Introduction
Semiconductor nanocrystals, also known as quantum
dots (QDs), which contain elements in groups III–V, II–VI or IV
(1), have become an important tool in
biomedical research. These nanometer-sized inorganic nanomaterials,
with notable optical and electronic properties, exhibit distinct
advantages over traditional fluorescent organic dyes, including
improved signal brightness, high quantum yield, increased
photostability, tunable broad excitation and narrow emission
spectra (2), particularly for
quantitative and long-term fluorescence imaging and detection
(3). QDs of various sizes emit light
of various wavelengths following excitation by the same light
source (4); therefore, compared with
conventional organic fluorescent dyes, QDs may be used effectively
as biomarkers, as well as unique optical properties, particularly
in cell labeling and clinical targeting bio-imaging (5). Upon entry into cells, QDs can be
localized in various subcellular compartments, such as the
cytoplasm and lysosomes, depending on their surface charge
(6). Fluorescent QDs can be
conjugated with bioactive moieties, including antibodies, peptides,
aptamers or small-molecule ligands, to target specific biological
events and cellular structures, including labeling neoplastic
cells, cell membrane receptors, DNA or peroxisomes (7). QDs have exhibited notable promise in
various biomedical applications, including labeling of cellular
proteins, sensitive cellular imaging, real-time tracking,
fluorescence resonance energy transfer sensors, visible drug
carriers, in vivo animal imaging and cancer theranostics
(8).
It is necessary to label the adoptive cells,
targeting agents or drugs that are injected in vivo in
biomedical research and clinical targeting therapies, to trace
their biological behavior or in vivo kinetics (9). Additionally, the in vivo
applications vary from in vitro studies, and rely markedly
on biocompatibility with monocytes and macrophages when used for
tracing in vivo (10).
Macrophages serve important roles in particle clearance and
inflammatory reactions (11), are
crucial effectors of innate immunity in the primary responses to
pathogens (12) and participate in
homeostasis and tissue regeneration (13). Upon activation, macrophages induce a
variety of biological effects, such as mediating in vivo
inflammatory responses and specific immune responses, thus they are
involved in the occurrence and development of a number of diseases
(14).
A previous study demonstrated that charged QDs may
enter macrophages more efficiently than neutral QDs, and negative
QDs are internalized more efficiently than positive QDs; however,
positive QDs exhibit severe cytotoxicity, compared with negative
QDs (15). Therefore, the aim of the
present study was to determine the biocompatibility of CdSe/ZnS QDs
with monocytes and macrophages to provide a theoretical and
experimental basis for future applications of in vivo QD
labeling.
Macrophage clonal stimulating factor induces
osteoclast differentiation factor, which in turn induces osteoclast
formation. The activity of a macrophage determines the destruction
of giant cell tumor of bone (16).
Giant cell tumor of bone can be surgically removed; however, other
treatments, including biological therapy and clinical targeted
therapy, remain in the initial stages of development. In the
present study, the intake of QDs by RAW 264.7 macrophages was
investigated in order to determine the role of macrophages as
markers of giant cell tumors of bone in vivo.
Materials and methods
Co-culture of QDs with RAW 264.7
cells
RAW 264.7 macrophages [Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China)] were
cultured and passaged in 10% fetal bovine serum-containing
high-glucose Dulbecco's modified Eagle's medium (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). When the cells reached 80%
confluence, they were digested using trypsin, sampled and counted
using an inverted microscope (×400 magnification; Ti-S; Nikon,
Tokyo, Japan) followed by seeding into 96-well plates at a density
of 2×104 cells/well. Subsequently, the cells were cultured in 10%
fetal bovine serum-containing high-glucose Dulbecco's modified
Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 4 h at room temperature and following adherence to the
wall of the plate, various concentrations (0, 10, 50 and 100 µg/ml)
of QDs (water-soluble CdSe/ZnS QDs; Q2565; emission wavelength, 565
nm; Wuhan Jiayuan Quantum Dot Technological Development Co., Ltd.,
Wuhan, China) were added for co-culture at room temperature. The
diameter of the CdSe/ZnS core/shell QDs was 9.79±2.185 nm, and the
CdSe/ZnS QDs were carboxylate functionalized. Following different
incubation periods (1 or 2 h), a fluorescence microscope (×400
magnification) was used to observe the cells, and images were
captured. Alternatively, the cells in the wells were washed with
PBS twice to remove the non-macrophage-ingested QDs and then
harvested for subsequent analysis. In subsequent experiments, the
duration of co-culture of QDs with macrophages was 18 h at room
temperature unless stated otherwise.
