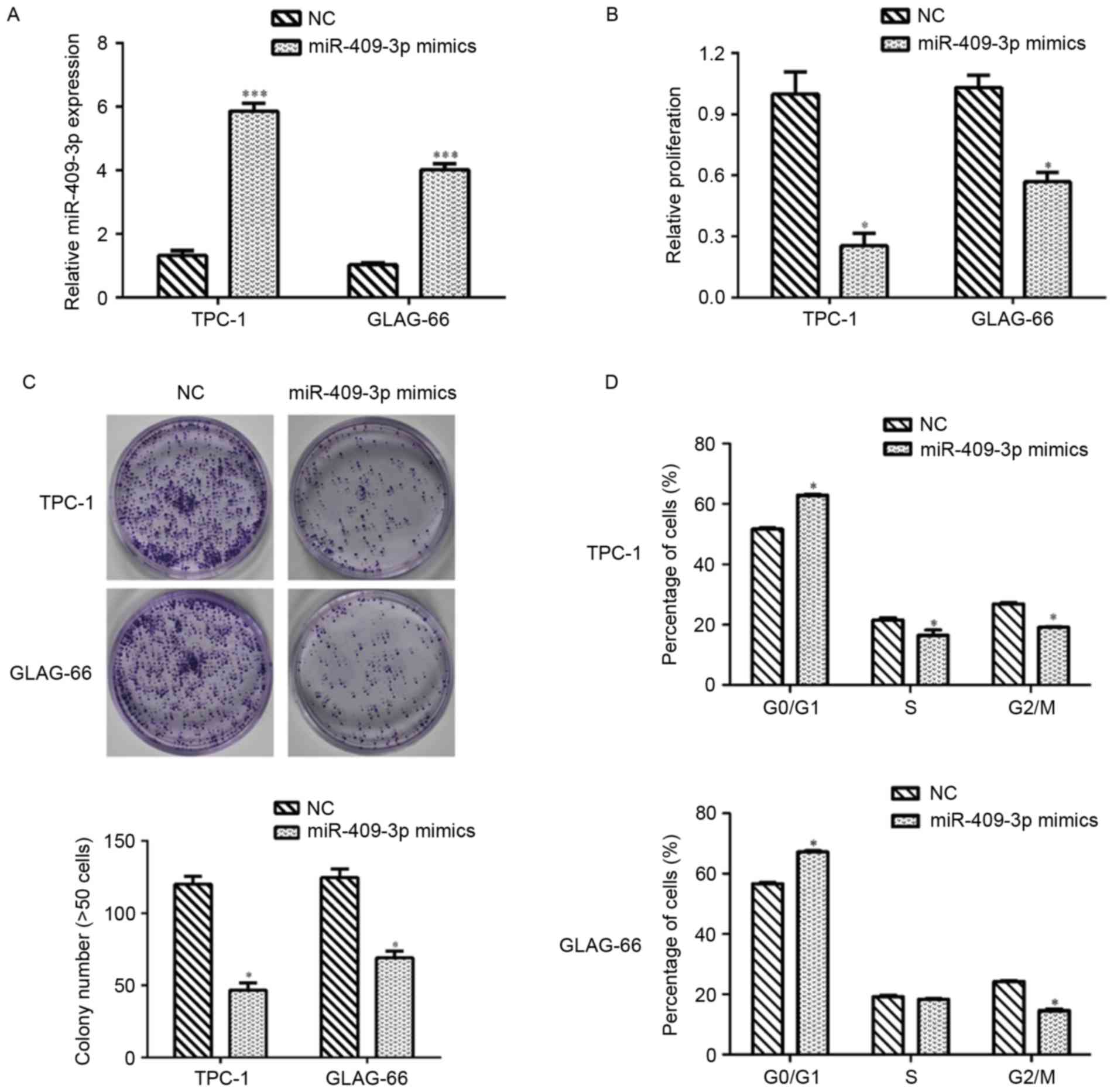
The results demonstrated that TPC-1 and GLAG-66
cells presented lower expression of miR-409-3p. Therefore,
synthetic miR-409-3p mimics and NC mimics were separately
transfected into TPC-1 and GLAG-66 cells to investigate the
biological function of miR-409-3p in PTC. RT-qPCR assays revealed
that miR-409-3p expression was significantly upregulated following
transfection with miR-409-3p mimics, compared with NC control cells
in TPC and GLAG-66 cells (P<0.001; Fig. 2A). The CCK-8 assay demonstrated that
transfection with miR-409-3p mimics significantly inhibited cell
proliferation in TPC-1 and GLAG-66 cells (P<0.05; Fig. 2B). The colony formation assay
indicated that the ability of colony formation in TPC-1 and GLAG-66
cells transient transfected with miR-409-3p mimics was impaired
compared with that in NC-transfected cells (P<0.05; Fig. 2C). Flow cytometric analysis revealed
that overexpression of miR-409-3p led to
G0/G1 phase arrest by elevating the
percentage of cells at G0/G1 phase in TPC-1
and GLAG-66 cells, but decreasing the cell numbers at S and
G2/M phase in TPC cells and cells at G2/M
phase in GLAG-66 cells (P<0.05; Fig.
