Introduction
Lung cancer continues to be the leading cause of
cancer-associated mortality globally and its rates of incidence and
mortality have increased rapidly in developing countries (1,2). Previous
studies have primarily focused on advancing the discovery and
understanding of the development of lung cancer and metastasis
(3–5).
Clinical applications of cancer immunotherapies, radio therapies,
gene therapies or target-oriented therapies have been suggested as
potential approaches to lung cancer treatment (6). However, due to the considerable toxicity
and in efficiency of these treatments, the results of standard
chemotherapy and radiotherapy for the treatment of patients with an
advanced stage of or locally recurring lung cancer remain
unsatisfactory (7). Metastasis is
responsible for a substantial number of the associated mortalities
and the poor prognosis of patients with all types of cancer
(4). Tumor metastasis is an
exceedingly complex process and prevailing models of metastasis
reflect that metastasis is a late, acquired event in tumorigenesis
(8). Increasingly, evidence supports
the view that the matrix metalloproteinases (MMPs) are proteolytic
enzymes that mediate a number of the changes in the
microenvironment during tumor progression (9–11). Among
the members of the MMP family, MMP-2 and MMP-9 are type IV
collagenases primarily involved in the degradation of the
extracellular matrix (ECM) (11). In
patients clinically diagnosed with lung cancer metastasis, serum
levels of MMP-2 and MMP-9 are significantly increased compared with
patients with an absence of distant metastasis or in healthy
volunteers (12,13). Additionally, the Wnt/β-catenin pathway
has been identified as providing tumor cells with the capacity to
become resistant to treatment, self-renewal and metastasis
(14). A study revealed that the
invasion of hepatocellular carcinoma BEL-7402 cells would be
suppressed by inhibiting the expression of phosphorylated-glycogen
synthase kinase (p-GSK), β-catenin and its target proteins
(15). A number of molecular
predictors, including β-catenin, MMPs, disheveled segment polarity
protein (Dvl) and protein kinase B (Akt) have been proposed and are
usually over expressed in non-small-cell lung cancer (NSCLC)
(16–18). Therefore, the specific inhibition of
expression of these proteins may serve as an effective method to
prolong the survival and improve the prognosis of patients with
lung cancer.
Cordyceps militaris has been extensively used
as in the formula of nutraceuticals and as a tonic supplement for
sub-healthy patients who are generally not completely healthy,
particularly in China and Korea (19,20). At
present, cultured C. militaris has been well established and
a variety of constituents extracted from C. militaris
(21). In addition to functional
foods and supplements, C. militaris also has various
pharmacological activities, including antioxidation (22), anti-inflammation (23), anti-proliferation (24) and anti-metastasis (25) in numerous tumor types. Therefore,
C. militaris has good development prospects not only for
healthcare but also for cancer treatment.
In our previous study, C. militaris fraction
(CMF) was demonstrated to inhibit the proliferation of K562 cells
and to induce apoptosis in addition to cell cycle arrest in the S
phase. The mechanism underlying CMF-induced apoptosis was involved
in mitochondrial dysfunction (26).
In the present study, the aim was to investigate the inhibitory
effects of CMF on the migration and invasion of NCI-H1299 and Lewis
lung cancer (LLC) cell lines, in addition to metastasis in a
xenograft model.
Materials and methods
Fraction preparation and
materials
Cultured C. militaris was purchased from
Shaanxi Honghao BioTech Co., Ltd. (Jiangmen, China). CMF was
prepared as previously described (26), dissolved in serum-free of RPMI-1640 or
DMEM medium to make a 1 mg/ml stock solution, and stored at −20°C
in multiple aliquots. RPMI-1640 medium and Dulbecco's modified
Eagle's medium (DMEM) were purchased from Thermo Fisher Scientific,
Inc., (Waltham, MA, USA). Fetal bovine serum (FBS) was purchased
from Biological Industries (Kibbutz Beit Haemek, Israel).
Anti-MMP-2 (cat no. 4022), MMP-9 (cat no. 3852), Akt (cat no.
9272), p-Akt (cat no. 9271), GSK-3β (cat no. 9832), p-GSK-3β (cat
no. 9323) and MYC proto-oncogene (c-Myc) (cat no. 9402) antibodies
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Anti-β-catenin (cat no. sc7963) and Dvl-2 (cat no. sc8026)
antibodies were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Anti-β-actin (cat no. ab16039) and GAPDH (cat
no. ab181602) were obtained from Abcam (Cambridge, UK). HRP, Rabbit
Anti-Goat IgG (H+L) (cat no. E030130) and HRP, Mouse Anti-Goat IgG
(H+L) (cat no. E030110-01) were obtained from Earth Ox Life
Sciences (Millbrae, CA, USA).
Cells and culture
NCI-H1299 and Lewis lung carcinoma (LLC) cell lines
were purchased from the American Type Culture Collection (Manassas,
VA, USA). NCI-H1299 cells were cultured in RPMI-1640 medium with
10% FBS and LLC cells in DMEM with 10% FBS, each of which was
supplemented with penicillin (100 U/ml) and streptomycin (100
mg/ml). Cells were maintained in a humidified atmosphere of 5%
CO2 in air at 37°C.
MTT assay
A total of 3×103 NCI-H1299 and LLC cells
per well were seeded onto 96-well plates (cat no. 3599; Corning
Incorporated, Corning, NY, USA) and treated with 100 µl of CMF at
0.3, 1, 3, 10, 30, 90 µ1/ml in NCI-H1299 cells and 0.03, 0.1, 0.3,
0.9 µg/ml in LLC cells. In a pre-screening experiment (data not
shown), the effect of CMF was stronger against LLC cells than
against NCI-H1299 cells. Therefore, the two cell lines were treated
with different concentrations of CMF to obtain the IC50
values. The same volume (100 µl) of corresponding complete medium
was used as a negative control. Following incubation for 24, 48, 72
h at 37°C, 5% CO2, 20 µl MTT solution (5 mg/ml) was
added into each well. Then 200 µl DMSO was used to dissolve the
formazan crystals and optical density (OD) absorbance was measured
at 570 nm using a 96-well microplate reader. The results were
presented as cell viability
(%)=ODtreatment/ODncx100% and 50% inhibitory
concentration (IC50) was calculated by linear-regression
analysis. All experiments were performed in triplicate.
Colony formation assay
NCI-H1299 and LLC cells were plated onto 6-well
plates (200 cells/well; cat no. 3516; Corning Incorporated) and
allowed to adhere overnight. The cells were then treated with 0.01,
0.03, 0.1, 0.3, 1 µg/ml of CMF or left untreated and cultured in
corresponding complete medium at 37°C, 5% CO2 in air for
up to 14 days. After 14 days, the cloned cells were fixed with
absolute methanol for 30 min and stained with 0.1% crystal violet
solution at room temperature for 20 min. Colonies of each well were
photographed and counted with the naked eye.
