Introduction
The results from the National Central Cancer
Registry (NCCR) collected registration data in 2013 from local
cancer registries (1) showed that the
incidence and mortality rates of lung cancer occupied the first
position among malignant tumors, and there are about 733,000 new
cases and 591,000 deaths each year in China. Platinum-based
therapy, which is used for clinical tumor treatment in the
chemotherapeutic regimen in China, was used in 80% of cases
(2). Cisplatin is one of the most
common platinum drugs; however, tumor drug-resistance (3), nephrotoxicity (4,5),
hepatotoxicity (6), ototoxicity
(7), and other side-effects are
associated with the long-term use of cisplatin. In addition,
advanced lung cancers are difficult to treat with the currently
available platinum-based chemotherapeutic regimens. Therefore,
novel therapeutic regimens that combine cisplatin-based therapy
with other methods to reduce side effects and increase the curative
effect are urgently needed.
The apoptin protein from chicken anemia virus (CAV)
exhibits selective cytotoxicity, which is enabled by its
phosphorylation and translocation to the nucleus for tumor cells
without affecting normal diploid cells (8). Apoptin aggregates mainly in the cancer
cell nucleus where it plays a role in specific cytotoxicity, while
it is mainly localized in the cytoplasm of normal cells where it is
degraded (9). The size of xenografted
tumors in mice could be reduced effectively by various delivery
strategies of apoptin (10–12). However, an effective cancer treatment
strategy also needs a safe delivery vehicle that can express
apoptin protein persistently and deliver it effectively. The human
adenovirus serotype 5 (Ad5) is one of such vector that has been
used widely to treat a variety of tumors (13–16). In
addition, satisfactory curative effects in the treatment of tumors
have been demonstrated by a combination of a recombinant Ad5 and
cisplatin (17,18).
A dual cancer-specific oncolytic adenovirus ATV
containing the apoptin gene (with the capacity for tumor specific
cytotoxicity) under the control of the hTERTp promoter (enabling
tumor-dependent, specific replication) was constructed in our
laboratory (19–23). In the present study, we used the
combination of ATV and cisplatin to inhibit tumor formation, and
the migration and invasion of A549 cells. The effects were assessed
using cell proliferation assays, wound healing assays, Transwell
migration assays, Matrigel invasion assay, and nude mouse models
with subcutaneous tumor xenografts of A549 cells.
Materials and methods
Virus, cell lines, reagents and
mice
ATV was constructed previously in our laboratory.
Human lung adenocarcinoma (A549) cells were purchased from the Cell
Bank, Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Hank's F12 medium, Dulbecco's modified Eagle's
medium (DMEM), fetal bovine serum, and 0.25% trypsin solution were
purchased from HyClone (GE Healthcare Life Sciences, Logan, UT,
USA). The Annexin V-FITC apoptosis detection kit was purchased from
Biomiga (Beijing, China). The cell proliferation detection kit was
purchased from Promega Corp. (Madison, WI, USA). Acridine
Orange/Ethidium Bromide (AO/EB), 4,6-Diamidino-2-phenylindole
(DAPI), propidium iodide (PI) were purchased from Beijing Solarbio
Science and Technology Co., Ltd., Beijing, China. Six-week-old
BALB/c nude mice were purchased from Experimental Animal Center,
Academy of Military Medical Sciences of PLA (Beijing, China) and
were housed under standard pathogen-free conditions. The animal
experimental protocols were approved by the Institutional Animal
Care and Use Committee (IACUC) of the Chinese Academy of Military
Medical Science (Changchun, China; 10ZDGG007). All surgeries were
performed under sodium pentobarbital anesthesia, and all efforts
were made to minimize suffering.
