Introduction
Lung cancer is the most frequently diagnosed cancer
and the leading cause of cancer mortality worldwide. Over 80% of
cases are histologically classified as non-small cell lung cancer
(NSCLC) (1,2). Surgery is generally the treatment of
choice for early stage NSCLC. However, 30–55% of patients develop
recurrence even following complete resection, and the mortality
rate is high (3). As early disease is
typically asymptomatic, the majority of patients are diagnosed at a
distant stage, resulting in extremely poor prognosis, even with
chemotherapy and/or radiotherapy (2).
Recently, computed tomography (CT)-guided iodine-125 interstitial
brachytherapy (125I-IBT) has been employed to treat
NSCLC at the inoperable advanced stage, and in cases with local
recurrence or metastasis following surgery or concurrent
chemoradiotherapy, alone or in combination with other treatments
(3–6).
It was demonstrated that 125I-IBT significantly improves
overall survival and local control, by maximizing the radiation
dose delivered to the tumor, and minimizing radiation injury of the
surrounding normal lung tissue. Certain preclinical studies have
further suggested that increased G2/M arrest, increased apoptosis
and enhanced bystander effect may serve key roles in the
anti-proliferative effects induced by 125I-IBT in NSCLC
(7–9).
However, the association between 125I-IBT and glucose
metabolism in NSCLC remains unclear.
The Warburg effect, a hallmark for tumor cells, was
discovered by Otto Warburg in 1924. It is well established that in
contrast to normal cells, which predominantly rely on mitochondrial
oxidative phosphorylation (OXPHOS) to generate the energy required
for cellular processes, the majority of tumor cells instead rely on
aerobic glycolysis. The Warburg effect is a determinant of tumor
cell proliferation (10). The rate of
glucose entry into tumor cells is at least 20–30-fold higher than
normal cells. This unique phenomenon is the basis for the use of
positron emission tomography (PET)/CT with
18F-fluorodeoxyglucose (18F-FDG), a
radioactive glucose analog that is intensely accumulated by tumor
cells (11). 18F-FDG
PET/CT is widely used for initial diagnosis, disease staging,
recurrence and metastasis detection, as well as for monitoring
chemotherapy and/or radiotherapy response in lung cancer (12–16).
In recent years, several studies have investigated
the Warburg effect in lung cancer progression, as well as for the
development of targeted therapies (17–19). It
was suggested that Sad1 and UNC84 domain containing 2 (SUN2)
protein may suppress lung cancer cell proliferation and migration
by attenuating the Warburg effect, whereas pyruvate dehydrogenase
kinase 1 (PDK1), may promote lung cancer cell proliferation and
migration, via the inverse mechanism. In addition, dichloroacetate,
a metabolic agent, may partially revert the Warburg effect in tumor
cells, increase X-ray sensitivity and inhibit the growth of lung
cancer cells. Based on these previous reports, the present study
aimed to investigate if and how the Warburg effect was involved in
125I-IBT for NSCLC. Herein, it was hypothesized that
125I-IBT may have suppressed tumor growth via Warburg
effect inhibition in NSCLC. Alterations in tumor growth and
18F-FDG uptake were monitored using micro-PET/CT
imaging. In addition, the relationships among these factors, the
expression of glycolysis-associated molecules and the radiation
dose were analyzed in NSCLC A549 ×enografts following
125I-IBT.
Materials and methods
Cell culture and animal model
The A549 cell line was purchased from Shanghai
Jianglin Biological Technology Co., Ltd. (Shanghai, China). Cells
were cultured in RPMI-1640 (GE Healthcare Life Sciences, Logan, UT,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and
0.1 mg/ml streptomycin at 37°C in a 5% CO2 atmosphere.
Cells were trypsinized and harvested once 80–90% confluence was
reached. Male BALB/c-nu mice (18–20 g; 4–6 weeks old) were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China) and allowed to acclimatize for 1 week in a specific
pathogen-free room under controlled temperature and humidity prior
to experimental initiation. Following this, ~0.2 ml A549 cell
suspension (1×107 cells/1 ml RPMI-1640) was injected
subcutaneously into the right armpits of the forelegs. After 4
weeks, mice with a xenograft diameter of ~10 mm were used for
study.
Study design
A total of 24 prior mice were randomly divided into
four groups (n=6 per group). The control group without
125I seed implantation, and three treatment groups with
a prescribed dose of 20, 40 and 60 Gy, via 1–2 125I seed
implantation with a radioactivity of 0.3–0.6 mCi, respectively. The
treatment period was 4 weeks. Tumor growth was monitored by
determining the xenograft size every 2 days with a calliper. Before
and 14, 28 days after receiving 125I-IBT, micro-PET/CT
imaging was performed to determine the baseline level and
post-treatment alterations in 18F-FDG uptake. At the end
of study, the mice were euthanized with dislocation of cervical
vertebra after being anesthetized with 3% isoflurane inhalation and
all xenografts were histologically analyzed with
immunohistochemical (IHC) staining.
Interstitial brachytherapy
125I seeds (model 6711; diameter, 0.8 mm;
length, 4.5 mm; half-life, 59.6 days; half value thickness, 1.7 cm
in tissue; main emission, 27.4–31.4 Kev X-ray and 35.5 Kev γ-ray)
were provided by Seeds Biological Pharmacy, Ltd. (Tianjin, China).
The 125I seeds were preloaded into 18-gauge needles and
subsequently implanted into the xenograft center. The radiation
prescribed dose was planned based on the axial micro-CT images
using a brachytherapy treatment planning system (Beijing Astro
Technology, Beijing, China).
Tumor volume
Based on measurements of the short (a) and long (b)
tumor diameters, tumor volume (TV) was calculated, according to the
formula of TV=1/2 × a2 × b. The TV and tumor inhibition
rate (TIR) on day 14 and 28 were defined as TV14,
TV28, TIR14 and TIR28,
respectively. TIR was calculated using the following equation:
TIR=(mean of TVc-mean of TVt)/mean of
TVc ×100%, where TVc was the control TV, and
TVt was the treatment group TV.