Cell adhesion assay
CI-H1299 and LLC cells (1×105) were
plated in 6-well culture dishes with or without CMF and allowed to
adhere for 1.5 h. Subsequently, corresponding medium with
non-adhered cells was discarded and cells were gently washed twice
with PBS in order to remove any loosely attached cells. Adhered
cells were then counted using a 0.1% crystal violet staining
solution at room temperature for 20 min subsequent to being fixed
with methanol for 30 min at room temperature. Data are expressed as
a percentage in adhered cells treated with CMF relative to the
control cells.
Wound healing assay
Cell migration was analyzed using a wound healing
assay. NCI-H1299 and LLC cells (2×105) were seeded onto
6-well plates until they reached confluence. A scratch wound in
confluent monolayer was made using a 10 µl sterile pipette tip.
Subsequent to washing away all detached cells with PBS, the
remaining cells were treated with or without CMF (NCI-H1299 cells
were treated at 1, 3, 10, 30 µg/ml at 1 and LLC cells at 0.1, 0.3,
1, 3 µg/ml) in serum-free RPIM-1640 or DMEM medium. Photographs
were taken at 0 and 24 h after treatment.
Transwell migration and invasion
assay
A Transwell assay was used to test the migration
ability of the cells. In order to test the invasion ability of
cells, 6.5-mm Transwell inserts with a 8.0-µm pore membrane (cat
no. 3422; Corning Incorporated) coated with 50 µl of a 1:4 diluted
Matrigel (BD Biosciences, San Jose, CA, USA) in cold RPIM-1640 or
DMEM medium to form a thin continuous film on the top of the filter
were used. The procedure was performed as previously described
(22). A total of 4×104
NCI-H1299 and LLC cells per well were seeded onto an insert with
200 µl serum-free RPIM-1640 or DMEM medium with or without CMF, and
600 µl corresponding medium containing 20% FBS was added into the
bottom wells. Following 24 h incubation at 37°C, 5% CO2
in air, non-migrating cells were removed from the upper surface by
wiping with a cotton swab. The bottom cells of the filter were
fixed with absolute methanol for 30 min at room temperature and
stained with 0.1% crystal violet for 20 min at room temperature.
Subsequently, cells in 4 randomly selected fields were counted by a
Digital Sight Inverted Light Microscope at ×100 magnification
(Nikon Corporation, Tokyo, Japan).
Western blot analysis
Whole cell lysate preparation and western blot
analysis were performed as previously described (27). NCI-H1299 and LLC cells treated with or
without CMF were collected and lysed in radio immunoprecipitation
assay buffer (cat no. 66016413; Biosharp, Hefei, China) with 1%
phenylmethylsulfonyl fluoride and 1% phosphatase inhibitor after 24
h cultivation. Cleared total cell lysate was quantified by BCA
assay kit (Thermo Fisher Scientific, Inc.) and denatured by boiling
for 10 min and loaded onto 10% SDS-PAGE with 40 µg per lane.
Following electrophoretic separation, proteins were transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked for 2 h at room temperature in
blocking buffer (5% skimmed milk in Tris-buffered saline with
Tween-20 buffer) and incubated with the primary antibodies (AKT,
p-AKT, GSK-3β, p-GSK-3β, c-MYC, MMP-2, MMP-9 were diluted to
1:1,000, DVL2 and β-catenin to 1:500, GAPDH and β-actin to 1:4,000)
overnight at 4°C. After washed with TBST three times, membranes
which were incubated with AKT, p-AKT, p-GSK-3β, c-MYC, MMP-2,
MMP-9, GAPDH and β-actin antibodies were then incubated with goat
anti-rabbit IgG secondary antibody (1:4,000 dilutions), while
membranes which were incubated with GSK-3β, DVL2 and β-catenin were
then incubated with goat anti-mouse IgG HRP secondary antibodies
(1:4,000 dilutions) for 2 h at room temperature. Finally, the
membranes were developed by electrochemiluminescence substrates
(Tanon Science and Technology Co., Ltd., Shanghai, China) and
exposed onto X-ray films in a dark room. Results were analyzed
using Image Lab Software (Version 5.1; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). GAPDH and β-actin were used as controls.
Animal experiments
A total of 40 C57BL/6 mice (male, aged 6–8 weeks,
weighted 16–18 g) were purchased from Guangdong Medical Laboratory
Animal Center (Guangzhou, China). All animal experiments were
carried out in compliance with the Animal Management Rules of the
Ministry of Health of the People's Republic of China and approved
by the Animal Care and Use Committee of Jinan University
(Guangzhou, China). The mice were housed and maintained under
sterile conditions at 23–27°C, 40–60% relative humidity in a 12 h
light/12 h dark cycle and ad libitum access to food and
water. Mice were randomly divided into five groups (8 mice per
group). A total of 6×106 LLC cells were injected
subcutaneously into the right armpit of the mice. After 24 h of
inoculation, the mice were treated with different doses of CMF (65,
130 or 260 mg/kg) by oral administration once a day and the
negative control group was administered the same volume of
distilled water. The positive control group was administered
cyclophosphamide (30 mg/kg) through intraperitoneal injection twice
a week. During the treatment, the body weight of the mice was
scaled and the volume of tumor was measured using a vernier
caliper. Following a 4-week treatment course, mice were sacrificed,
and tumors were removed and weighed. Half of each tumor was frozen
in liquid nitrogen for western blot analysis (as previously
described). The lung tissues were fixed in Bouin's solution
(picranisic acid:formalin:glacial acetic acid=15:5:1) for 24 h at
room temperature and then tumor nodules on the lung surface were
counted. Hematoxylin and eosin staining of lung and liver tissues
was performed at room temperature for 20 min to evaluate the
morphology and then examined under a light microscope with ×100
magnification (Nikon Corporation, Tokyo, Japan).
Statistical analysis
The data are presented as the mean ± the standard
deviation. GraphPad Prism 6.0 software (GraphPad Software, Inc., La
Jolla, CA, USA) was used for statistical analysis. The data for
concentration and dosage effects were analyzed using a one-way
analysis of variance followed by Tukey's multiple comparisons test.
P<0.05 and P<0.01 were considered to indicate a statistically
significant difference.