Plaque formation assay and cell
proliferation assay
The A549 human lung adenocarcinoma cells were
dispensed at 5×103 cells per well in a 96-well plate at
37°C with 5% CO2 for 24 h. A549 cells were treated with
0.1, 0.2, 0.4, and 0.8 µg cisplatin, which determined the optimum
does of cisplatin as 0.4 µg. The formation of virus plaques in A549
cells treated with ATV and cisplatin was measured via crystal
violet staining. A549 cells were seeded in 12-well cell culture
plates at 1×105 per well and cultured at 37°C with 5%
CO2 for 24 h. The cells were then treated with ATV at 1
multiplicity of infection (MOI), 10 MOI, and 100 MOI together with
0.4 µg cisplatin. Along with Ad5-infected control wells, the
infected cells were cultured at 37°C with 5% CO2 for 24,
48, and 72 h. The plaques that were stained by crystal violet were
examined under an optical microscope. A549 cells were infected with
either ATV (1, 10, and 100 MOI) or a combination of ATV (1, 10, and
100 MOI) and cisplatin (0.4 µg). Cell viability was measured by the
MTS cell proliferation kit at 24, 36, 48, 72, and 96 h, following
the manufacturer's instructions. Ad5-infected cells were used as
negative controls.
Transwell migration and Matrigel
invasion assay
Human A549 lung adenocarcinoma cells were dispensed
at 5×104 cells per well in a 24-well plate at 37°C with
5% CO2 for 24 h. The cells were infected with either ATV
at an MOI of 10 and 100 or ATV at an MOI of 10 and 100 combined
with 0.4 µg cisplatin for 24 and 48 h. The A549 cells were then
seeded in the upper chamber of the cell culture inserts after
trypsinization, and cultured for 24 h. Cells that had migrated
through the membrane were counted under a microscope after they
were fixated by carbinol and stained with crystal violet. The
experimental procedure of Matrigel invasion assay was the same as
that for the Transwell migration assay except for incubation with
Matrigel of the upper chamber for 1 h before seeding the cells.
Ad5-infected cells were used as negative controls. And Transwell
migration and Matrigel invasion assay was performed as described
previously (24).
Xenograft and orthotopic tumor model
BALB/c nude mice and their treatment strategy
The xenograft models were established via
subcutaneous injection of A549 cells (1×106/100 µl) into
the right legs of the mice. The orthotopic model was established
via subcutaneous injection of A549 cells (1×106/100 µl)
into the caudal vein. When the tumors had formed clearly (usually 4
days), the mice were divided randomly into five groups (n=50).
Group 1 was injected with 1×108 plaque forming units
(PFU) of ATV in 100 µl of phosphate-buffered saline (PBS). Group 2
was injected with 0.06 mg of cisplatin in 100 µl of PBS. Group 3
was injected with ATV (1×108 PFU) and cisplatin (0.06
mg) in 100 µl of PBS. Group 4 was injected with 1×108
PFU of Ad5 in 100 µl of PBS. Group 5 was injected with 100 µl of
PBS. All groups were treated from the 0th day. The xenograft models
were infected with ATV via intratumoral injection and treated with
cisplatin via intraperitoneal injection, while the orthotopic
models were infected with ATV via caudal vein injection and treated
with cisplatin via intraperitoneal injection. Injections were given
every four days for 16 days. The length and width of the xenograft
tumors were measured every four days for 24 days using Vernier
calipers. Survival of the xenograft and orthotopic mice models was
observed every four days for 30 days. All the mice models were
sacrificed at day 30, and the xenografted tumors were removed and
measured. Meanwhile, the lungs from the orthotopic models were
removed and observed.
The analysis of A549 cells inhibition
pathway by ATV
The key difference between apoptosis and cells death
is the integrity of the cell membrane. Therefore, apoptosis and
cells death could be obviously distinguished via DAPI, AO/EB and
Annexin V staining. Human A549 lung adenocarcinoma cells were
dispensed at 1×106 cells per well in a 6-well plate and
incubated at 37°C with 5% CO2 for 24 h. The cells were
then infected with 100 MOI ATV for 48 h. The ATV-infected A549
cells were stained by AO/EB and DAPI to confirm the inhibition
pathway of A549 cells by ATV. The A549 cells (1×106
cells) were treated with 100 MOI ATV for 24, 48, and 72 h, and then
the uninfected and ATV-infected A549 cells were harvested, washed
with PBS three times, and incubated in the presence of 5 µl Annexin
V-FITC (early apoptotic marker appearing green) and 5 µl of PI
(late apoptotic marker appearing red) in 100 µl of 1X binding
buffer at room temperature in the dark for 15 min, and then the
apoptosis of A549 cells were analyzed by laser scanning confocal
microscopy and flow cytometry. Ad5-infected cells were used as
negative controls.