Micro-PET/CT imaging
18F-FDG was synthesized by the PET/CT
center in our institution. Following an overnight fast, mice were
injected with ~0.1 mCi 18F-FDG via the tail vein. After
1 h, mice were anesthetized with 3% isoflurane inhalation and
placed in the prone position in the center of a Siemens Inveon
combined micro-PET/CT scanner (Siemens Preclinical Solution USA,
Inc., Knoxville, TN, USA). Micro-CT scans were performed with a 80
kV X-ray tube voltage, 500 µA current, 150 ms ec exposure time and
120 rotation steps. Micro-PET static acquisition was subsequently
performed for 10 min and the data were processed using the ordered
set expectation maximization algorithm for three dimensional PET
reconstruction. Micro-PET/CT images were analyzed with an Inveon
Research Workplace 4.1 (Siemens, Erlangen, Germany). The maximum
standardized uptake value (SUVmax; g/ml) of the xenograft was
measured on day 0, 14 and 28 and referred to as SUVmax0,
SUVmax14, SUVmax28, respectively. The
18F-FDG uptake attenuation rate (FUAR) of the xenograft
on day 14 and 28 was defined as FUAR14 and
FUAR28, respectively, using the following equation:
FUAR=(mean SUVmaxc value-mean SUVmaxt
value)/mean SUVmaxc value ×100%, where
SUVmaxc was the control group SUVmax, and the
SUVmaxt was the treatment group SUVmax.
Histological evaluation
All xenografts were fixed in 10% formalin overnight
at 4°C and subsequently embedded in paraffin for histological
section preparation (4 µm). The sections were deparaffinized and
stained with hematoxylin and eosin for normal histological
evaluation. IHC was also performed with antibodies to detect
mammalian target of rapamycin (mTOR), c-Myc, hypoxia inducible
factor-1α (HIF-1α) and glucose transporter 1 (GLUT1) expression,
according to manufacturer's instructions. Primary antibodies
(Abcam, Cambridge, UK) used were anti-mTOR ab2732 (diluted 1:200),
anti-c-Myc ab32072 (diluted 1:100), anti-HIF-1α ab16066 (diluted
1:400) and anti-GLUT1 ab652 (diluted 1:200). In brief, sections
were dewaxed and rehydrated prior to quenching in 3% hydrogen
peroxide to block endogenous peroxidase activity for 10 min.
Antigen retrieval was performed by immersing the sections in
EDTA/Tris solution (pH 9.0) in a microwave oven for 2×15 min.
Sections were cooled at room temperature and washed in PBS for 3×10
min. Following this, sections were blocked in 5% bovine serum
albumin (BSA) for 30 min and subsequently incubated overnight with
primary antibodies at 4°C and rewarmed for 30 min and washed in PBS
for 3×10 min. Then sections were incubated with biotinylated
secondary antibodies at 37°C for 45 min and washed in PBS for 3×10
min, followed by exposure to streptavidin-biotin complex
(Ready-to-use SABC-POD kits: SA1021, goat anti-mouse IgG and
SA1022, goat anti-rabbit IgG) for 45 min at 37°C and
diaminobenzidine tetrahydrochloride (DAB AR1022; both Boster,
Wuhan, China). Staining without primary antibodies was performed in
parallel and served as a negative control. Finally, sections were
counterstained with hematoxylin, dehydrated in ethanol and xylene
and mounted with cover slips using neutral balsam. Each section was
assessed in three random microscopic fields (×200). The expression
of mTOR, c-Myc, HIF-1α and GLUT1 was quantified by determining the
mean optical density (MOD) using Image-Pro Plus software (version
6.0; Media Cybernetics, Inc. Maryland, USA). The expression
suppression rate (ESR) of mTOR, c-Myc, HIF-1α and GLUT1 was defined
as ESRmTOR, ESRc-Myc, ESRHIF-1α
and ESRGLUT1, respectively, using the following
equations: ESR=(mean MODc-mean MODt)/mean
MODc ×100%, where MODc was the control group
MOD, and MODt was the MOD of the treatment groups.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 software (IBM Corp., Armonk, NY, USA). The continuous
variables were described as the mean ± standard deviation.
Significant differences were evaluated using one-way analysis of
variance. If variances were equal, all pairwise comparisons between
group means were performed with Fisher's Least Significant
Difference test; if unequal, Dunnett's T3 test was used. Pearson's
correlation analysis was used to evaluate the relationships among
TV, SUVmax and the MOD of mTOR, c-Myc, HIF-1α and GLUT1. P<0.05
was considered to indicate a statistically significant
difference.
Results
125I-IBT inhibits A549
×enograft growth
A549 ×enograft growth over time following
125I-IBT was presented in Fig.
1. Overall, the tumor gradually enlarged over time in all
groups, although TV decreased as the radiation dose increased. The
mean TV of the 60 Gy group (377.3±227.2 mm3) and 40 Gy
group (410.7±174.42 mm3) was significantly reduced
compared with controls since day 14 (691.0±258.5 mm3)
and 16 (717.5±212.9 mm3), respectively (P<0.05).
However, no statistical differences were observed between the 20 Gy
group and controls or among all treatment groups (P>0.05). In
addition, the TIR14 of the 20, 40 and 60 Gy groups was
19.9, 39.8 and 45.4%; and the TIR28 were 18.0, 60.5 and
61.7%, respectively. These data suggested that 125I-IBT
may have inhibited the growth of A549 ×enografts, and inhibition
was enhanced as the radiation dose increased.