Results
CMF inhibits the viability of
NCI-H1299 and LLC cells
The inhibitory effect of CMF on the viability of
NCI-H1299 and LLC cells was investigated using an MTT assay at
varying concentrations (0.3, 1.0, 3.0, 10.0, 30.0 or 90.0 µg/ml in
NCI-H1299 cells; 0.03, 0.10, 0.30, 1.00, 3.00 or 9.00 µg/ml in LLC
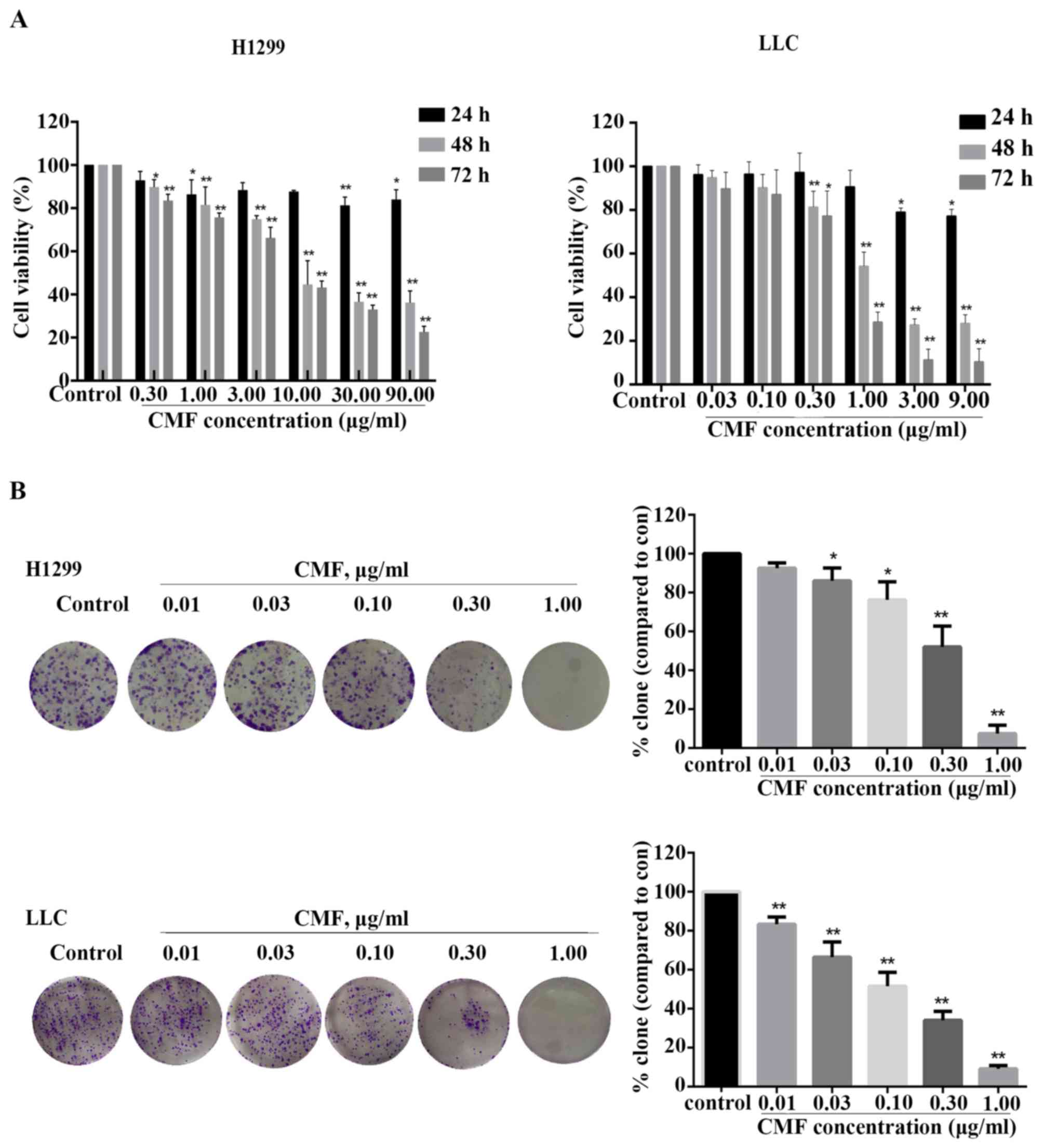
cells) and time periods (24, 48 or 72 h). As shown in Fig. 1A, CMF inhibited the viability of
NCI-H1299 and LLC cells in a time- and concentration-dependent
manner compared with the control. The half maximal inhibitory
concentration (IC50) values of CMF in NCI-H1299 cells
were 16.58 and 7.95 µg/ml for 48 and 72 h, respectively. For the
LLC cells, the IC50 values were only 1.63 µg/ml (48 h)
and 0.58 µg/ml (72 h). Furthermore, a clone formation assay was
used to evaluate the ability of single cell viability. As shown in
Fig. 1B, that cell clone formation
was significantly inhibited in a concentration-dependent manner in
NCI-H1299 cells treated with ≥0.03 µg/ml CMF compared with the
control (P<0.05) and in all CMF-treated LLC cells compared with
the control (P<0.01). Subsequent to incubation with CMF at 1
µg/ml, the number of cells forming colonies in the two cell lines
was decreased by almost 90% compared with that of control group.
These results suggested that CMF might efficiently inhibit the
viability of NCI-H1299 and LLC cells.
CMF suppresses the adhesion, migration
and invasion of NCI-H1299 and LLC cells
Two-dimensional and physiological three-dimensional
culture systems have been constructed to perform cell motility
in vitro, which contributes to current understanding of the
mechanisms of cell migration (28).