Flow cytometry
Trypsin-digested RAW 264.7 macrophages were filtered
to prepare a single cell suspension and the fluorescence signal
intensity of the QDs in the cells was detected directly using a
flow cytometer (FACSCalibur™; BD Biosciences, Franklin
Lakes, NJ, USA). Alternatively, an annexin V-fluorescein
isothiocyanate kit (eBioscience; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was added to the cell suspension for 30 min of
labeling at 4°C. Following washing with buffer (NaCl 137 mmol/l,
KCl 2.7 mmol/l, Na2HPO4 10 mmol/l,
KH2PO4 2 mmol/l, pH 7.2~7.4) twice, propidium
iodide (eBioscience; Thermo Fisher Scientific, Inc.) was added,
followed by the immediate detection of apoptosis of RAW 264.7 cells
using a flow cytometer and analyzed by FlowJo (version 7.2; FlowJo
LLC, Ashland, OR, USA)
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cultured cells were centrifuged (6,000 × g, 10
min at 4°C) to discard the medium, washed twice with PBS and then
added to 1 ml RNAiso Plus reagent (Takara Biotechnology Co., Ltd.,
Dalian, China), followed by repetitive pipetting to ensure complete
contact of the cells with the reagent for ~10 min. Following lysis
using trypsin on ice for 5 min, the cell mixture was transferred
into RNase-free Eppendorf tubes, gently agitated for 5 min with 200
µl pre-cooled chloroform (4°C) and left to stand for 10 min at 4°C.
Following centrifugation at 6,500 × g for 10 min at 4°C, the
mixture was divided into three layers, among which the supernatant
was carefully withdrawn and transferred into another clean
Eppendorf tube. To this new Eppendorf tube, 500 µl isopropanol was
added. The tube was shaken gently at 4°C and left to stand for 10
min at 4°C. The new Eppendorf tube was centrifuged at 6,500 × g for
10 min at 4°C. The supernatant was discarded and 1 ml 75% ethanol
solution [750 µl of ethanol and 250 µl of diethyl pyrocarbonate
(DEPC)-treated water] was added to the precipitate, RNA was
dissolved again for 2–3 min and centrifuged at 6,500 × g for 5 min
at 4°C and. The precipitate was then dried and the RNA pellet was
dissolved in the appropriate amount of DEPC-treated water (5–20
µl). The concentration of the total RNA was detected using a
nucleic acid detector (CFX96, Bio-Rad Laboratories, Inc., Hercules,
CA, USA), and the A260/A280 ratio of between
1.8 and 2.0 was determined.
First-strand cDNA was produced using an Invitrogen
SuperScript cDNA synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc.). The reaction comprised 2 µg total RNA template,
1 µl oligo(dT)18 primer, 1 µl 10 mM dNTP mixture and
enzyme-free ultrapure water to a final volume of 12 µl. The
reaction tube was incubated at 65°C for 5 min and then immediately
placed on ice for ~5 min. Following centrifugation (6,000 × g, 10
min at 4°C), 4 µl 5X Reaction Buffer, 1 µl RiboLock™
nuclease inhibitor and 2 µl 0.1 M dithiothreitol were added,
followed by mixing gently and incubating for 2 min at 37°C. Moloney
murine leukemia virus reverse transcriptase (1 µl) was then added
and mixed gently for reverse transcription in a LightCycler 480
System RT-PCR instrument (Roche Diagnostics, Basel, Switzerland)
with alternating cycles of 37°C for 50 min and 70°C for 15 min.