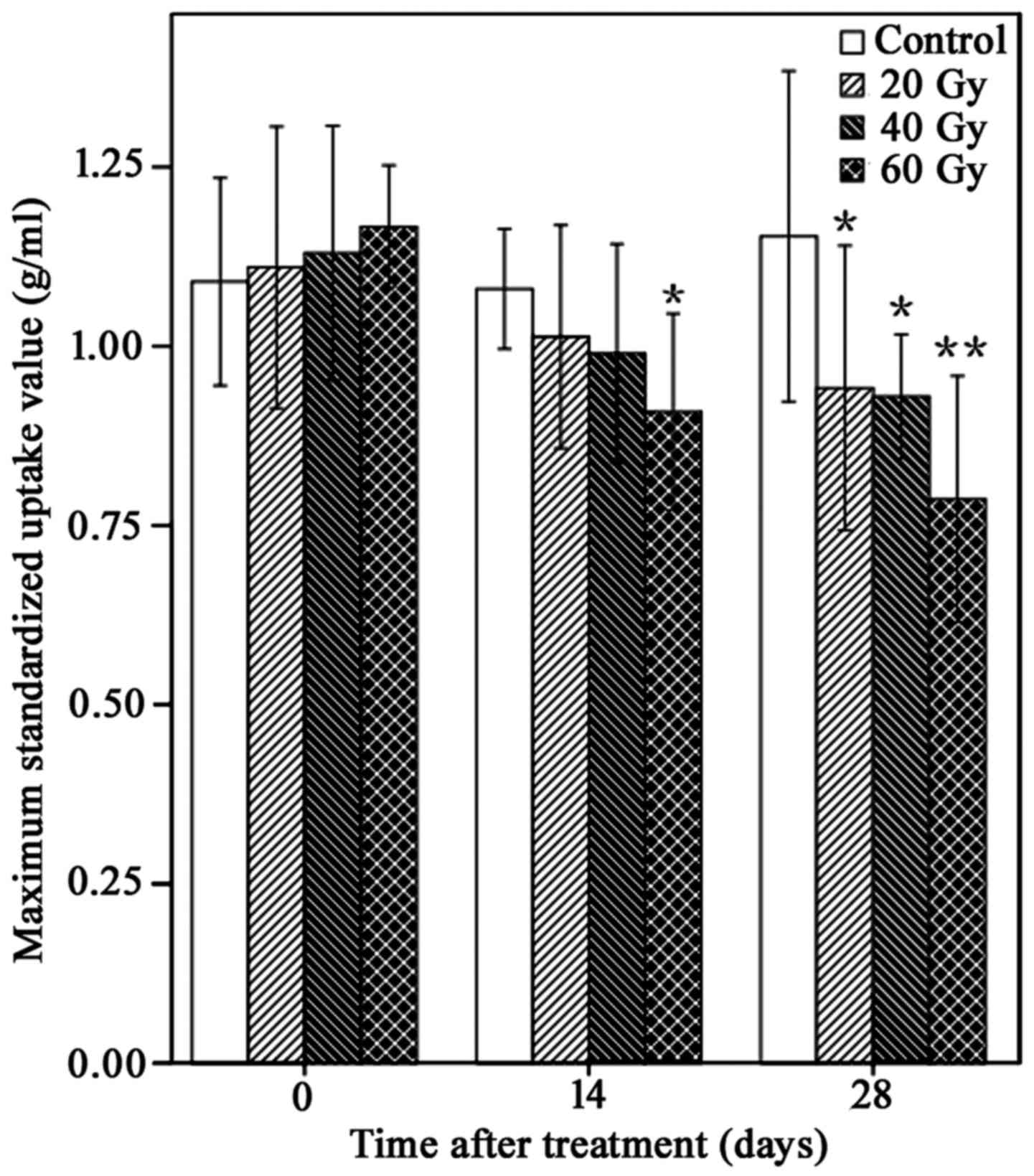
125I-IBT reduces
18F-FDG uptake in A549 ×enografts
18F-FDG uptake within A549 ×enografts
following 125I-IBT was presented in Fig. 2. In general, compared with controls,
the 18F-FDG uptake of xenografts gradually reduced over
time in 125I-IBT treatment groups. Furthermore, the
reduction in SUVmax was increased as the radiation dose increased
(Fig. 3). The mean of
SUVmax14 of the 60 Gy-group was significantly lower than
the control group (0.91±0.13 g/ml vs. 1.08±0.08 g/ml; P<0.05).
The mean SUVmax28 of all treatment groups was
significantly lower than the control group (20 Gy, 0.94±0.19 g/ml;
40 Gy, 0.93±0.08 g/ml; 60 Gy, 0.79±0.16 g/ml vs. control group,
1.15±0.22 g/ml; P<0.05), although there were no significant
differences among treatment groups on day 14 and 28 (P>0.05).
Furthermore, the FUAR14 of the 20, 40 and 60 Gy groups
was 6.5, 8.3 and 15.7%, and the FUAR28 of the 20, 40 and
60 Gy groups was 18.3, 19.1 and 31.3%, respectively. These data
suggested that 125I-IBT may have reduced
18F-FDG uptake within A549 ×enografts, and this effect
was more marked as the radiation dose increased.