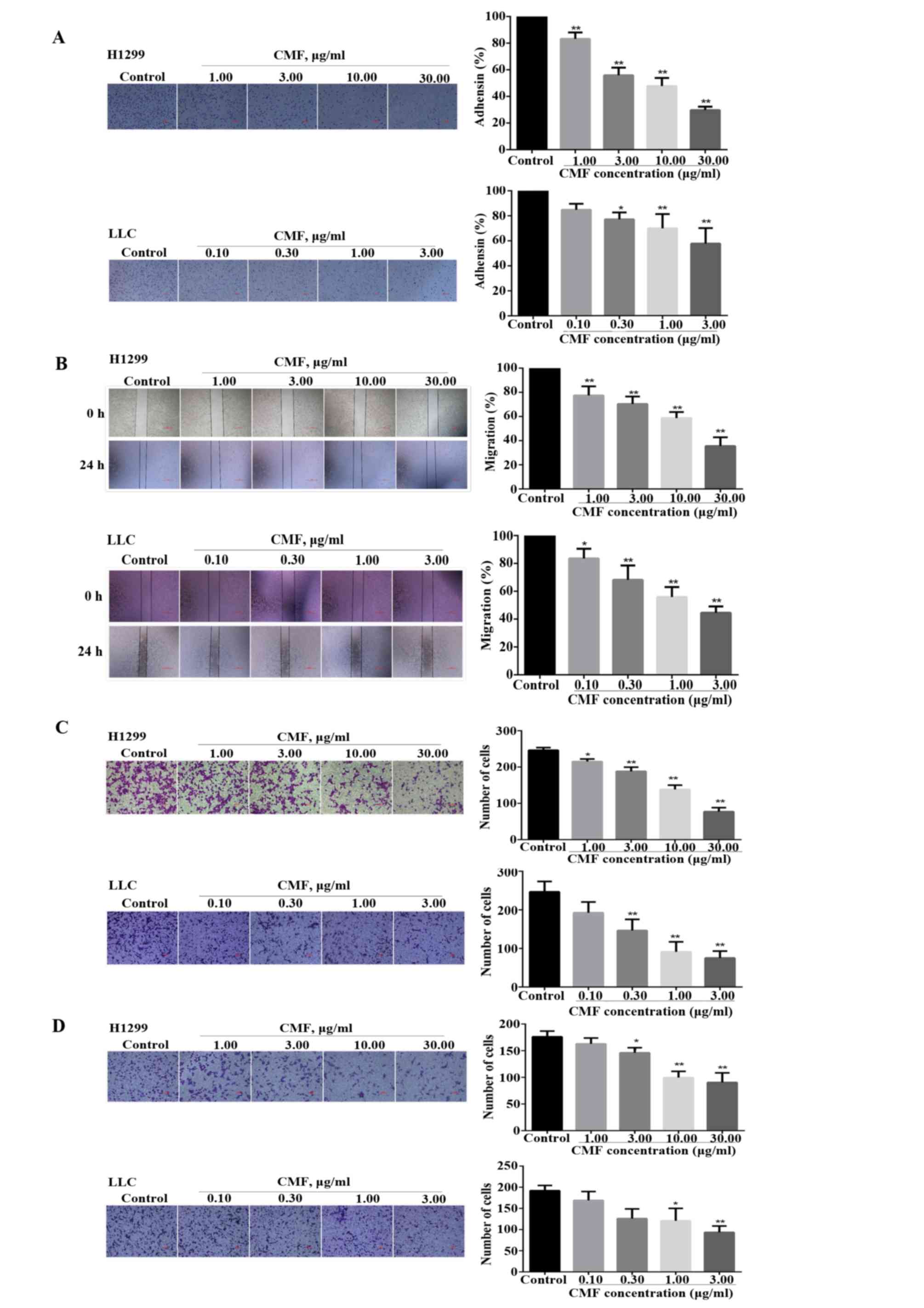
In the present study, a non-specific cell adhesion assay was used
to investigate the effect of CMF on cell attachment. As shown in
Fig. 2A, the number of NCI-H1299 and
LLC cells that adhered was significantly decreased by 44 and 42.5%,
respectively, compared with the control group at 3 µg/ml CMF
(P<0.01). In addition, the migratory abilities of the two cell
lines from one end of the wound to the other also significantly
decreased following treatment with CMF for 24 h compared with the
control (P<0.05; Fig. 2B). The
results were further detected using a Transwell assay. As presented
in Fig. 2C, CMF significantly reduced
the numbers of NCI-H1299 cells (at all CMF concentrations) and LLC
cells (CMF concentrations ≥0.3 µg/ml) migrating through the
Transwell membrane to the lower chamber in a
concentration-dependent manner, compared with the control
(P<0.05), in which CMF at the concentration of 30 µg/ml
decreased the number of migrating NCI-H1299 cells by >50%, and
the number of LLC cells decreased 63.1% at the CMF concentration of
1 µg/ml, compared with the control. In a tumor micro-environment,
an invaded cell must modify its shape and stiffness to interact
with the surrounding tissue structures to migrate through a
physical barrier of dense extracellular matrix (ECM) (29). A matrigel-coated chamber was used to
simulate the ECM in order to study the effect of CMF on the
invasive capacity of the two cell lines. As shown in Fig. 2D, the invasion of NCI-H1299 cells into
the lower chamber was significantly decreased when treated with CMF
compared with the control group (≥3 µg/ml; P<0.05). Furthermore,
the number of LLC cells was also inhibited by ~37.2% at the CMF
concentration of 1 µg/ml, demonstrating a significant difference
compared with the control group (P<0.05). In the presence of the
respective highest concentrations of CMF, NCI-H1299 and LLC cells
barely invaded into the lower part of the insert. Taken together,
these results demonstrated that CMF functions by acting directly on
NCI-H1299 and LLC cells to inhibit the processes of adhesion,
migration and invasion.
CMF inhibits the Akt/GSK-3β/β-catenin
signaling pathway in NCI-H1299 cells
In order to investigate the mechanism of CMF on
NCI-H1299 cells in vitro, western blot analysis was used to
assay cell signaling transduction. CMF inhibited the
phosphorylation of Akt at Ser473, which resulted in the down
regulation of the expression of its target protein p-GSK-3β
compared with the untreated cells (Fig.
3A). As GSK-3β also served an important role in the Wnt
signaling pathway, its downstream protein β-catenin was
investigated. Consistent with a previous study (30), the expression of β-catenin was
attenuated when cells were treated with CMF at 40 µg/ml compared
with untreated cells. It was reported that the overexpression of
Dvl was evidence of the activation of the Wnt pathway in NSCLC
(17). As a critical mediator of Wnt
signaling, Dvl is hyperphosphorylated following linking to
Wnt/Frizzled and prevents GSK-3β from phosphorylating β-catenin
through its association with a cytoplasmic protein complex
[Axin/APC, WNT signaling pathway regulator (APC)/GSK-3β complex]
(31). The results of the present
study revealed that CMF inhibited Dvl-2 expression at a
concentration of ≥20 µg/ml, which indicated that Dvl protein also
served a function in the regulation of GSK-3β activity.
Additionally, these results were further confirmed by treatment
with CMF at 40 µg/ml for different durations (0, 6, 12 or 24 h).
The expression of p-Akt was decreased after 12 h incubation with
CMF compared with cells treated for 0 h, while its downstream
protein GSK-3β was activated even at 6 h, suggesting that Dvl-2 was
also implicated in the regulation of GSK-3β (Fig. 3B). In addition, c-Myc, one of the
β-catenin target proteins, decreased in expression with the
increasing concentrations of CMF compared with the untreated cells.
Taken together, these results illustrated that CMF may inhibit the
migration and invasion of NCI-H1299 cells by blocking the
Akt/GSK-3β/β-catenin signaling pathway. Furthermore, Dvl-2 was
partially involved in the regulation of GSK-3β.