Statistical analysis
The statistical analysis was performed using data
from at least three independent experiments using the Statistical
Package for the Social Sciences (SPSS) statistical software package
(version 15.0; SPSS, Inc., Chicago, IL, USA), and the results were
obtained using GraphPad Prism version 7.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). Student's t-test or one-way analysis of
variance followed by Tukey's post hoc test were used. Differences
with a P<0.05 or P<0.01 were considered to indicate a
statistically significant difference. Data are presented as the
mean ± standard deviation (SD) values.
Results
Proliferation inhibition effect of
A549 cells
The results for the inhibition of A549 cell
proliferation are shown in Fig. 1A-E.
The plaque formation assay is shown in Fig. 1A. The MTS assays showed that the
proliferation of A549 cells was suppressed significantly at 24, 48
and 72 h by 0.8 µg cisplatin (P<0.01). However, this inhibitory
effect may be caused by itself toxic. The proliferation of A549
cells was suppressed significantly at 24 and 48 h by 0.4 µg
cisplatin (P<0.05) and the inhibitory effect of A549 cells in
0.4, 0.1 and 0.2 µg cisplatin had no significant difference
(P>0.05). This result indicated that the inhibitory effect of
0.4 µg was not induced by itself toxic. Therefore the optimum dose
of cisplatin was 0.4 µg. The growth of A549 cells could be
suppressed by ATV or ATV combined with cisplatin in a dose- and
time-dependent manner. The inhibition rate of A549 cells was
increased significantly by 0.4 µg (P<0.05) and 0.8 µg
(P<0.01) cisplatin compared to 0.1 and 0.2 µg cisplatin, while
the higher toxicity of 0.8 µg cisplatin made it unfit for
subsequent experiments (Fig. 1B). The
growth suppression of A549 cells was not increased by 1 MOI ATV.
However, increased growth suppression of A549 cells was observed by
1 MOI ATV and 0.4 µg cisplatin, which reached a peak of 3.11-fold
greater than that of the 1 MOI ATV-infected cells at 72 h (Fig. 1C). The growth suppression induced by
10 MOI ATV and 0.4 µg cisplatin was significantly higher than that
of the control group (P<0.01). Meanwhile, the combination of 10
MOI and 0.4 µg cisplatin caused increasing growth suppression from