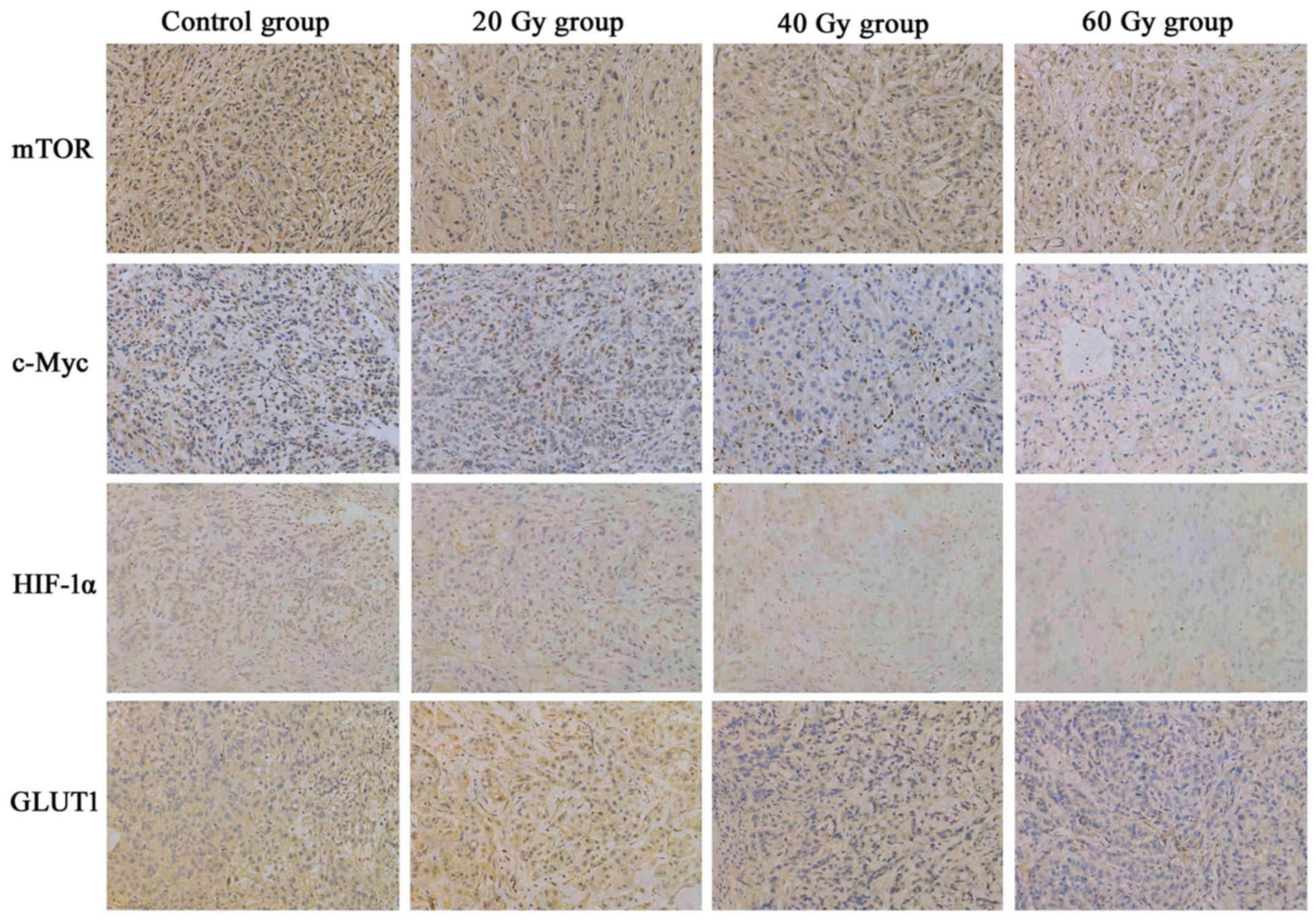
125I-IBT suppresses mTOR,
c-Myc, HIF-1α and GLUT1 expression in A549 ×enografts
The IHC staining patterns of mTOR, c-Myc, HIF-1α and
GLUT1 in A549 ×enografts following 125I-IBT were
presented in Fig. 4. In tumor cells,
mTOR, c-Myc and HIF-1α were expressed in the nucleus and cytoplasm,
whereas GLUT1 was predominantly expressed in the cytomembrane and
cytoplasm. Overall, the expression of mTOR, c-Myc, HIF-1α and GLUT1
in xenografts was gradually downregulated as the radiation dose
increased in the 125I-IBT treatment groups, compared
with the controls (Fig. 5). The MOD
of mTOR staining in the 60 Gy-group (0.0092±0.0062) was markedly
decreased compared with the other groups (control, 0.0198±0.0053;
20 Gy, 0.0191±0.0096 and 40 Gy, 0.0194±0.0062; P<0.05). In
addition, the MOD of c-Myc staining was significantly lower in the
40 (0.0025±0.0010) and 60 Gy (0.0006±0.0006) treatment groups,
compared with the other groups (control, 0.0077±0.0020 and 20 Gy,
0.0055±0.0029; P<0.01). Furthermore, the MOD of HIF-1α staining
in the 40 (0.0024±0.0021) and 60 Gy (0.0018±0.0006) treatment
groups was significantly than that of the control group
(0.0070±0.0058; P<0.05). Finally, the MOD of GLUT1 staining in
the 60 Gy (0.0024±0.0025) group was significantly decreased
compared with the other (control, 0.0150±0.0043; 20 Gy,
0.0139±0.0046; 40 Gy, 0.0130±0.0043; P<0.01). However, there
were no significant differences between 20 Gy group and the control
for mTOR, c-Myc, HIF-1α or GLUT1 expression in A549 ×enografts
(P>0.05). Additionally, the ESRmTOR, in the 20, 40
and 60 Gy groups was 4.0, 2.5 and 53.8%, respectively; the
ESRc-Myc was 28.6, 67.5 and 92.2%, respectively; the
ESRHIF-1α was 51.4, 65.7 and 74.3%, respectively; and
the ESRGLUT1 was 7.3, 13.3 and 84.0%, respectively.
These data suggested that 125I-IBT may have suppressed
the expression of mTOR, c-Myc, HIF-1α and GLUT1 in A549 ×enografts,
and the suppression effect was increased as the radiation dose
increased.
 | Figure 5.Comparison of mTOR, c-Myc, HIF-1α and
GLUT1 staining in A549 ×enografts following 125I-IBT.
The mean optical densities of mTOR, c-Myc, HIF-1α and GLUT1
gradually decreased as the radiation dose increased in treatment
groups, compared with the controls. *P<0.05, **P<0.01 vs.
controls; ▲P<0.05, ▲▲P<0.01 vs. 20 Gy
group; ΔP<0.05, ΔΔP<0.01 vs. 40 Gy
group. mTOR, mammalian target of rapamycin; HIF-1α, hypoxia
inducible factor-1α; GLUT1, glucose transporter 1;
125I-IBT, iodine-125 interstitial brachytherapy. |
Relationships among tumor growth,
18F-FDG uptake and expression of mTOR, c-Myc, HIF-1α and
GLUT1
The correlations among tumor growth,
18F-FDG uptake and expression of mTOR, c-Myc, HIF-1α and
GLUT1 were presented in Fig. 6. There
was a positive correlation between TV14 and
SUVmax14 (r=0.711), TV28 and
SUVmax14 (r=0.682) and TV28 and
SUVmax28 (r=0.586), respectively (P<0.01). In
addition, SUVmax28 positively correlated to the MOD of
c-Myc (r=0.621) and GLUT1 (r=0.546), respectively (P<0.01).