The reaction was then terminated, and the products
were collected, and the qPCR mixture contained: 1 µl cDNA; 0.5 µl
each of upstream and downstream primers; 5 µl SYBR®
Green (Takara Biotechnology Co., Ltd.); and 3 µl double-distilled
water. The reaction conditions were: Pre-denaturation at 95°C for
30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec,
annealing for 20 sec (TNF-α: 58°C, IL-1β: 59°C, β-actin: 57°C) and
extension at 72°C for 30 sec. The primer sequences were: Tumor
necrosis factor (TNF)-α, sense 5′-GAACTGGCAGAAGAGGCACT-3′, and
antisense 5′-GGTCTGGGCCATAGAACTGA-3′; interleukin (IL)-1β, sense
5′-TGTGAAATGCCACCTTTTGA-3′, and antisense
5′-TGAGTGATACTGCCTGCCTG-3′; and β-actin (internal reference), sense
5′-TGGAATCCTGTGGCATCCATGAAAC-3′, and antisense
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. For relative quantitative
analysis, the 2−ΔΔCq method was used (17).
Detection of cell proliferation
RAW 264.7 cells in the exponential growth phase were
sampled, a cell suspension was prepared once the DMEM was washed
away using HBSS and digested by 0.25% pancreatin for 5–10 min and
the cells were seeded into 96-well plates at a volume as ~100
µl/well for 4 h culture at 37°C and an atmosphere containing 5%
CO2. When the cells adhered to the wall, QDs were added
and co-cultured at 37°C for 18 h. At 4 h prior to the end of the
incubation, 10 µl Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added to each well and
incubated at 37°C for 18 h. The optical density of each well was
determined at 450 nm using an ELISA subsequent to culture (BioTek
Instruments, Inc., Winooski, VT, USA).
Statistical analysis
GraphPad Prism software (version 6.0; GraphPad
Software, Inc., La Jolla, CA, USA) was used to analyze the
experimental data. The data are presented as the mean ± the
standard error of the mean. The multiple group comparison used a
two-way analysis of variance with Fisher's least significant
difference post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Macrophages engulf QDs
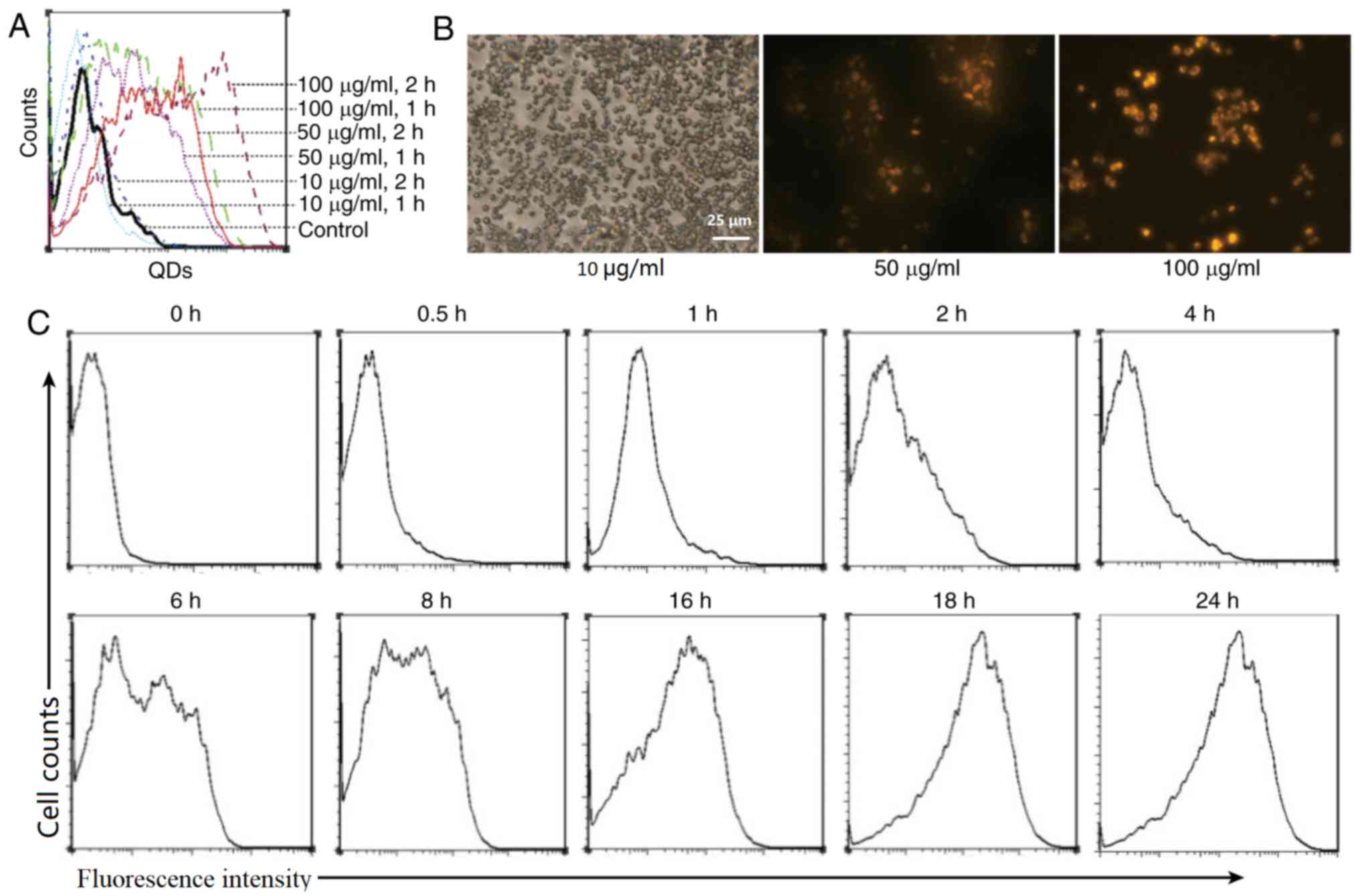
Fig. 1A demonstrates
that the co-culture of RAW 264.7 cells with various concentrations
of QDs enabled the macrophages to become labeled with fluorescent