24 to 72 h, which reached a peak of 2.5-fold greater than that of
the 10 MOI ATV-infected cells at 72 h (Fig. 1D). The growth suppression rate of 100
MOI ATV infected-cells was also significantly higher than that of
the control group (P<0.01). However, the growth suppression was
not significantly different between the combination of 100 MOI ATV
100 with 0.4 µg and 100 MOI ATV (P>0.05) (Fig. 1E). Therefore, the combination of ATV
at an MOI of 10 or 100 with 0.4 µg cisplatin showed a synergistic
effect on the inhibition of the growth of A549 cells.
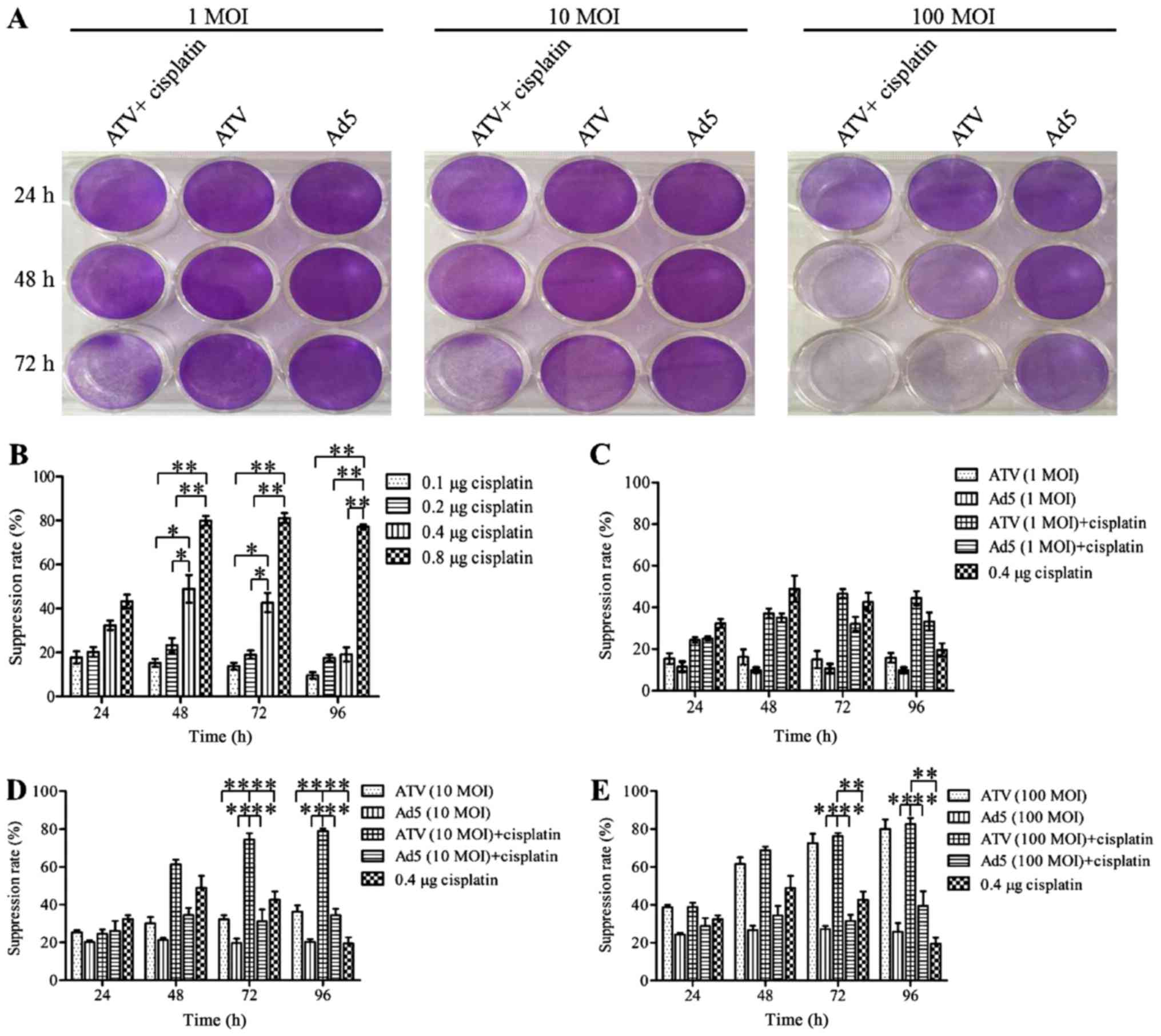 | Figure 1.Plaque formation assay by crystal
violet staining and cell proliferation assay by MTS. (A) A549 cells
in 12-well plates treated with 1 MOI, 10 MOI, and 100 MOI of ATV
and 0.4 µg cisplatin for 24, 48, 72 h and then stained with 0.4%
crystal violet after plaque phenotypes formed. (B) The suppression
of proliferation of A549 cells treated by 0.1, 0.2, 0.4, or 0.8 µg
of cisplatin. (C) The suppression of proliferation of A549 cells
infected with 1 MOI ATV or a combination of the 1 MOI ATV and 0.4
µg cisplatin. (D) The suppression of proliferation of A549 cells
infected with 10 MOI ATV or a combination of the 10 MOI ATV and 0.4
µg cisplatin. (E) The suppression of proliferation of A549 cells
infected with 100 MOI ATV or a combination of the 100 MOI ATV and
0.4 µg cisplatin. All values represent the mean ± SD. **P<0.01
and *P<0.05. MOI, multiplicity of infection; SD, standard
deviation; Ad5, human adenovirus serotype 5. |
Migration and invasion suppression
effect of A549 cells
The results of A549 cell migration and Matrigel
invasion are shown in Fig. 2A-B. The
migration inhibition effect of 10 MOI ATV and 0.4 µg
cisplatin-treatment A549 cells for 48 h was significantly higher
than those of the other groups at 48 h (P<0.05) (Fig. 2A). The migration inhibition effects of
100 MOI ATV-infected A549 cells and the combination of 100 MOI ATV-
with 0.4 µg cisplatin-treatment A549 cells showed no significant
differences, but were significantly higher than those of the
control groups at 48 h (P<0.01) (Fig.
2B). The Matrigel invasion assay showed similar results to the
migration assay (Fig. 2C-D).