However, the MOD of mTOR (r=0.243) and HIF-1α (r=0.029) had no
significant correlation with SUVmax28 (P>0.05).
Furthermore, the MOD of mTOR, c-Myc and GLUT1 had a significant
correlation with each other (P<0.05; Table I).
 | Figure 6.Relationships among tumor growth,
18F-FDG uptake and expression of mTOR, c-Myc, HIF-1α and
GLUT1 in A549 ×enografts following 125I-IBT. (A) TV
positively correlated to SUVmax on day 14 (r=0.711; P<0.01) and
day 28 (r=0.586; P<0.01). TV on day 28 also correlated to SUVmax
on day 14 (r=0.682; P<0.01). (B) SUVmax positively correlated
with the mean optical densities of c-Myc (r=0.621) and GLUT1
(r=0.546) on day 28, respectively (P<0.01). TV, tumor volume;
SUVmax, maximum standardized uptake value; mTOR, mammalian target
of rapamycin; HIF-1α, hypoxia inducible factor-1α; GLUT1, glucose
transporter 1; 125I-IBT, iodine-125 interstitial
brachytherapy. |
 | Table I.Associations among expression of
mTOR, c-Myc, HIF-1α and GLUT1 in A549 ×enograft after
125I-IBT. |
Table I.
Associations among expression of
mTOR, c-Myc, HIF-1α and GLUT1 in A549 ×enograft after
125I-IBT.
| Protein name | mTOR, correlation
coefficient (P-value) | c-Myc, correlation
coefficient (P-value) | HIF-1α, correlation
coefficient (P-value) |
|---|
| c-Myc | 0.500a (0.013) |
|
|
| HIF-1α | −0.003 (0.988) | 0.365 (0.079) |
|
| GLUT1 | 0.526b (0.008) | 0.614b (0.001) | 0.264 (0.212) |
Discussion
In the present study, it was postulated that
125I-IBT may have suppressed NSCLC tumor growth by
inhibiting the Warburg effect. Tumor growth and 18F-FDG
uptake was monitored in NSCLC A549 ×enografts following
125I-IBT with a radiation dose of 20, 40 or 60 Gy, and
the relationships among them were also evaluated. It was
demonstrated that both the TV and SUVmax of all treatment groups
were decreased compared with controls following
125I-IBT, and these reductions were more marked as the
time and radiation dose increased, which was consistent with
results obtained in previous studies of pancreatic carcinoma
xenografts (20,21). In addition, the TIR and FUAR increased
more or less as the radiation dose increased, and TV positively
correlated to SUVmax on day 14 and 28. On the basis of these
results, it can be inferred that 125I-IBT may have
reduced tumor growth by inhibiting the Warburg effect in NSCLC, and
this inhibition increased in a dose-dependent manner more or less.
Notably, it was revealed that SUVmax14 was positively
correlated with TV28 (P<0.05). This suggested that
interim 18F-FDG PET/CT may be a promising strategy to
predict the anti-tumor efficacy of 125I-IBT in NSCLC in
the early stages. 18F-FDG PET/CT is currently used to
predict chemotherapy and/or radiotherapy efficacy in lung cancer
(16), as well as other malignancies
(22,23).
The Warburg effect is considered to involve multiple
intricate mechanisms, including tumor microenvironment support, HIF
stabilization, oncogene activation, tumor suppressor gene loss,
cancer cell mitochondrial dysfunction, nuclear DNA mutations,
epigenetic modifications, microRNA regulation, glutamine metabolism
and post-translational modifications (24). HIF-1, a heterodimeric transcription
factor consisting of α and β subunits, is known to contribute to
the adaptability of tumor cells to hypoxic conditions. It may
increase the efficacy of the glycolytic pathway by upregulating the
expression of several GLUTs and several other genes linked to
aerobic glycolysis (25). For
example, GLUT1 is ubiquitously expressed, but overexpressed in
tumor cells and therefore increases the uptake of glucose. HIF
regulation is predominantly dependent on the HIF-1α subunit, that
has a short half-life under normoxic conditions due to proteasomal
degradation (24,26,27). The
phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and the
Ras/Raf/mitogen-activated protein kinase kinase
(MAPKK)/extracellular regulated protein kinase (ERK) pathways are
the major signaling pathways downstream from growth factor-bound
receptor tyrosine kinases, which are linked to both growth control
and glucose metabolism in tumor cells (28,29). mTOR,
a serine/threonine kinase, is an important downstream effector of
AKT that stimulates the synthesis of HIF-1α under normoxic
conditions, further enhancing the expression of GLUT1 and
glycolytic enzymes, including lactate dehydrogenase B and pyruvate
kinase M2 (PKM2) (10,24,30). The
suppression of mTOR expression reduces glucose uptake in tumor
cells (31). Furthermore, activated
ERK induces the expression of MYC oncogene, which encodes a
transcription factor c-Myc that is known to be aberrantly
overexpressed in more than half of all human cancers (32). Similar to HIF-1, c-Myc activates GLUT1
and several glycolytic genes, resulting in increased glucose influx
and higher glycolytic rates and contributes to the Warburg effect
(33). In addition, activated c-Myc
may work together with HIF-1 in glycolysis and OXPHOS regulation.
Both HIF and c-Myc activate hexokinase 2 (HK2) and PDK1, leading to
increased glycolytic rates and conversion of glucose to lactate
(27).
In the present study, alterations in mTOR, c-Myc,
HIF-1α and GLUT1 expression was analyzed by IHC techniques, and the
relationship among them was determined. The findings revealed that
the xenograft expression of mTOR, c-Myc, HIF-1α and GLUT1 decreased
as the radiation dose increased, with the largest decrease observed
in the 60 Gy group, compared with the controls. Furthermore, the
ESRmTOR, ESRc-Myc, ESRHIF-1α and
ESRGLUT1 was dose-dependently increased, and the MOD of
mTOR, c-Myc and GLUT1 were significantly correlated with each
other. These findings indicated that the Warburg effect was
inhibited by 125I-IBT, which may have resulted from
mTOR, c-Myc, HIF-1α and GLUT1 downregulation in NSCLC A549
×enografts.