signals, which were detected using flow cytometry. The intensity of
the fluorescence signal was notably associated with the
concentration of the co-cultured QDs. At a QD concentration of 10
µg/ml, the fluorescence intensity was weak, whereas at 50 µg/ml,
the fluorescence intensity inside the macrophages was notably
increased. The results also indicated that the fluorescence
intensity was stronger in the 1 h co-culture group, compared with
the 2 h co-culture group. Microscopic observations (Fig. 1B) also demonstrated that the
macrophages exhibited strong fluorescence signals following
engulfment of QDs. The results indicated that macrophages are able
to engulf a specific concentration of QDs, and are thus labeled
with strong fluorescence signals.
Furthermore, the dynamic changes of fluorescence
signal intensity, following QDs (50 µg/ml) being co-cultured with
the macrophages, were observed. The results (Fig. 1C) demonstrated that with extended
co-incubation time, the fluorescence signals inside the macrophages
increased, reaching a peak at 18 h. The results indicated that the
QD labeling increased in a time-dependent manner.
QDs promote macrophages to secrete
inflammatory cytokines
Macrophages are important natural immune cells,
which can be activated to secrete inflammatory cytokines (18) To observe whether QDs were able to
activate the macrophages directly, the expression levels of the
genes encoding the inflammatory cytokines TNF-α and IL-1β in
QD-labeled RAW 264.7 cells were examined. The results (Fig. 2) indicated that QDs at 50 µg/ml
significantly increased mRNA expression levels of TNF-α (4.2-fold
change) and IL-1β (21.5-fold change) in the macrophages. When the
QD concentration was 100 µg/ml, the expression of TNF-α mRNA was
significantly increased, compared with the 0 (P=0.04328) and 50
µg/ml groups (P=0.00032); additionally, 100 µg/ml QDs increased
IL-1β mRNA expression levels, compared with the 0 and 50 µg/ml
groups, but this was only significantly increased compared with the
0 µg/ml group (P=0.00016). The results indicated that QDs have the
ability to activate macrophages, thus promoting the secretion of
certain inflammatory factors.