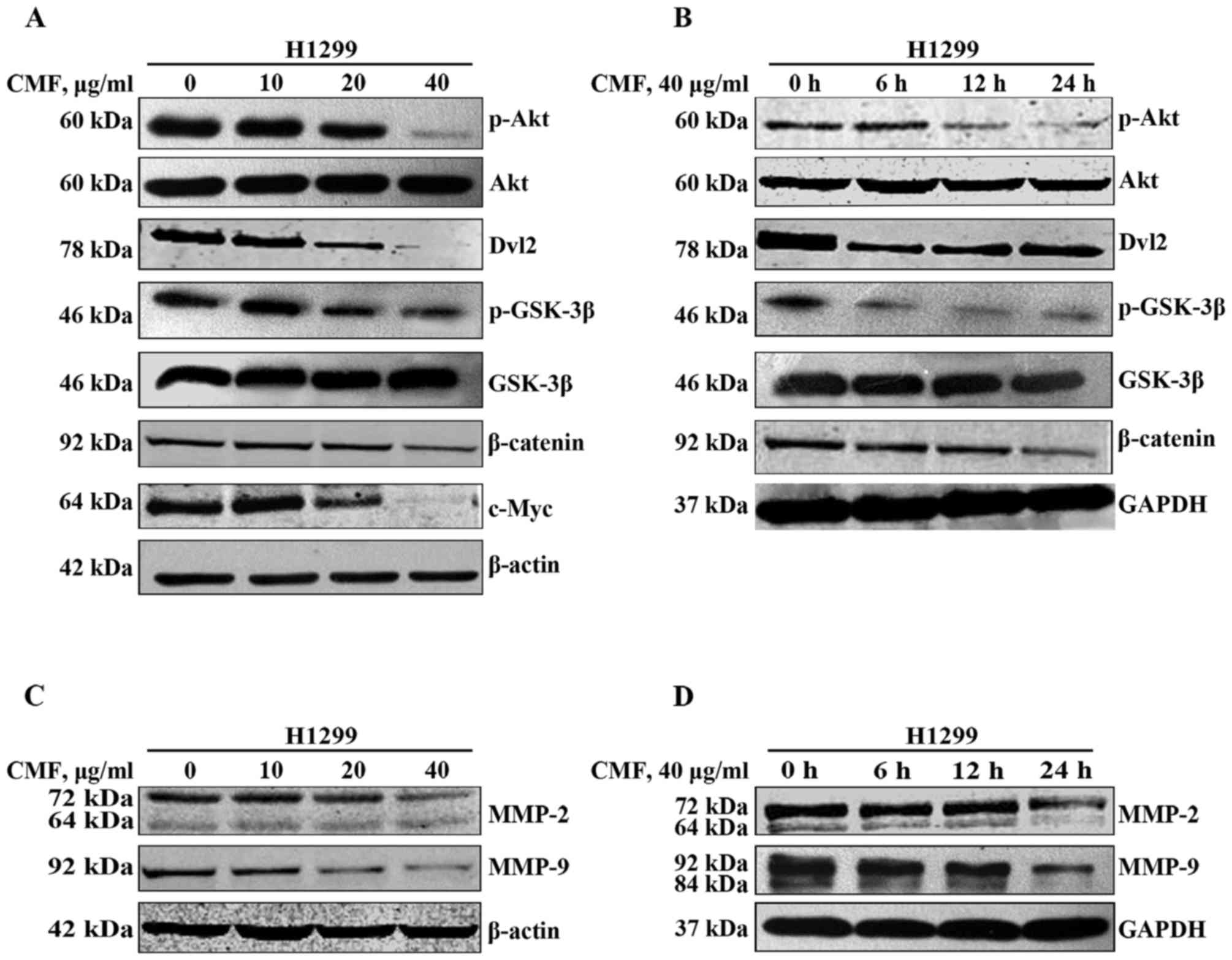 | Figure 3.CMF suppresses Akt/GSK-3β/β-catenin
signaling in addition to the expression of MMP-2 and MMP-9 in
NCI-H1299 cells. (A) NCI-H1299 cells were treated with 10, 20 and
40 µg/ml CMF for 24 h. The protein expression of p-Akt, Akt,
p-GSK-3β, GSK-3β, Dvl-2, β-catenin and c-Myc was determined in
whole cell lysates by western blot analysis. (B) NCI-H1299 cells
were treated with 40 µg/ml CMF for 6, 12 and 24 h. The protein
expression of p-Akt, Akt, p-GSK-3β, GSK-3β, Dvl-2 and β-catenin
were determined in whole cell lysates by western blot analysis. (C)
NCI-H1299 cells were treated with 10, 20 or 40 µg/ml CMF for 24 h,
and tested for MMP-2 and MMP-9 by western blot analysis. (D)
NCI-H1299 cells were treated with 40 µg/ml CMF for 6, 12 or 24 h,
and tested for MMP-2 and MMP-9 by western blot analysis. β-actin
and GAPDH were used as loading controls. CMF, Cordyceps
militaris fraction; Akt, protein kinase B; Dvl, Disheveled
segment polarity protein; GSK-3β, glycogen synthase kinase 3β;
c-Myc, MYC proto-oncogene, BHLH transcription factor; MMP, matrix
metalloproteinase; p-, phosphorylated. |
CMF down regulates the expression of
MMP-2 and MMP-9
MMPs have long been associated with cancer cell
invasion and metastasis (32).
Elevated levels of MMP-2 and MMP-9 have been demonstrated to
promote metastasis and worsen the prognosis of patients with lung
cancer (16,33). The results of the present study
revealed that the expression of MMP-2 and MMP-9 was inhibited
subsequent to treatment with CMF, in a concentration- and
time-dependent manner (Fig. 3C and
D). This suggested that MMP-2 and MMP-9 were implicated in the
effects of CMF on the migration and invasion of NCI-H1299
cells.