Although the downregulation of the above molecules
by 125I-IBT has not been explicitly defined in the
present study, possible involvement of AKT and ERK-mediated
signaling pathways may be suggested in accordance with a previous
report demonstrating that 125I-IBT may downregulate
epidermal growth factor receptor, inhibiting AKT activation in
colorectal tumor cells (34).
125I-IBT has also been demonstrated to suppress vascular
endothelial growth factor-A expression, and consequently inhibit
ERK activation in nasopharyngeal tumor cells (35). It is plausible that AKT and ERK
inactivation may have reduced the expression of mTOR and c-Myc,
resulting in the downregulation of HIF-1α and GLUT1 expression in
the present study. In addition, Cron et al (36) reported that 125I seeds may
significantly increase oxygen partial pressure within 24 h and even
on day 3. Furthermore, it may elevate blood perfusion from day 3,
in 2–4 mm of the surrounding liver tumor region (36). As a result, the improvement of the
tumor hypoxic microenvironment by 125I-IBT may have also
partially reduced the expression and/or accelerated the degradation
of HIF-1α. However, Hu et al (37) demonstrated that 125I-IBT
increases mitochondrial reactive oxygen species (ROS) production
and consequently upregulates the expression of HIF-1α in human
colorectal cancer cells (37).
Therefore, the downregulation of HIF-1α by 125I-IBT may
have been a synthetic action of above factors in NSCLC.
In addition, the correlation analysis between
glucose metabolism and the expression of mTOR, c-Myc, HIF-1α and
GLUT1 in the present study revealed that the SUVmax28
positively correlated with the MOD of c-Myc (r=0.621) and GLUT1
(r=0.546). This suggested that 18F-FDG uptake may depend
upon the overexpression of c-Myc and GLUT1 in xenografts,
particularly c-Myc. GLUT1 is the major glucose transporter
expressed in NSCLC. Although its overexpression is considered to be
linked to 18F-FDG uptake within tumors, it is unlikely
that this is the sole or rate-limiting step in this process
(38). The role of GLUT1 remains
controversial in NSCLC; certain studies have reported a significant
correlation (39,40), whereas others have detected no
correlation (41,42). This may be attributed to the fact that
18F-FDG uptake involves tumor blood flow and tracer
delivery rate, in addition to expression of GLUT1. Once
18F-FDG enters tumor cells, hexokinase and
glucose-6-phosphatase (G6Pase) activity determines how much
phosphorylated 18F-FDG is trapped in cells (38). c-Myc, as a central regulator of cell
growth and proliferation (43),
targets genes involved in glucose transport, glycolysis,
glutaminolysis and fatty acid synthesis (44). c-Myc activates GLUT1, as well as HK2
(27) and G6Pase (45), leading to increased uptake of
18F-FDG. Downregulation of c-Myc and GLUT1 suppresses
the glycolytic pathway and glycolytic enzyme activity, leading to
18F-FDG uptake inhibition (46).
However, it was noted that in the present study, no
statistical differences between TV, SUVmax14 and
SUVmax28 were observed among the treatment groups, or
between the TV and the MOD of mTOR, c-Myc, HIF-1α and GLUT1 in the
20 Gy and control group. This may have been due to the short
investigation duration of 28 days, as 125I-IBT is
typically delivered over >180 days (21). Additionally, 60 Gy is a relatively low
radiation dose, considering that the clinically effective dose is
typically 100–160 Gy (3–6). Furthermore, there were no significant
correlations between HIF-1α expression and the other detected
proteins, or between the SUVmax28 and the MOD of mTOR
and HIF-1α (P>0.05). This may be attributed in part to the
complex, multifactorial regulation of HIF-1α as detailed above. In
addition, it suggested that the AKT/mTOR pathway may have less
influence on the Warburg effect in NSCLC, compared with the
ERK/c-Myc pathway.
Certain limitations of the present study should be
considered. First, other key molecules associated with glycolysis
were not examined, such as PKM2 and HK2. Second, the expression of
glycolysis-associated molecules was only evaluated using IHC rather
than via western blotting and/or reverse transcription-quantitative
polymerase chain reaction assays for transcriptional and
translational expression analyses, respectively. Third, A549 cells
harbor the mutant K-ras gene, and the Ras/Raf/ERK signaling pathway
is critical for tumor proliferation. Therefore, multiple NSCLC
cells, including those with the wild-type K-ras gene, should be
used to confirm the results of the present study.
In conclusion, the present study suggested that
125I-IBT may have reduced tumor growth via Warburg
effect inhibition, and this inhibitory effect increased as the
radiation dose increased. Warburg effect inhibition may have
resulted from mTOR, c-Myc, HIF-1α and GLUT1 expression
downregulation, particularly c-Myc and GLUT1, in NSCLC A549
×enografts. Further research is required to clearly delineate the
exact mechanisms underlying Warburg effect inhibition by
125I-IBT in NSCLC.
Acknowledgements
The authors would like to thank Mr. Jiong Yu for the
cell culture assistance, Mr. Li Jiang for helping establishing the
animal model and Mr. Hui Chen for assistance in animal imaging.