QDs promote the proliferation of
macrophages
Cell proliferation is an important biological
property of macrophages, which, to a certain extent, reflects the
activation state of macrophages. The results of the CCK-8 assay
demonstrated that co-culture with 50 µg/ml QDs significantly
increased the proliferation of RAW 264.7 cells, compared with the 0
µg/ml group. However, at concentration of 100 µg/ml QDs, the
proliferation was similar to that of the 50 µg/ml QDs and was only
significantly different when compared with the 0 µg/ml group (100
µg/ml: P=0.04681; 50 µg/ml: P=0.04329; Fig. 3). The results indicated that QDs have
the ability to increase the proliferation of macrophages.
Impact of QDs on the apoptosis of
macrophages
Apoptosis initiates cell death (19), and is notably associated with cell
biological functions including activation and proliferation
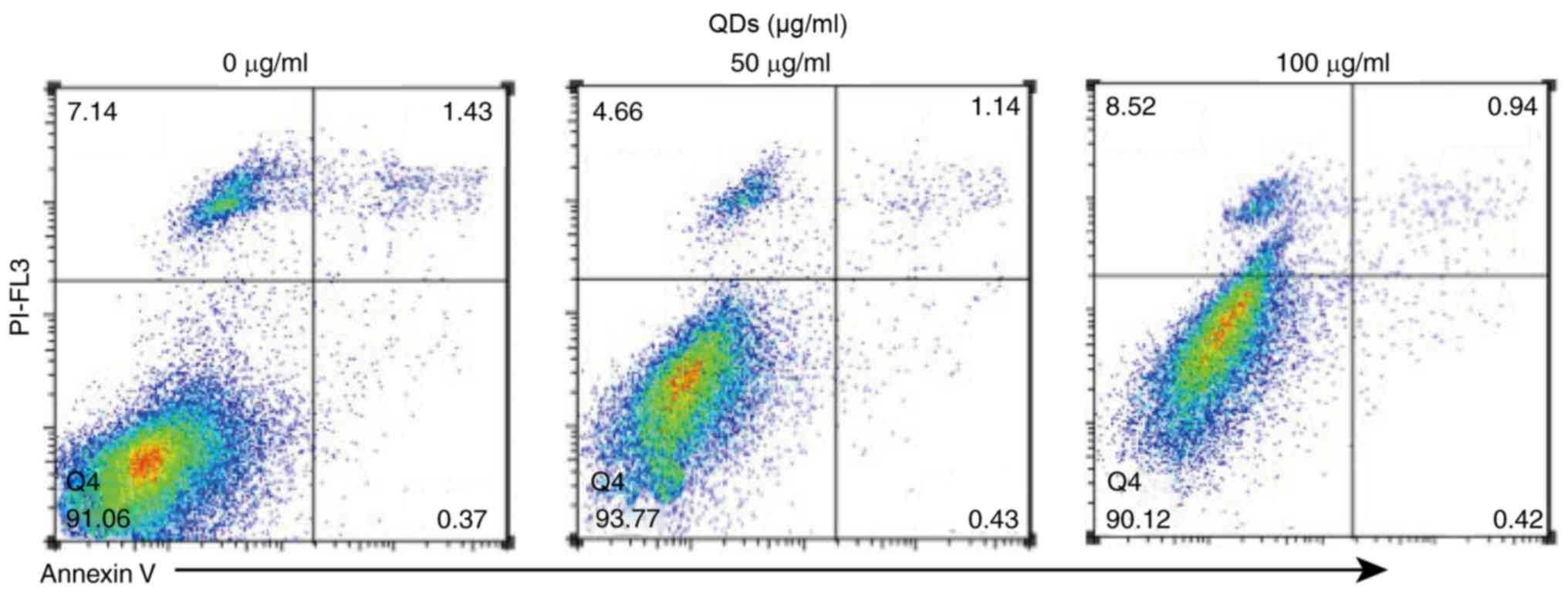
(20). The results of annexin V
labeling (Fig. 4) demonstrated that
QDs were not able to increase the apoptosis rate of RAW 264.7
cells. When the concentration of QDs was 100 µg/ml, the apoptosis
rate of RAW 264.7 cells decreased from 1.43% (0 µg/ml group) to
1.14% (50 µg/ml group) and 0.94% (100 µg/ml group), indicating that
QDs do not promote the apoptosis of macrophages.