Oral administration of CMF inhibits
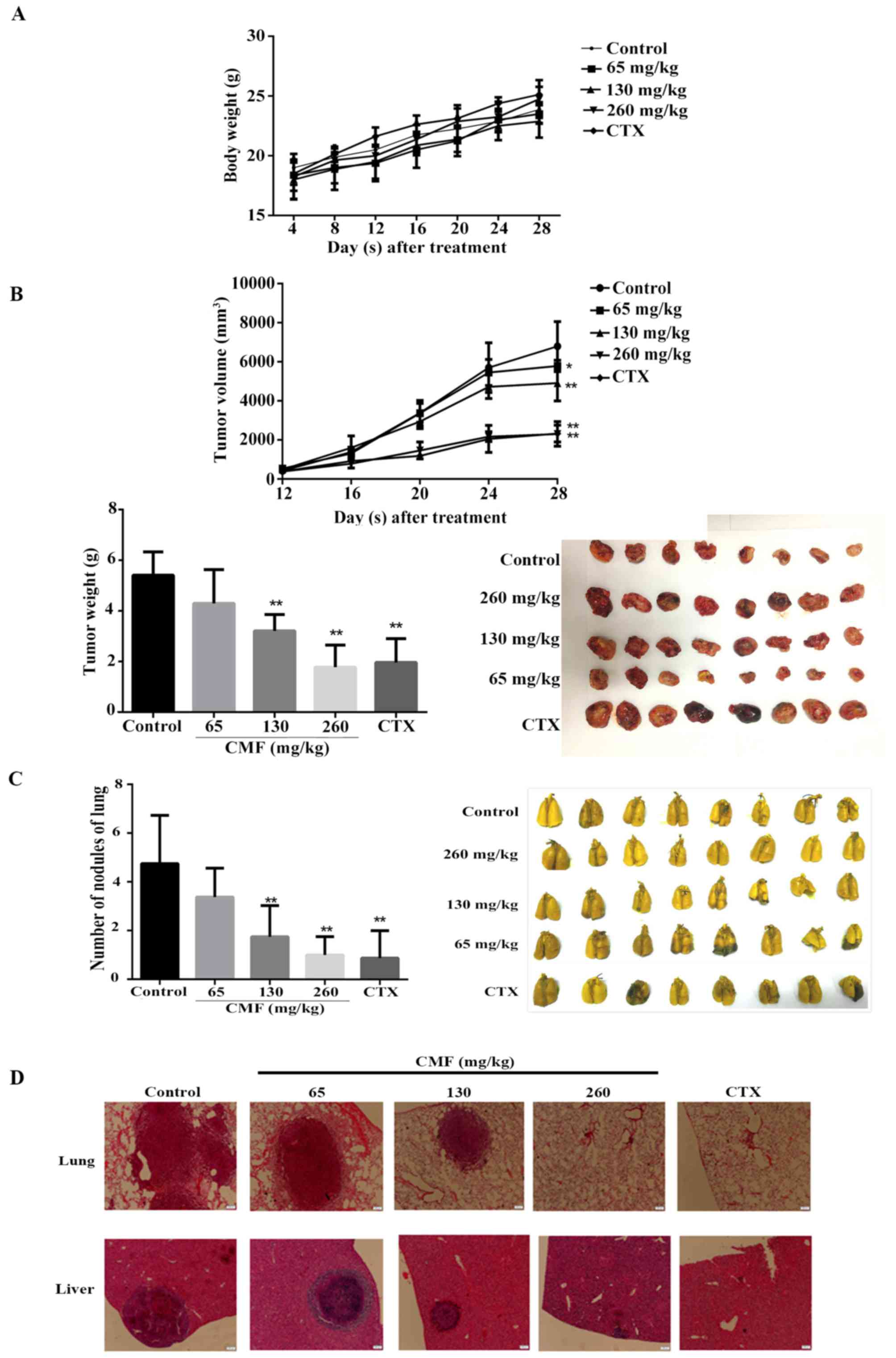
tumor growth and metastasis in an LLC xenograft model
To determine the effect of CMF on tumor growth and
metastasis in vivo, LLC cells were injected into the right
armpit of C57BL/6 mice to establish an animal lung cancer model.
Subsequent to intragastric administration of CMF once a day for 4
weeks, the volume and weight of the tumor were significantly
inhibited in a dose-dependent manner compared with those of control
group with no significant difference in body weight (P<0.05;
Fig. 4A). In the group treated with
260 mg/kg CMF, tumor volumes were inhibited after 12 days of being
injected with the cells, resulting in the final average volume
being significantly smaller than that of the untreated group
(P<0.01). Similarly, tumor weights were decreased to 57.5% of
the control group when administered with 130 mg/kg (Fig. 4B).
Certain organs, including the lung (34) and liver (35) are prone to be sites for the formation
of metastatic colonization. To investigate the effect of CMF on
metastasis in vivo, whole lung tissue was collected once the
mice were sacrificed and the nodules on the surface were counted
(Fig. 4C). The results revealed that
CMF significantly decreased the number of nodules in mice at
dosages of 130 and 260 mg/kg compared with the control (P<0.01).
Furthermore, CMF treatment also substantially decreased the size
and number of metastatic clones in the lung and liver tissue
(Fig. 4D). Western blot analysis
results revealed that the expression of MMP-2 and MMP-9 was
substantially down regulated in the tumor tissue of mice treated
with CMF at a dose of 260 mg/kg (Fig.
5). Additionally, consistent with the in vitro results,
the expression of p-Akt, p-GSK-3β and β-catenin was also decreased
compared with that in control mice. Taken together, the results
from the in vitro and in vivo assays revealed that
CMF may inhibit the invasion and metastasis of lung carcinoma cells
through the Akt/GSK-3β/β-catenin signaling pathway.
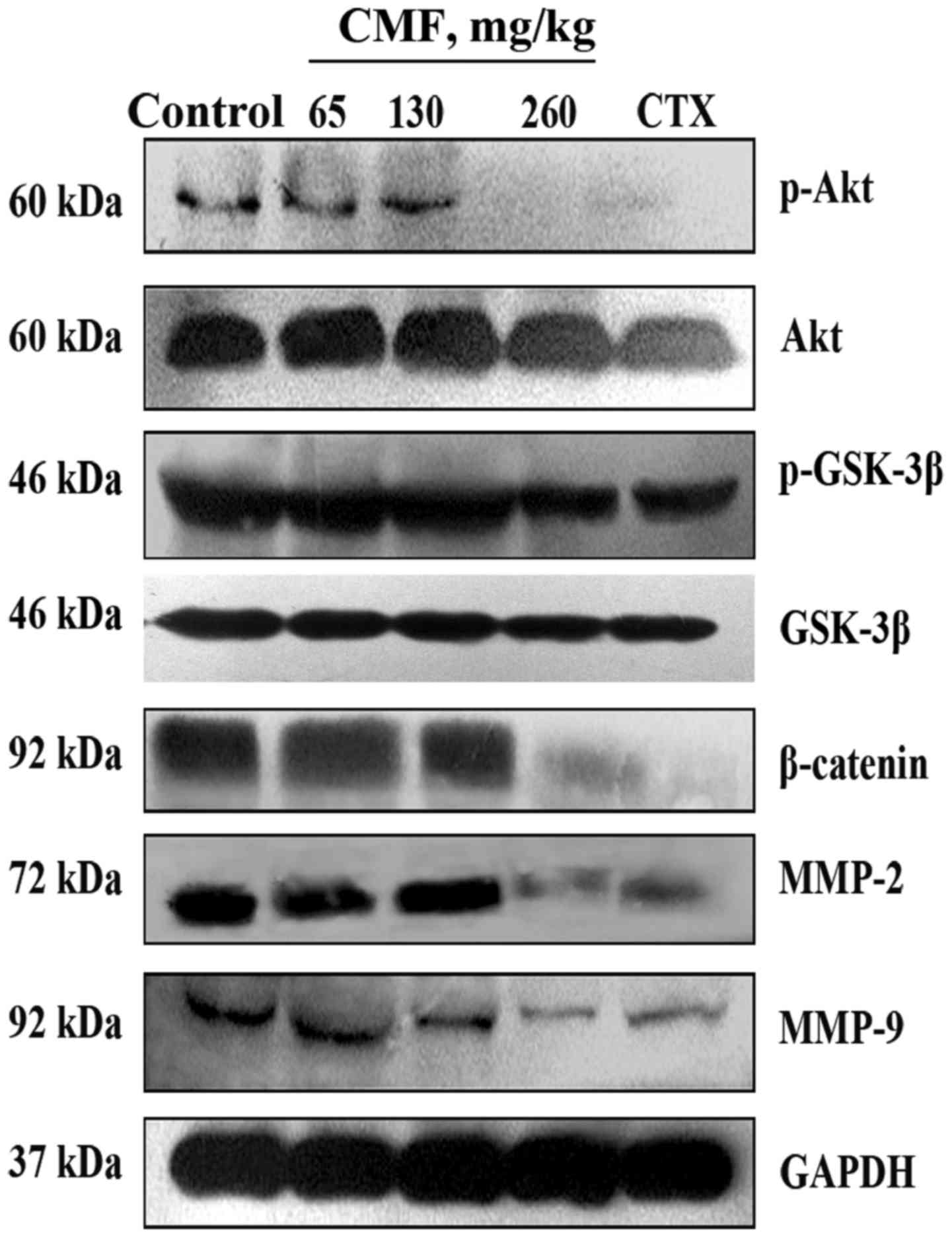 | Figure 5.CMF induces the down regulation of
MMP-2 and MMP-9 through Akt/GSK-3β/β-catenin signaling in
vivo. Tumor tissue from the five groups of mice was randomly
selected and the protein was obtained by lysis buffer. Protein
expression of p-Akt, Akt, p-GSK-3β, GSK-3β, β-catenin, MMP-2 and
MMP-9 was detected using a western blot analysis assay. GAPDH was
used as a loading control. CMF, Cordyceps militaris
fraction; Akt, protein kinase B; GSK-3β, glycogen synthase kinase
3β; MMP, matrix metalloproteinase; p-, phosphorylated; CTX,
cyclophosphamide. |
Discussion
Lung cancer develops more commonly in humans and
usually has a poor clinical outcome and low survival rate due to
high rates of metastasis at a late stage of the tumor development
(36). Therefore, novel approaches
are urgently required for the treatment of this disease and the
prevention of its metastasis. Over previous years, natural
compounds that are extracted and purified from herbal plants have
widely attracted attention due to their preventative and treatment
effects on cancer without serious side effects (37). The results of the present study
demonstrated that CMF served an important functional role in
suppressing the invasion and metastasis of lung cancer cells.