2D).
miR-409-3p downregulates cyclin D2
expression by targeting its 3′UTR
On the basis of the aforementioned results, a
possible role for miR-409-3p in repressing
G0/G1-associated genes was hypothesized. As
shown in Fig. 3A, CCND2
exhibited miR-409-3p-binding sequences in its 3′-UTR, which was
identified as a putative target of miR-409-3p by miRNA target gene
prediction. To confirm the direct targeting of CCND2 by
miR-409-3p, a dual-luciferase reporter assay was performed by
integrating sequences of the CCND2 3′UTR containing the
binding sites for miR-409-3p or corresponding mutated sequences
into 293T cells. Luciferase assays revealed that miR-409-3p mimics
significantly decreased the relative activity of the luciferase
reporter containing the wild-type 3′-UTR of CCND2 mRNA
(P<0.05), but did not affect the luciferase activity of the
mutant CCND2 3′-UTR (Fig. 3B).
Next, the effect of miR-409-3p on the expression of CCND2
was measured at the mRNA and protein levels in PTC cells. As shown
in Fig. 3C, transfection with
miR-409-3p mimics downregulated the mRNA and protein expression
levels of CCND2 and cyclin D2, respectively, compared with
cells transfected with NC controls (P<0.05). These results
indicated that CCND2 may be a direct target of miR-409-3p in
PTC.
Enhanced expression of CCND2 partially
rescues miR-409-3p-induced cell growth inhibition and
G0/G1 phase arrest in TPC cells
To determine whether CCND2 mediated the
inhibitory effect of miR-409-3p on cell proliferation and cell
cycle progression, rescue experiments were performed by
transfecting pcDNA/CCND2 into PTC cells with higher
miR-409-3p expression levels and cell proliferation and cell cycle
were then examined. The results revealed that overexpression of
CCND2 partially rescued the impaired cell proliferation
induced by miR-409-3p mimics in TPC-1 and GLAG-66 cells (P<0.05;
Fig. 4A). It was also found that
restoration of CCND2 reversed the cell cycle
G0/G1 phase arrest imposed by miR-409-3p
mimics in TPC-1 (P<0.05; Fig. 4B)
and GLAG-66 cells (P<0.05; Fig.
4C). These data indicated that CCND2 may mediate the
suppressive functions of miR-409-3p in PTC cells.
Discussion
In recent years, many miRNA signatures have been
well characterized for PTC (17);
however, but further investigation on the roles of dysregulated
miRs in PTC development and progression is necessary for seeking
more effective therapeutic strategies. In the present study, it was
observed that miR-409-3p was frequently downregulated in PTC
samples specimens and cell lines, consistent with its reduced
expression in lung (18) and bladder
cancer (19). Functional analysis
revealed that overexpression of miR-409-3p caused a reduction in
proliferation rate, which was accompanied by
G0/G1 phase arrest of the cell cycle,
indicating its suppressive role in PTC cells.
Evidence of miR-409-3p as a tumor growth inhibitor
has been well documented in several types of cancer. For example,
miR-409-3p inhibited growth and induced apoptosis in gastric cancer
(20). In breast cancer, miR-409-3p
has been demonstrated to suppress cell growth by targeting protein
kinase B (15). Consistent with the
role of miR-409-3p in PTC cell proliferation, the present study
identified CCND2 as a target gene of miR-409-3p. Further
analysis demonstrated that the enforced expression of miR-409-3p
significantly decreased cyclin D2 protein expression levels,
indicating that miR-409-3p may be able to negatively regulate the
expression of CCND2. The identification of CCND2 may
account for one of the underlying mechanisms by which miR-409-3p is
involved in PTC cell proliferation.
D-type cyclins are reported to be closely associated
with cell cycle progression (21), of
which CCND2 is a key player in the cell cycle from the
G0/G1 phase to S phase (22). The CCND2 oncogene has been well
noted in human thyroid cancer, which was downregulated by miR-1
(23). Similarly, upregulation of
CCND2 has been documented in breast cancer (24) and prostate cancer (25). To confirm the role of CCND2 in
PTC further, the present study also demonstrated that when
miR-409-3p-expressing cells resumed CCND2 expression, the
proliferation deficiencies and G0/G1 phase
arrest were partly reversed. Therefore, it was concluded that
miR-409-3p may inhibit cell proliferation and cell cycle
progression in PTC by downregulating, at least partially, the
protein expression of cyclin D2.