Funding
The present study was supported by the Zhejiang
Provincial Natural Science Foundation (grant no. LY15H180007) and
Zhejiang Province Medical Health Science Foundation of China (grant
nos. 2015KYB153, 2016KYB099 and 2017KY061).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ established animal models, performed animal
imaging and drafted the manuscript. YZ was responsible for cell
culture and participated in preparation of animal model and
imaging. MD, JY and WW participated in the iodine-125 seed
implantation and immunohistochemistry. LT conceived and designed
the study, performed the statistical analysis and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animals used in the present study received
humane care in compliance with the Guideline to the Care and Use of
Experimental Animals established by the Medical Ethical Committee
on animal experiments of the Zhejiang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
125I-IBT
|
iodine-125 interstitial
brachytherapy
|
|
OXPHOS
|
oxidative phosphorylation
|
|
PET/CT
|
positron emission tomography/computed
tomography
|
|
18F-FDG
|
18F-fluorodeoxyglucose
|
|
PDK1
|
pyruvate dehydrogenase kinase 1
|
|
TV
|
tumor volume
|
|
TIR
|
tumor inhibition rate
|
|
SUVmax
|
maximum standardized uptake value
|
|
FUAR
|
18F-FDG uptake attenuation
rate
|
|
IHC
|
immunohistochemistry
|
|
mTOR
|
mammalian target of rapamycin
|
|
HIF-1α
|
hypoxia inducible factor-1α
|
|
GLUT1
|
glucose transporter 1
|
|
MOD
|
mean optical density
|
|
ESR
|
expression suppression rate
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
AKT
|
protein kinase B
|
|
MAPKK
|
mitogen-activated protein kinase
kinase
|
|
ERK
|
extracellular regulated protein
kinase
|
|
PKM2
|
pyruvate kinase M2
|
|
HK2
|
hexokinase 2
|
|
ROS
|
reactive oxygen species
|
|
G6Pase
|
glucose-6-phosphatase
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huo X, Wang H, Yang J, Li X, Yan W, Huo B,
Zheng G, Chai S, Wang J, Guan Z and Yu Z: Effectiveness and safety
of CT-guided (125)I seed brachytherapy for postoperative
locoregional recurrence in patients with non-small cell lung
cancer. Brachytherapy. 15:370–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Guan J, Yang L, Zheng X, Yu Y and
Jiang J: Iodine-125 brachytherapy improved overall survival of
patients with inoperable stage III/IV non-small cell lung cancer
versus the conventional radiotherapy. Med Oncol. 32:3952015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu X, Li J, Zhong X and He J: Combination
of Iodine-125 brachytherapy and chemotherapy for locally recurrent
stage III non-small cell lung cancer after concurrent
chemoradiotherapy. BMC Cancer. 15:6562015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Dan G, Jiang J, Zheng Y, Zheng X and
Deng D: Repeated iodine-125 seed implantations combined with
external beam radiotherapy for the treatment of locally recurrent
or metastatic stage III/IV non-small cell lung cancer: A
retrospective study. Radiat Oncol. 11:1192016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen HH, Jia RF, Yu L, Zhao MJ, Shao CL
and Cheng WY: Bystander effects induced by continuous low-dose-rate
125I seeds potentiate the killing action of irradiation on human
lung cancer cells in vitro. Int J Radiat Oncol Biol Phys.
72:1560–1566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu A, Wang H, Li J, Wang J, Liu J, Hou Y,
Huang L and Zhao Y: Biological effects of (125)i seeds radiation on
A549 lung cancer cells: G2/M arrest and enhanced cell death. Cancer
Invest. 32:209–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Zhao Z, Lu J, Chen Z, Mao A, Teng
G and Liu F: A comparison of the biological effects of 125I seeds
continuous low-dose-rate radiation and 60Co high-dose-rate gamma
radiation on non-small cell lung cancer cells. PLoS One.
10:e1337282015.
|
|
10
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ganapathy V, Thangaraju M and Prasad PD:
Nutrient transporters in cancer: Relevance to Warburg hypothesis
and beyond. Pharmacol Ther. 121:29–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Dong M, Sun X, Li W, Xing L and Yu
J: Prognostic value of 18F-FDG PET/CT in surgical non-small cell
lung cancer: A meta-analysis. PLoS One. 11:e1461952016.
|
|
13
|
Madsen PH, Holdgaard PC, Christensen JB
and Høilund-Carlsen PF: Clinical utility of F-18 FDG PET-CT in the
initial evaluation of lung cancer. Eur J Nucl Med Mol Imaging.
43:2084–2097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruilong Z, Daohai X, Li G, Xiaohong W,
Chunjie W and Lei T: Diagnostic value of 18F-FDG-PET/CT for the
evaluation of solitary pulmonary nodules: A systematic review and
meta-analysis. Nucl Med Commun. 38:67–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheikhbahaei S, Mena E, Yanamadala A,
Reddy S, Solnes LB, Wachsmann J and Subramaniam RM: The value of
FDG PET/CT in treatment response assessment, follow-up, and
surveillance of lung cancer. AJR Am J Roentgenol. 208:420–433.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cremonesi M, Gilardi L, Ferrari ME,
Piperno G, Travaini LL, Timmerman R, Botta F, Baroni G, Grana CM,
Ronchi S, et al: Role of interim 18F-FDG-PET/CT for the
early prediction of clinical outcomes of non-small cell lung cancer
(NSCLC) during radiotherapy or chemo-radiotherapy. A systematic
review. Eur J Nucl Med Mol Imaging. 44:1915–1927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen KT, Chin-Sinex H, DeLuca T,
Pomerening JR, Sherer J, Watkins JR, Foley J, Jesseph JM and
Mendonca MS: Dichloroacetate alters Warburg metabolism, inhibits
cell growth, and increases the X-ray sensitivity of human A549 and
H1299 NSC lung cancer cells. Free Radic Biol Med. 89:263–273. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv XB, Liu L, Cheng C, Yu B, Xiong L, Hu
K, Tang J, Zeng L and Sang Y: SUN2 exerts tumor suppressor
functions by suppressing the Warburg effect in lung cancer. Sci
Rep. 5:179402015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu T and Yin H: PDK1 promotes tumor cell
proliferation and migration by enhancing the Warburg effect in
non-small cell lung cancer. Oncol Rep. 37:193–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma JX, Jin ZD, Si PR, Liu Y, Lu Z, Wu HY,
Pan X, Wang LW, Gong YF, Gao J and Zhao-shen L: Continuous and
low-energy 125I seed irradiation changes DNA methyltransferases
expression patterns and inhibits pancreatic cancer tumor growth. J
Exp Clin Cancer Res. 30:352011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jian L, Zhongmin W, Kemin C, Yunfeng Z and
Gang H: MicroPET-CT evaluation of interstitial brachytherapy in
pancreatic carcinoma xenografts. Acta Radiol. 54:800–804. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subocz E, Hałka J and Dziuk M: The role of
FDG-PET in Hodgkin lymphoma. Contemp Oncol (Pozn). 21:104–114.