Discussion
In the present study, it was determined that
CdSe/ZnS QDs could be phagocytosed by macrophages. The co-culture
of QDs and macrophages did not lead to an increase in the apoptosis
of RAW 264.7 cells. This indicated that CdSe/ZnS QDs could be used
as tracers.
As a novel type of nanoparticle material, QDs have
notable prospects in biomedical fields for in vivo labeling
and tracing, as well as for in vitro multi-fluorescence
labeling; however, a number of previous studies have identified
in vivo toxicity of QDs (21–23). The
cytotoxicity of CdSe and CdSe/ZnS nanoparticles has been
investigated for different surface modifications, including coating
with mercaptopropionic acid, silanization and polymer coating
(24), when targeting liver cells,
red blood cells and other cell types (25,26). The
cytotoxicity of Cd/Se QDs or Cd/Te QDs is mainly associated with
the Cd2+ ions carried in QDs that produce oxidative free
radicals. Heavy metal Cd2+ ions exhibit cell toxicity
through a variety of mechanisms, such as interfering with cellular
DNA repair and promoting oxidative stress. Furthermore, the
cytotoxicity of QDs is notably associated with multiple parameters,
including the particle size, the number of surface charges, the
redox activity, the surface coating component, the mechanical
stability of QDs and the external environmental conditions
(27,28). It has been demonstrated that the
toxicity and biological activities of QDs are associated mainly
with their surface coating materials (29). Primarily, when QDs are coated with
cytotoxic reagents, significant toxicity is observed; however, when
they are coated with bioactive materials, the QDs exhibited the
biological characteristics of the conjugates, with decreased
toxicity (30). For example,
β-cyclodextrin-modified Cd/Se QDs exhibited a broader light
spectrum and lower cytotoxicity compared with Cd/Se QDs alone
(31).
The results of the present in vitro study
indicated that QDs could be engulfed effectively by macrophages,
thus marking them with fluorescence signals. This labeling process
is notably associated with the concentration and incubation time of
the QDs; however, the increase in mRNA expression levels of TNF-α
and IL-1β indicated that the intake of QDs was also able to promote
macrophages to secrete inflammatory cytokines, which could promote
cell proliferation, while having no significant effect on
apoptosis. When QDs are coated with bioactive molecules, their
activation of macrophages may be decreased; therefore, when QDs are
used for in vivo labeling and tracing, their
macrophage-activating effect would be decreased with less
macrophages activated to prolong their in vivo labeling
duration (10). The results of that
study are agreement with the present results.
The biocompatibilities of QDs with macrophages are
also beneficial for the treatment of macrophage-associated diseases
and to regulate the immune system. CdSe/CdS/ZnS QDs cross-linked to
Adriamycin can be engulfed by alveolar macrophages in the lungs,
the accumulation of Adriamycin by alveolar macrophages would thus
induce apoptosis (32). In this
process, the phagocytosis of QDs by macrophages is exploited to
form effective carriers for in vivo macrophage-targeted
therapies (33). A variety of
pattern-recognition receptors are expressed on the surface of
macrophage. When these receptors bind with their corresponding
ligands, such as Toll-like receptors, macrophages can be
effectively activated, thus causing immunomodulatory effects
(33). Lipopolysaccharide (LPS)-QDs,
prepared by cross-linking the pattern-recognition molecule
Kdo2-Lipid A, an LPS from Escherichia coli, with QDs, were
engulfed by macrophages, resulting in their in vitro
activation, which may have immunomodulatory effects in vivo
(34). In addition, when the novel
anti-tuberculosis drug Zn-RIF, a complex of zinc with rifampicin,
was combined with transferrin protein-coupled silver QDs,
Zn-RIF-Tf-QD, targeting was towards the inner macrophages improved,
and the anti-tuberculosis activity was increased >10-fold
(35). The results of these studies
are consistent with the theory that the biocompatibility of QDs and
macrophages, and the phagocytosis of QDs, does not result in
apoptosis, and that different modifications may promote cell
proliferation.
In conclusion, the results of the present study
supported the hypothesis that macrophages engulf QDs effectively
in vitro. The surface of QDs can be modified with different
molecules according to the application purposes, such as to prolong
the in vivo duration of QDs or to use QDs as carriers to
cause the targeted activation of macrophages.