In conclusion, the results of the present study
indicated that miR-409-3p is frequently downregulated in PTC and
may negatively regulate PTC cell proliferation and cell cycle
progression by downregulating its target gene CCND2. The
data may help to further elucidate the molecular mechanisms
underlying PTC progression and provide a strategy for targeting the
miR-409-3p/CCND2 interaction as a novel prevention and
treatment of PTC.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
ZZ, FY and YL performed the experiments. KF
participated in the interpretation of data. SJ is major contributor
and participated in the design of study. All authors revised the
manuscript, read and approved the final manuscript.
Ethics approval and consent to
participate
Each patient voluntarily signed written informed
consent and the collection and the current study was approved by
the Fourth Hospital of Hebei Medical University.
Patient consent for publication
The written informed consent for publication was
signed by all patients in advance and they gave their consent for
the publication of any associated data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carneiro RM, Carneiro BA, Agulnik M, Kopp
PA and Giles FJ: Targeted therapies in advanced differentiated
thyroid cancer. Cancer Treat Rev. 41:690–698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lloyd RV, Buehler D and Khanafshar E:
Papillary thyroid carcinoma variants. Head Neck Pathol. 5:51–56.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi X, Liu R, Basolo F, Giannini R, Shen
X, Teng D, Guan H, Shan Z, Teng W, Musholt TJ, et al: Differential
clinicopathological risk and prognosis of major papillary thyroid
cancer variants. J Clin Endocrinol Metab. 101:264–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Srivastava A, Goldberger H, Dimtchev A,
Ramalinga M, Chijioke J, Marian C, Oermann EK, Uhm S, Kim JS, Chen
LN, et al: MicroRNA profiling in prostate cancer-the diagnostic
potential of urinary miR-205 and miR-214. PLoS One. 8:e769942013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minna E, Romeo P, De Cecco L, Dugo M,
Cassinelli G, Pilotti S, Degl'Innocenti D, Lanzi C, Casalini P,
Pierotti MA, et al: miR-199a-3p displays tumor suppressor functions
in papillary thyroid carcinoma. Oncotarget. 5:2513–2528. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin X, Guan H, Li H, Liu L, Liu J, Wei G,
Huang Z, Liao Z and Li Y: miR-101 inhibits cell proliferation by
targeting Rac1 in papillary thyroid carcinoma. Biomed Rep.
2:122–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma Y, Qin H and Cui Y: miR-34a targets
GAS1 to promote cell proliferation and inhibit apoptosis in
papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem
Biophys Res Commun. 441:958–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai R, Weng C, Dong H, Li S, Chen G and Xu
Z: MicroRNA-409-3p suppresses colorectal cancer invasion and
metastasis partly by targeting GAB1 expression. Int J Cancer.
137:2310–2322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang G, Liu Z, Xu H and Yang Q:
miR-409-3p suppresses breast cancer cell growth and invasion by
targeting Akt1. Biochem Biophys Res Commun. 469:189–195. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu G, Xie L and Miller D: Expression of
MicroRNAs in thyroid carcinoma. Methods Mol Biol. 1617:261–280.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan L, Zhu L, Xu J, Lu B, Yang Y, Liu F
and Wang Z: MicroRNA-409-3p functions as a tumor suppressor in
human lung adenocarcinoma by targeting c-Met. Cell Physiol Biochem.
34:1273–1290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Chen H, Lin Y, Hu Z, Mao Y, Wu J, Xu
X, Zhu Y, Li S, Zheng X and Xie L: MicroRNA-409-3p inhibits
migration and invasion of bladder cancer cells via targeting c-Met.
Mol Cells. 36:62–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Nie H, Wang M, Su L, Li J, Yu B, Wei
M, Ju J, Yu Y, Yan M, et al: MicroRNA-409-3p regulates cell
proliferation and apoptosis by targeting PHF10 in gastric cancer.
Cancer Lett. 320:189–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ando K, Ajchenbaum-Cymbalista F and
Griffin JD: Regulation of G1/S transition by cyclins D2 and D3 in
hematopoietic cells. Proc Natl Acad Sci USA. 90:9571–9575. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leone V, D'Angelo D, Rubio I, de Freitas
PM, Federico A, Colamaio M, Pallante P, Medeiros-Neto G and Fusco
A: MiR-1 is a tumor suppressor in thyroid carcinogenesis targeting
CCND2, CXCR4, and SDF-1alpha. J Clin Endocrinol Metab.
96:E1388–E1398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Tian Y, Li J, Lu B, Sun M, Zou Y,
Kong R, Luo Y, Shi Y, Wang K and Ji G: miR-206 is down-regulated in
breast cancer and inhibits cell proliferation through the
up-regulation of cyclinD2. Biochem Biophys Res Commun. 433:207–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong Q, Meng P, Wang T, Qin W, Qin W, Wang
F, Yuan J, Chen Z, Yang A and Wang H: MicroRNA let-7a inhibits
proliferation of human prostate cancer cells in vitro and in vivo
by targeting E2F2 and CCND2. PLoS One. 5:e101472010. View Article : Google Scholar : PubMed/NCBI
|