Tumor growth inhibition and survival
rate of BALB/c nude mice model
The results of the tumor growth inhibition
experiment are shown in Fig. 3A and
the tumor growth inhibition of ATV and cisplatin group was
significantly lower than that of the PBS group (P<0.01). The
results of for the survival rate over 30 days are shown in Fig. 3B-C. The photographs of the
subcutaneous tumors and lungs from the orthotopic models excised
from the nude mice are shown in Fig.
3D. The inhibition of tumor growth in the treatment group was
higher than those of the other groups. Although the comparison
between treatment groups showed no significant differences in terms
of tumor growth inhibition, the survival rate of the combination
group was significantly higher than that of the cisplatin group and
indicated that the toxicity of cisplatin could be reduced by
combination of ATV and cisplatin.
Mechanism of action of ATV on A549
cells
The results of the apoptosis detection assays of
A549 cells are shown in Fig. 4A-F.
The results of AO/EB (Fig. 4A) and
DAPI (Fig. 4B) staining proved that
ATV induced apoptosis in the A549 cells. The Annexin V-FITC and PI
double-labeled positive cells could be observed among the
ATV-infected A549 cells under the laser scanning confocal
microscope (Fig. 4C), which indicated
that apoptotic morphological characteristics of the A549 cells was
caused by apoptin expressed from ATV. The degree of apoptosis in
the ATV-infected A549 cells at various time-points was evaluated
under flow cytometry (Fig. 4D-F),
which showed that the apoptosis rate of ATV-infected A549 cells at
48 h was 3.8-fold greater than that of ATV-infected A549 cells at
24 h (P<0.05); however, there was no further significant
increase from 48 to 72 h. The trend in the variation of the death
rate of ATV-infected A549 cells was the same as the apoptosis rate.
Thus, in A549 cells, ATV induced mainly apoptosis.
 | Figure 4.The mode of action of ATV on A549
cells. (A) AO/EB staining of ATV-infected A549 cells
(magnification, ×100). (B) DAPI staining of ATV-infected A549 cells
(magnification, ×200). (C) Apoptosis assay of ATV-infected A549
cells by laser scanning confocal microscope (magnification, ×400).
(D) The apoptosis rate of ATV-infected A549 cells at 24, 48 and 72
h. (E) The death rate of ATV-infected A549 cells at 24, 48, and 72
h. (F) Apoptosis assay of ATV-infected A549 cells by flow
cytometry. All values represent the mean ± SD. **P<0.01 and
*P<0.05. AO/EB, Acridine Orange/Ethidium Bromide; DAPI,
4,6-Diamidino-2-phenylindole; SD, standard deviation; Ad5, human
adenovirus serotype 5. |
Discussion
Cancer cells can be recognized and killed
specifically by oncolytic viruses. Many clinical studies have
demonstrated the good antitumor activity of oncolytic viruses
(25–28). Oncolytic viruses have been used as
delivery vectors that carry specific therapeutic proteins to
destroy malignant cells during replication. Thus, oncolytic viruses
have emerged as important methods in cancer treatment. Adenovirus,
herpes virus, and orthopoxviruse, as oncolytic virus vehicles, have
been applied successfully to antitumor therapy. Apoptin is
considered as one of the most promising antitumor proteins because
it induces specific apoptosis of cancer cells without effecting
normal cells. Oncolytic viruses expressing apoptin proteins have
been constructed and tested, including a dual cancer-specific
oncolytic adenovirus containing apoptin, poly A, hTERTp, and E1a.
Experiments have proved that tumor cell growth could be suppressed
significantly by a dual cancer-specific oncolytic adenovirus both
in vitro and in vivo (19–22).
Currently, chemotherapy, radiotherapy, and
biotherapy are the main strategies for cancer treatment. However,
because of the specificity of tumors and the side effects of these
methods, treatment is far from satisfactory. With the emergence of,
for example, drug resistance (3) and
DNA damage repair (29,30) during the treatment, the therapeutic
effect is compromised in cancers when using monotherapy. Therefore,
it is necessary to use combinations of two or more therapeutic
methods that act via different mechanisms to produce synergistic
effects to treat cancers. The ideal combination treatment should
reduce toxicity and increase the therapeutic effect. Some clinical
studies have shown that a good antitumor ability, reduced toxicity,
and increased treatment effect could be exerted by combining
oncolytic adenovirus with a chemotherapy drug such as cisplatin
(17,18).