The limitations of the present study included: Using
a single monocyte/macrophage cell line, which limited its value for
extrapolation into potential clinical applications of QDs; and that
the protein levels of TNF-α and IL-1β were measured in the cells,
but not in culture supernatants. Since it is possible that
inflammatory factors may be released into the culture supernatant,
a measure including secreted factors may be more accurate.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CL designed the experiment and wrote the manuscript.
PZ, YH, DH, YS and RL performed the experiment. YH and RL evaluated
the experiment. DH performed experimental data collection. YH
reviewed the manuscript thoroughly.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hild WA, Breunig M and Goepferich A:
Quantum dots-nano-sized probes for the exploration of cellular and
intracellular targeting. Eur J Pharm Biopharm. 68:153–168. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michalet X, Pinaud FF, Bentolila LA, Tsay
JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS and Weiss S:
Quantum dots for live cells, in vivo, imaging, and diagnostics.
Science. 307:538–544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joo KI, Fang Y, Liu Y, Xiao L, Gu Z, Tai
A, Lee CL, Tang Y and Wang P: Enhanced real-time monitoring of
adeno-associated virus trafficking by virus-quantum dot conjugates.
ACS Nano. 5:3523–3535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abbasi E, Kafshdooz T, Bakhtiary M,
Nikzamir N, Nikzamir N, Nikzamir M, Mohammadian M and Akbarzadeh A:
Biomedical and biological applications of quantum dots. Artif Cells
Nanomed Biotechnol. 44:885–891. 2016.PubMed/NCBI
|
|
5
|
Kamila S, McEwan C, Costley D, Atchison J,
Sheng Y, Hamilton GR, Fowley C and Callan JF: Diagnostic and
therapeutic applications of quantum dots in nanomedicine. Top Curr
Chem. 370:203–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Delehanty JB, Mattoussi H and Medintz IL:
Delivering quantum dots into cells: Strategies, progress and
remaining issues. Anal Bioanal Chem. 393:1091–1105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farlow J, Seo D, Broaders KE, Taylor MJ,
Gartner ZJ and Jun YW: Formation of targeted monovalent quantum
dots by steric exclusion. Nat Methods. 10:1203–1205. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bajwa N, Mehra NK, Jain K and Jain NK:
Pharmaceutical and biomedical applications of quantum dots. Artif
Cells Nanomed Biotechnol. 44:758–768. 2016.PubMed/NCBI
|
|
9
|
Cesar CL: Quantum dots as biophotonics
tools. Methods Mol Biol. 1199:3–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jayagopal A, Su YR, Blakemore JL, Linton
MF, Fazio S and Haselton FR: Quantum dot mediated imaging of
atherosclerosis. Nanotechnology. 20:1651022009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rabolli V, Lison D and Huaux F: The
complex cascade of cellular events governing inflammasome
activation and IL-1β processing in response to inhaled particles.
Part Fibre Toxicol. 13:402016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Egners A, Erdem M and Cramer T: The
response of macrophages and neutrophils to hypoxia in the context
of cancer and other inflammatory diseases. Mediators Inflamm.
2016:20536462016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaur S, Raggatt LJ, Batoon L, Hume DA,
Levesque JP and Pettit AR: Role of bone marrow macrophages in
controlling homeostasis and repair in bone and bone marrow niches.
Semin Cell Dev Biol. 61:12–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel U, Rajasingh S, Samanta S, Cao T,
Dawn B and Rajasingh J: Macrophage polarization in response to
epigenetic modifiers during infection and inflammation. Drug Discov
Today. 22:186–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Q, Li H, Xia Q, Liu Y and Xiao K: Role
of surface charge in determining the biological effects of CdSe/ZnS
quantum dots. Int J Nanomedicine. 10:7073–7088. 2015.PubMed/NCBI
|
|
16
|
Xiao Y, Zijl S, Wang L, de Groot DC, van
Tol MJ, Lankester AC and Borst J: Identification of the common
origins of osteoclasts, macrophages, and dendritic cells in human
hematopoiesis. Stem Cell Reports. 4:984–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohamed MM, El-Ghonaimy EA, Nouh MA,
Schneider RJ, Sloane BF and El-Shinawi M: Cytokines secreted by
macrophages isolated from tumor microenvironment of inflammatory
breast cancer patients possess chemotactic properties. Int J
Biochem Cell Biol. 46:138–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Donjerković D and Scott DW:
Activation-induced cell death in B lymphocytes. Cell Res.