Cancer metastasis is a complicated multistep process
involving the dissociation of malignant cells at the primary sites,
invasion through the extracellular matrix, intravasation of
invading cells into the vasculature or lymphatic systems, survival
and finally proliferation at a distant organ (38,39). In
the in vitro experiments in the present study, it was
revealed that the cell adhesion, migration, invasion and clone
formation in NCI-H1299 and LLC cells decreased in a
concentration-dependent manner when treated with CMF (Fig. 2).
MMPs have been associated with cancer risk, clinical
prognosis, metastasis and recurrence (10). A previous study in genetic mouse
models of cancer has suggested that an MMP deficiency may result in
decreased or increased tumor progression, incidence, size and
metastasis (10). In the results of
the present study, the expression of MMP-2 and MMP-9 was reduced by
CMF in NCI-H1299 cells in vitro and LLC cells in C57BL/6
mice, suggesting that the two MMPs were associated with the
metastasis of the lung cancer cells. Akt is a serine/threonine
kinase that may phosphorylate and inactivate GSK-3β which may be
involved in the regulation of the Wnt/β-catenin pathways by
facilitating phosphorylation within other proteins (including APC
and Axin) and promoting the degradation of β-catenin (15,40). It
has been reported that the Akt/GSK-3β/β-catenin pathway is required
for the epithelial-mesenchymal transition process induced by
soluble interleukin-15 receptor α (41). It was revealed in the present study
that CMF may inhibit the phosphorylation of the Akt protein at the
Ser473 site which promoted the activation of its downstream protein
GSK-3β in vitro and in vivo. Dvl proteins combine
with Axin and Frat1, WNT signaling pathway regulator to form a
complex to prevent β-catenin from degradation mediated by GSK-3β
(42). The results demonstrated that
Dvl-2 was also inhibited and the expression of β-catenin and its
target protein c-Myc decreased following GSK-3β protein activation
(Fig. 3), which were consistent with
results previously reported (43).
However, the association between Akt and Dvl-2, which regulate the
activity of GSK-3β by CMF, remains unknown. Furthermore, the
anti-metastatic effect of CMF in vivo was investigated using
an LLC cell line xenograft model of C57BL/6 mice. Tumor sizes in
addition to lung and liver metastases were reduced following the
oral administration of CMF.
To the best of our knowledge, the present study was
the first to demonstrate that CMF may inhibit the viability,
invasion and metastasis of lung carcinoma cells in vitro and
in vivo. Furthermore, it was revealed that the inhibitory
effect of CMF was primarily associated with the suppression of the
phosphorylation of upstream Akt and an increase in the activity of
GSK-3β, which promoted the degradation of downstream protein
β-catenin. These results suggested that CMF may possess great
potential for the treatment of lung cancer metastasis partially
through Akt/GSK-3β/β-catenin signaling.
Acknowledgements
The authors would like to thank Dr Dongbo Yu of The
University of Chicago (Chicago, IL, USA) for proofreading the
manuscript.
Funding
The present study was supported by the Major
National Science and Technology Products/Significant New Drugs
Creation (grant no. 2011ZX09102-001-33) and National Natural
Science Foundation of China (grant nos. 81374015 and 81503303).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on request.
Authors' contributions
QZ conducted the majority of experiments and drafted
the manuscript. CH, QC and SB also conducted the experiments. ZZ
and XH provided support during the experiments and technical
assistance. RY and LS defined and guided the experiments, wrote and
revised the manuscript.
Ethics approval and consent to
participate
All animal experiments were carried out in
compliance with the Animal Management Rules of the Ministry of
Health of the People's Republic of China and approved by the Animal
Care and Use Committee of Jinan University.
Competing interests
The authors declare that there are no conflicts of
interest.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Youlden DR, Cramb SM and Baade PD: The
international epidemiology of lung cancer: Geographical
distribution and secular trends. J Thorac Oncol. 3:819–831. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riihimaki M, Hemminki A, Fallah M, Thomsen
H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and
survival in lung cancer. Lung Cancer. 86:78–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosell R and Karachaliou N: Relationship
between gene mutation and lung cancer metastasis. Cancer Metastasis
Rev. 34:243–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen QY, Jiao DM, Yan L, Wu YQ, Hu HZ,
Song J, Yan J, Wu LJ, Xu LQ and Shi JG: Comprehensive gene and
microRNA expression profiling reveals miR-206 inhibits MET in lung
cancer metastasis. Mol Biosyst. 11:2290–2302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X and Adjei AA: Lung cancer and
metastasis: New opportunities and challenges. Cancer Metastasis
Rev. 34:169–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Novaes FT, Cataneo DC, Junior Ruiz RL,
Defaveri J, Michelin OC and Cataneo AJ: Lung cancer: Histology,
staging, treatment and survival. J Bras Pneumol. 34:595–600.