In this study, we developed a synergistic antitumor
strategy, which combined a dual cancer-specific oncolytic
adenovirus (ATV) with cisplatin. We confirmed the enhanced
antitumor and reduced toxicity of ATV with cisplatin in A549 cells
in vitro and in vivo. In our in vitro
experiments, the MTS assays were used to detect the suppression of
the ATV, Ad5 and cisplatin in A549 cells. We found that 0.8 µg
cisplatin significantly inhibited A549 cells proliferation at 48,
72 and 96 h, while 0.4 µg cisplatin significantly inhibited A549
cells proliferation at 48 and 72 h, however, considering the
relationship between toxicity and efficacy of cisplatin, we finally
determined the dosage of cisplatin was 0.4 µg. The ATV and
cisplatin combination group have no significant difference with ATV
alone group, but significantly higher than the other experimental
groups. In addition, 10 MOI ATV and cisplatin combination group can
significantly inhibit the proliferation of A549 cells, the
inhibitory effect was significantly higher than the other
experimental groups. These results show that combined application
of ATV and cisplatin can effectively improve the suppression effect
of the tumor.
In our in vivo tumor model experiments in
nude mice, compared with the PBS control group and the Ad5 control
group use cisplatin alone can significantly inhibit the growth of
the tumor and prolong the survival time of nude mice, but due to
its toxicity, it can lead to the death of individual nude mice in
the early stages of treatment, and the result also appeared in the
Ad5 and cisplatin combination group. In ATV and cisplatin
combination group, the combined application of ATV and cisplatin
can obviously inhibit the growth of tumor, and prolong the survival
time of nude mice, while avoiding the death of individual nude mice
due to toxicity of cisplatin in the early stages of treatment. This
result suggests that cisplatin combined with Ad5 can not reduce the
toxicity of cisplatin, but combination of cisplatin and the dual
cancer-specific oncolytic adenovirus (ATV) can effectively reduce
the toxicity of cisplatin, improve the survival rate of nude mice
in the early stages of treatment. In the metastatic tumor model,
through comparing the size of the isolated tumor volume, it is
obvious that although the tumor volume of ATV and cisplatin
combination group has no significant difference with the tumor
volume of ATV alone group, but it is much smaller than that of the
other experimental groups. In the in-situ tumor model,
uncancerous lungs were isolated from nude mice in the ATV and
cisplatin combination groups, and there was a slight canceration of
the lungs in ATV alone group. In the remaining groups, the lungs of
nude mice had become cancerous, the lung tissue was swollen and
congested and lost the original form, suggesting that the combined
application of ATV and cisplatin was better than that of ATV or
cisplatin alone. In the experiments in mechanism of action of ATV
on A549 cells, the characteristic of apoptosis of A549 cells is
that integrity of the cell membrane does not damage and the cell
nuclear membrane and nucleolus fragmentations has been appeared.
Cell nucleus state could be obviously observed by DAPI staining.
After AO/EB staining, bright green fluorescence in early apoptotic
cells, orange fluorescence in late apoptotic cells and red
fluorescence in death cells could be observed under a fluorescence
microscope. The cytomembrane of apoptotic cells could be dyed green
and the nuclear of apoptotic cells could be dyed red after Annexin
V staining. The apoptosis rate and death rate of A549 cells were
almost the same at 24, 48 and 72 h. The present study showed that
the death of A549 cells was mainly induced by apoptosis. These
results showed that ATV inhibits the proliferation of A549 cells by
inducing apoptosis. Experimental results show that combined
application of ATV and cisplatin can effectively reduce the
toxicity of cisplatin and can improve the therapeutic effect of the
tumor. The results demonstrated a promising therapeutic potential
for this combined antitumor drug formulation and provided a
foundation for future preclinical studies of antitumor
treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
Research and Development Program of China (grant no.
2016YFC1200900), the Major Technological Program of Changchun City
(grant no. 16ss11), the National Science and Technology Major
Project (Major New Drugs Innovation and Development) (grant no.
2018ZX09301053-004-001) and the Shandong Provincial Key Research
and Development Program (grant no. 2016CYJS01A03).