2017.PubMed/NCBI
|
|
23
|
Cremonesi M, Garibaldi C, Timmerman R,
Ferrari M, Ronchi S, Grana CM, Travaini L, Gilardi L, Starzynska A,
Ciardo D, et al: Interim 18F-FDG-PET/CT during
chemo-radiotherapy in the management of oesophageal cancer
patients. A systematic review. Radiother Oncol. 125:200–212. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Upadhyay M, Samal J, Kandpal M, Singh OV
and Vivekanandan P: The Warburg effect: Insights from the past
decade. Pharmacol Ther. 137:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thorens B and Mueckler M: Glucose
transporters in the 21st Century. Am J Physiol Endocrinol Metab.
298:E141–E145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu H, Forbes RA and Verma A:
Hypoxia-inducible factor 1 activation by aerobic glycolysis
implicates the Warburg effect in carcinogenesis. J Biol Chem.
277:23111–23115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Qian Y and Wu S: The Warburg
effect: Evolving interpretations of an established concept. Free
Radic Biol Med. 79:253–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dang CV: Links between metabolism and
cancer. Genes Dev. 26:877–890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh AL, Walton ZE, Altman BJ, Stine ZE
and Dang CV: MYC and metabolism on the path to cancer. Semin Cell
Dev Biol. 43:11–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makinoshima H, Takita M, Saruwatari K,
Umemura S, Obata Y, Ishii G, Matsumoto S, Sugiyama E, Ochiai A, Abe
R, et al: Signaling through the phosphatidylinositol 3-kinase
(PI3K)/mammalian target of rapamycin (mTOR) axis is responsible for
aerobic glycolysis mediated by glucose transporter in epidermal
growth factor receptor (EGFR)-mutated lung adenocarcinoma. J Biol
Chem. 290:17495–17504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toschi A, Lee E, Thompson S, Gadir N,
Yellen P, Drain CM, Ohh M and Foster DA: Phospholipase D-mTOR
requirement for the Warburg effect in human cancer cells. Cancer
Lett. 299:72–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dang CV, Kim JW, Gao P and Yustein J: The
interplay between MYC and HIF in cancer. Nat Rev Cancer. 8:51–56.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Wang H, Qu A, Li J, Zhao Y and Wang
J: Combined effects of C225 and 125-iodine seed radiation on
colorectal cancer cells. Radiat Oncol. 8:2192013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tian Y, Xie Q, Tian Y, Liu Y, Huang Z, Fan
C, Hou B, Sun D, Yao K and Chen T: Radioactive 125I seed
inhibits the cell growth, migration, and invasion of nasopharyngeal
carcinoma by triggering DNA damage and inactivating VEGF-A/ERK
signaling. PLoS One. 8:e740382013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cron GO, Beghein N, Crokart N, Chavée E,
Bernard S, Vynckier S, Scalliet P and Gallez B: Changes in the
tumor microenvironment during low-dose-rate permanent seed
implantation iodine-125 brachytherapy. Int J Radiat Oncol Biol
Phys. 63:1245–1251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu L, Wang H, Huang L, Zhao Y and Wang J:
The protective roles of ROS-mediated mitophagy on 125I
seeds radiation induced cell death in HCT116 cells. Oxid Med Cell
Longev. 2016:94604622016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brown RS, Leung JY, Kison PV, Zasadny KR,
Flint A and Wahl RL: Glucose transporters and FDG uptake in
untreated primary human non-small cell lung cancer. J Nucl Med.
40:556–565. 1999.PubMed/NCBI
|
|
39
|
van Baardwijk A, Dooms C, van Suylen RJ,
Verbeken E, Hochstenbag M, Dehing-Oberije C, Rupa D, Pastorekova S,
Stroobants S, Buell U, et al: The maximum uptake of
(18)F-deoxyglucose on positron emission tomography scan correlates
with survival, hypoxia inducible factor-1alpha and GLUT-1 in
non-small cell lung cancer. Eur J Cancer. 43:1392–1398. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suzawa N, Ito M, Qiao S, Uchida K, Takao
M, Yamada T, Takeda K and Murashima S: Assessment of factors
influencing FDG uptake in non-small cell lung cancer on PET/CT by
investigating histological differences in expression of glucose
transporters 1 and 3 and tumour size. Lung Cancer. 72:191–198.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chung JK, Lee YJ, Kim SK, Jeong JM, Lee DS
and Lee MC: Comparison of [18F]fluorodeoxyglucose uptake with
glucose transporter-1 expression and proliferation rate in human
glioma and non-small-cell lung cancer. Nucl Med Commun. 25:11–17.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
de Geus-Oei LF, van Krieken JH, Aliredjo
RP, Krabbe PF, Frielink C, Verhagen AF, Boerman OC and Oyen WJ:
Biological correlates of FDG uptake in non-small cell lung cancer.
Lung Cancer. 55:79–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dang CV: Rethinking the Warburg effect
with Myc micromanaging glutamine metabolism. Cancer Res.
70:859–862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morrish F, Isern N, Sadilek M, Jeffrey M
and Hockenbery DM: c-Myc activates multiple metabolic networks to
generate substrates for cell-cycle entry. Oncogene. 28:2485–2491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Collier JJ, Doan TT, Daniels MC, Schurr
JR, Kolls JK and Scott DK: c-Myc is required for the
glucose-mediated induction of metabolic enzyme genes. J Biol Chem.
278:6588–6595. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Broecker-Preuss M, Becher-Boveleth N,
Bockisch A, Dührsen U and Müller S: Regulation of glucose uptake in
lymphoma cell lines by c-MYC- and PI3K-dependent signaling pathways
and impact of glycolytic pathways on cell viability. J Transl Med.
15:1582017. View Article : Google Scholar : PubMed/NCBI
|