10:179–192. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galluzzi L, Kepp O, Trojel-Hansen C and
Kroemer G: Non-apoptotic functions of apoptosis-regulatory
proteins. EMBO Rep. 13:322–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu T and Tang M: Toxicity of quantum dots
on respiratory system. Inhal Toxicol. 26:128–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chong Y, Ma Y, Shen H, Tu X, Zhou X, Xu J,
Dai J, Fan S and Zhang Z: The in vitro and in vivo toxicity of
graphene quantum dots. Biomaterials. 35:5041–5048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hauck TS, Anderson RE, Fischer HC,
Newbigging S and Chan WC: In vivo quantum-dot toxicity assessment.
Small. 6:138–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kirchner C, Liedl T, Kudera S, Pellegrino
T, Muñoz Javier A, Gaub HE, Stölzle S, Fertig N and Parak WJ:
Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano
Lett. 5:331–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hardman R: A toxicologic review of quantum
dots: Toxicity depends on physicochemical and environmental
factors. Environ Health Perspect. 114:165–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu G, Wang X, Wang Z, Liu S and Jiang G:
Cytotoxicity of quantum dots and graphene oxide to erythroid cells
and macrophages. Nanoscale Res Lett. 8:1982013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin CH, Chang LW, Wei YH, Wu SB, Yang CS,
Chang WH, Chen YC and Lin PP: Electronic microscopy evidence for
mitochondria as targets for Cd/Se/Te-based quantum dot 705 toxicity
in vivo. Kaohsiung J Med Sci. 28:S53–S62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith WE, Brownell J, White CC,
Afsharinejad Z, Tsai J, Hu X, Polyak SJ, Gao X, Kavanagh TJ and
Eaton DL: In vitro toxicity assessment of amphiphillic
polymer-coated CdSe/ZnS quantum dots in two human liver cell
models. ACS Nano. 6:9475–9484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Yang L, Kuang H, Yang P, Aguilar
ZP, Wang A, Fu F and Xu H: Acute toxicity of quantum dots on late
pregnancy mice: Effects of nanoscale size and surface coating. J
Hazard Mater. 318:61–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bakalova R, Zhelev Z, Kokuryo D, Spasov L,
Aoki I and Saga T: Chemical nature and structure of organic coating
of quantum dots is crucial for their application in imaging
diagnostics. Int J Nanomedicine. 6:1719–1732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guleria A, Rath MC, Singh AK and Adhikari
S: Rapid and one-pot synthesis of self-assembled CdSe quantum dots
functionalized with β-Cyclodextrin: Reduced cytotoxicity and band
gap engineering. J Nanosci Nanotechnol. 15:9341–9357. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chakravarthy KV, Davidson BA, Helinski JD,
Ding H, Law WC, Yong KT, Prasad PN and Knight PR:
Doxorubicin-conjugated quantum dots to target alveolar macrophages
and inflammation. Nanomedicine. 7:88–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yeo JC, Wall AA, Luo L, Condon ND and Stow
JL: Distinct roles for APPL1 and APPL2 in regulating toll-like
receptor 4 signaling in macrophages. Traffic. 17:1014–1026. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barr TA, Krembuszewski M, Gupta M, Gray D
and Mareque-Rivas JC: Quantum dots decorated with pathogen
associated molecular patterns as fluorescent synthetic pathogen
models. Mol Biosyst. 6:1572–1575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pati R, Sahu R, Panda J and Sonawane A:
Encapsulation of zinc-rifampicin complex into
transferrin-conjugated silver quantum-dots improves its
antimycobacterial activity and stability and facilitates drug
delivery into macrophages. Sci Rep. 6:241842016. View Article : Google Scholar : PubMed/NCBI
|