Availability of data and materials
All datasets generated or analyzed during this
current study are included in this published article.
Authors' contributions
XL and NJ conceived and designed the experiments.
JJ, YZ, FS, ZC, SC, YL, WL, XY, ML and CC performed the
experiments. YC, SL, JZ and GY analyzed the data. YL, NJ and YZ
wrote the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experimental protocols were approved by
the IACUC of the Chinese Academy of Military Medical Science
(10ZDGG007). All surgeries were performed under sodium
pentobarbital anesthesia, and all efforts were made to minimize
suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Zhang S, Zeng H, Zou X
and He J: Report of Cancer Incidence and Mortality in China, 2013.
China Cancer. 25:1–8. 2017.(In Chinese). View Article : Google Scholar
|
|
2
|
Min YC: The development in the study of
platinum-based antineoplastic drugs. Chin J Mod Drug Appl.
9:2822015.
|
|
3
|
Brabec V and Kasparkova J: Molecular
aspects of resistance to antitumor platinum drugs. Drug Resist
Updat. 5:147–161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arany I and Safirstein RL: Cisplatin
nephrotoxicity. Semin Nephrol. 23:460–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ali BH and Al Moundhri MS: Agents
ameliorating or augmenting the nephrotoxicity of cisplatin and
other platinum compounds: A review of some recent research. Food
Chem Toxicol. 44:1173–1183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Christova TY, Gorneva GA, Taxirov SI,
Duridanova DB and Setchenska MS: Effect of cisplatin and cobalt
chloride on antioxidant enzymes in the livers of Lewis lung
carcinoma-bearing mice: Protective role of heme oxygenase. Toxicol
Lett. 138:235–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spracklen TF, Vorster AA, Ramma L, Dalvie
S and Ramesar RS: Promoter region variation in NFE2L2 influences
susceptibility to ototoxicity in patients exposed to high
cumulative doses of cisplatin. Pharmacogenomics J. 17:515–520.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maddika S, Mendoza FJ, Hauff K, Zamzow CR,
Paranjothy T and Los M: Cancer-selective therapy of the future:
Apoptin and its mechanism of action. Cancer Biol Ther. 5:10–19.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leliveld SR, Dame RT, Mommaas MA, Koerten
HK, Wyman C, Danen-van Oorschot AA, Rohn JL, Noteborn MH and
Abrahams JP: Apoptin protein multimers form distinct higher-order
nucleoprotein complexes with DNA. Nucleic Acids Res. 31:4805–4813.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong X, Zhao H, Liang S, Zhou D, Zhang W
and Yuan L: Gene delivery of apoptin-derived peptide using an
adeno-associated virus vector inhibits glioma and prolongs animal
survival. Biochem Biophys Res Commun. 482:506–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Hou L, Wu X, Zhao D, Wang Z, Hu
H, Fu Y and He J: Inhibitory effect of genetically engineered
mesenchymal stem cells with Apoptin on hepatoma cells in vitro and
in vivo. Mol Cell Biochem. 416:193–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta SK, Tiwari AK, Gandham RK and Sahoo
AP: Combined administration of the apoptin gene and poly (I:C)
induces potent anti-tumor immune response and inhibits growth of
mouse mammary tumors. Int Immunopharmacol. 35:163–173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vragniau C, Hubner JM, Beidler P, Gil S,
Saydaminova K, Lu ZZ, Yumul R, Wang H, Richter M, Sova P, et al:
Studies on the interaction of tumor-derived HD5 alpha defensins
with adenoviruses and implications for oncolytic adenovirus
therapy. J Virol. 91:pii: e02030–16. 2017. View Article : Google Scholar
|
|
14
|
Mato-Berciano A, Raimondi G, Maliandi MV,
Alemany R, Montoliu L and Fillat C: A NOTCH-sensitive
uPAR-regulated oncolytic adenovirus effectively suppresses
pancreatic tumor growth and triggers synergistic anticancer effects
with gemcitabine and nab-paclitaxel. Oncotarget. 8:22700–22715.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Wang P, Li H, Du X, Liu M, Huang Q,
Wang Y and Wang S: The efficacy of oncolytic adenovirus is mediated
by T-cell responses against virus and tumor in syrian hamster
model. Clin Cancer Res. 23:239–249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SY, Kang D, Choi HJ, Joo Y, Kim JH and
Song JJ: Prime-boost immunization by both DNA vaccine and oncolytic
adenovirus expressing GM-CSF and shRNA of TGF-β2 induces anti-tumor
immune activation. Oncotarget. 8:15858–15877. 2017.PubMed/NCBI
|
|
17
|
Wang S, Shu J, Chen L, Chen X, Zhao J, Li
S, Mou X and Tong X: Synergistic suppression effect on tumor growth
of ovarian cancer by combining cisplatin with a manganese
superoxide dismutase-armed oncolytic adenovirus. Onco Targets Ther.