2008.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wieczorek E, Jablonska E, Wasowicz W and
Reszka E: Matrix metalloproteinases and genetic mouse models in
cancer research: A mini-review. Tumour Biol. 36:163–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garbisa S, Scagliotti G, Masiero L, Di
Francesco C, Caenazzo C, Onisto M, Micela M, Stetler-Stevenson WG
and Liotta LA: Correlation of serum metalloproteinase levels with
lung cancer metastasis and response to therapy. Cancer Res.
52:4548–4549. 1992.PubMed/NCBI
|
|
13
|
Hrabec E, Strek M, Nowak D and Hrabec Z:
Elevated level of circulating matrix metalloproteinase-9 in
patients with lung cancer. Respir Med. 95:1–4. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vidal SJ, Rodriguez-Bravo V, Galsky M,
Cordon-Cardo C and Domingo-Domenech J: Targeting cancer stem cells
to suppress acquired chemotherapy resistance. Oncogene.
33:4451–4463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gai JQ, Sheng X, Qin JM, Sun K, Zhao W and
Ni L: The effect and mechanism of bufalin on regulating
hepatocellular carcinoma cell invasion and metastasis via
Wnt/β-catenin signaling pathway. Int J Oncol. 48:338–348. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin J and Beer DG: Molecular predictors of
prognosis in lung cancer. Ann Surg Oncol. 19:669–676. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uematsu K, He B, You L, Xu Z, McCormick F
and Jablons DM: Activation of the Wnt pathway in non small cell
lung cancer: Evidence of dishevelled overexpression. Oncogene.
22:7218–7221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
19
|
Cui JD: Biotechnological production and
applications of Cordyceps militaris, a valued traditional Chinese
medicine. Crit Rev Biotechnol. 35:475–484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang HJ, Baik HW, Kim SJ, Lee SG, Ahn HY,
Park JS, Park SJ, Jang EJ, Park SW, Choi JY, et al: Cordyceps
militaris enhances cell-mediated immunity in healthy korean men. J
Med Food. 18:1164–1172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yue K, Ye M, Zhou Z, Sun W and Lin X: The
genus Cordyceps: A chemical and pharmacological review. J Pharm
Pharmacol. 65:474–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu JY, Feng CP, Li X, Chang MC, Meng JL
and Xu LJ: Immunomodulatory and antioxidative activity of Cordyceps
militaris polysaccharides in mice. Int J Biol Macromol. 86:594–598.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiu CP, Liu SC, Tang CH, Chan Y,
El-Shazly M, Lee CL, Du YC, Wu TY, Chang FR and Wu YC:
Anti-inflammatory Cerebrosides from Cultivated Cordyceps militaris.
J Agric Food Chem. 64:1540–1548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao Y, Ling J, Zhang G, Liu F, Tao S, Han
Z, Chen S, Chen Z and Le H: Cordycepin induces cell cycle arrest
and apoptosis by inducing DNA damage and up-regulation of p53 in
Leukemia cells. Cell Cycle. 14:761–771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong JW, Jin CY, Park C, Han MH, Kim GY,
Moon SK, Kim CG, Jeong YK, Kim WJ, Lee JD and Choi YH: Inhibition
of migration and invasion of LNCaP human prostate carcinoma cells
by cordycepin through inactivation of Akt. Int J Oncol.
40:1697–1704. 2012.PubMed/NCBI
|
|
26
|
Tian T, Song L, Zheng Q, Hu X and Yu R:
Induction of apoptosis by Cordyceps militaris fraction in human
chronic myeloid leukemia K562 cells involved with mitochondrial
dysfunction. Pharmacogn Mag. 10:325–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo Q, Shen S, Liao M, Lian P and Wang X:
NGX6 inhibits cell invasion and adhesion through suppression of
Wnt/beta-catenin signal pathway in colon cancer. Acta Biochim
Biophys Sin (Shanghai). 42:450–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamaguchi H, Wyckoff J and Condeelis J:
Cell migration in tumors. Curr Opin Cell Biol. 17:559–564. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gu S, Honisch S, Kounenidakis M, Alkahtani
S, Alarifi S, Alevizopoulos K, Stournaras C and Lang F: Membrane
androgen receptor down-regulates c-src-activity and beta-catenin
transcription and triggers GSK-3beta-phosphorylation in colon tumor
cells. Cell Physiol Biochem. 34:1402–1412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei Q, Zhao Y, Yang ZQ, Dong QZ, Dong XJ,
Han Y, Zhao C and Wang EH: Dishevelled family proteins are
expressed in non-small cell lung cancer and function differentially
on tumor progression. Lung Cancer. 62:181–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shay G, Lynch CC and Fingleton B: Moving
targets: Emerging roles for MMPs in cancer progression and
metastasis. Matrix Biol. 44–46:1–206. 2015.
|
|
33
|
Leinonen T, Pirinen R, Böhm J, Johansson R
and Kosma VM: Increased expression of matrix metalloproteinase-2
(MMP-2) predicts tumour recurrence and unfavourable outcome in
non-small cell lung cancer. Histol Histopathol. 23:693–700.
2008.PubMed/NCBI
|
|
34
|
Maru Y: The lung metastatic niche. J Mol
Med (Berl). 93:1185–1192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kruger A: Premetastatic niche formation in
the liver: Emerging mechanisms and mouse models. J Mol Med (Berl).
93:1193–1201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reck M, Popat S, Reinmuth N, De Ruysscher
D, Kerr KM and Peters S; and ESMO Guidelines Working Group, :
Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 25 Suppl 3:iii27–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye L, Jia Y, Ji KE, Sanders AJ, Xue K, Ji
J, Mason MD and Jiang WG: Traditional Chinese medicine in the
prevention and treatment of cancer and cancer metastasis. Oncol
Lett. 10:1240–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mehlen P and Puisieux A: Metastasis: A
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duffy MJ, McGowan PM and Gallagher WM:
Cancer invasion and metastasis: Changing views. J Pathol.
214:283–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luu HH, Zhang R, Haydon RC, Rayburn E,
Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W and He TC:
Wnt/beta-catenin signaling pathway as novel cancer drug targets.
Curr Cancer Drug Targets. 4:653–671. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuan H, Meng X, Guo W, Cai P, Li W, Li Q,
Wang W, Sun Y, Xu Q and Gu Y: Transmembrane-bound IL-15-promoted
epithelial-mesenchymal transition in renal cancer cells requires
the Src-dependent Akt/GSK-3β/β-catenin pathway. Neoplasia.
17:410–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song X, Wang S and Li L: New insights into
the regulation of Axin function in canonical Wnt signaling pathway.
Protein Cell. 5:186–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rayasam GV, Tulasi VK, Sodhi R, Davis JA
and Ray A: Glycogen synthase kinase 3: More than a namesake. Br J
Pharmacol. 156:885–898. 2009. View Article : Google Scholar : PubMed/NCBI
|