9:6381–6388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma B and Wang Y, Zhou X, Huang P, Zhang R,
Liu T, Cui C, Liu X and Wang Y: Synergistic suppression effect on
tumor growth of hepatocellular carcinoma by combining oncolytic
adenovirus carrying XAF1 with cisplatin. J Cancer Res Clin Oncol.
141:419–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi Y, Guo H, Hu N, He D, Zhang S, Chu Y,
Huang Y, Li X, Sun L and Jin N: Preclinical pharmacology and
toxicology study of Ad-hTERT-E1a-Apoptin, a novel dual
cancer-specific oncolytic adenovirus. Toxicol Appl Pharmacol.
280:362–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, Wang J, Li C, Hu N, Wang K, Ji H,
He D, Quan C, Li X, Jin N and Li Y: Potent growth-inhibitory effect
of a dual cancer-specific oncolytic adenovirus expressing apoptin
on prostate carcinoma. Int J Oncol. 42:1052–1060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu L, Wu W, Zhu G, Liu L, Guan G, Li X,
Jin N and Chi B: Therapeutic efficacy of an hTERT promoter-driven
oncolytic adenovirus that expresses apoptin in gastric carcinoma.
Int J Mol Med. 30:747–754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Liu Y, Wen Z, Li C, Lu H, Tian M,
Jin K, Sun L, Gao P, Yang E, et al: Potent anti-tumor effects of a
dual specific oncolytic adenovirus expressing apoptin in vitro and
in vivo. Mol Cancer. 9:102010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin XZ, Chen S, Li WJ, Zhu YL, Li YQ, Cui
CX, Li M, Cui YL, Zhao J, Li SZ, et al: Inhibitory effect of
apopotin-loaded oncolytic adenovirus ATV on human cervical
carcinoma HeLa cells. Chin J Cancer Biother. 24:1356–1361. 2017.(In
Chinese).
|
|
24
|
Wang X, Xu L, Wu Q, Liu M, Tang F, Cai Y,
Fan W, Huang H and Gu X: Inhibition of LDHA deliver potential
anticancer performance in renal cell carcinoma. Urol Int.
99:237–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robinson S and Galanis E: Potential and
clinical translation of oncolytic measles viruses. Expert Opin Biol
Ther. 17:353–363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ungerechts G, Bossow S, Leuchs B, Holm PS,
Rommelaere J, Coffey M, Coffin R, Bell J and Nettelbeck DM: Moving
oncolytic viruses into the clinic: Clinical-grade production,
purification, and characterization of diverse oncolytic viruses.
Mol Ther Methods Clin Dev. 3:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patil S, Rao RS and Majumdar B: Clinical
trials with oncolytic viruses: Current and future prospects. J
Contemp Dent Pract. 16:i–ii. 2015.
|
|
28
|
Burke J, Nieva J, Borad MJ and Breitbach
CJ: Oncolytic viruses: Perspectives on clinical development. Curr
Opin Virol. 13:55–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Štefančíková L, Lacombe S, Salado D,
Porcel E, Pagáčová E, Tillement O, Lux F, Depeš D, Kozubek S and
Falk M: Effect of gadolinium-based nanoparticles on nuclear DNA
damage and repair in glioblastoma tumor cells. J Nanobiotechnology.
14:632016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sengupta S, Mantha AK, Song H,
Roychoudhury S, Nath S, Ray S and Bhakat KK: Elevated level of
acetylation of APE1 in tumor cells modulates DNA damage repair.
Oncotarget. 7:75197–75209. 2016. View Article : Google Scholar : PubMed/NCBI
|


















