Introduction
Dendritic cells (DCs) are antigen-presenting cells
with an important role in the innate and adaptive immune system. In
skin lesions, cutaneous DCs (Langerhans cells, dermal DCs and
plasmacytoid DCs) are involved in immune activation in inflammatory
benign lesions (psoriasis, lupus erythematosus (LE), spongiotic
dermatitis), as well as in malignant lymphoid proliferations
(mycosis fungoides, cutaneous T-cell lymphoma). DCs are a modulator
of skin immunity, with a significant role in immunological
reactions as well as in immune tolerance to various factors,
including tumor cells and auto-immune stimuli (1). In antitumor immune defense, DCs are
antigen-presenting cells for CD8+ T-lymphocytes via
histocompatibility complex class I (2).
Recent studies have defined DCs as immune regulators
of the skin with a key role in various complex reactions such as
antitumoral immunity, maintenance of immune homeostasis, modulation
of T-cell function, wound healing and interaction with skin grafts
and immune evasion of HIV (3–6).
Since DCs are located within the epidermis and
dermis as well, their activation intervenes early during the
interaction with environmental factors. Their migrating capacity to
local lymph nodes is involved in regional and systemic response to
skin injuries (5).
From our point of view, the study of DCs in
inflammatory and tumoral skin lesions should concern two
interconnected aspects: the distribution of DCs and their role in
lesional mechanisms.
Their special pattern of distribution in
inflammatory dermatitis and skin lymphomas is useful not only to
better understand pathophysiologic mechanisms involved in these
diseases, but also as an adjuvant tool for diagnosis. In addition,
they may play an important role in therapeutical approaches in
inflammatory skin diseases (7,8).
Our team previously presented studies concerning DC
distribution in cutaneous melanoma (1) as well as in mycosis fungoides vs.
inflammatory dermatoses (9,10). This preoccupation for DCs revealed
interesting results that prompted the initiation of this study.
Thus, in melanoma, DCs are especially found in areas
of regression, being involved in the immune mechanisms that
determine tumor cell destruction. Moreover, DCs have significant
patterns of distribution in areas of regression (nodular pattern)
compared with areas without regression (predominantly diffuse
pattern) (2,11). These data suggest that DCs are active
players in melanoma's regression and could be used as therapeutical
targets to enhance this natural process of tumor clearance
(2,12).
On the other hand, in spongiotic dermatitis, we
described a nodular pattern of DC distribution, while in the early
stage mycosis fungoides (patch/plaque stage), DCs were distributed
in clusters with arachnoid extensions. To the best of our
knowledge, this feature is very useful in difficult differential
diagnosis of mycosis fungoides vs. spongiotic dermatitis (9).
Different studies describe specific roles of skin
DCs in various inflammatory and tumoral conditions.
In psoriasis, a chronic inflammatory skin model type
1 autoimmune disease with a strong interferon-γ (IFN-γ) T helper 1
(Th1) signal (13–15), DCs interact with CD4+ T
cells and keratinocytes, controlling the fate of resident memory T
cells and their response to various pro-inflammatory cytokines [as
interleukin (IL)-17, IL-1β, IL-23] (3,13).
In LE, a chronic autoimmune disease (16,17), DCs
(especially the plasmocytoid subtype) have an important role by
producing type I IFN (16–18).
In spongiotic dermatitis, DCs initiate the
inflammatory process after the ones located in epidermis are
activated by environmental allergens (19–21).
Also, they have a high affinity for IgE, determining the increase
of antigen uptake (5).
In mycosis fungoides, a common cutaneous T-cell
lymphoma with dermal infiltrate T cells, DCs have an unclear role
in pathogenesis and progression (22). Their number is increased in all
stages of the disease, with a greater emphasis on the tumor stage
of the disease. They probably enhance tumor progression using the
same pathway as regulatory T cells (23).
In cutaneous T-cell lymphoma, a neoplasm of memory
inducer T cells (24–26), usually, neoplastic T cells, are mixed
with a significantly increased number of Langerhans cells
suggesting that the neoplastic process is triggered by DCs
(22). In addition, immature DCs
facilitate the survival of malignant T cells (27).
Patients and methods
Patients
We performed a retrospective study including 149
patients: 35 with mycosis fungoides, 35 with spongiotic dermatitis,
35 with psoriasis, 35 with lupus and 9 with cutaneous T-cell
lymphomas (other than mycosis fungoides) diagnosed using
histopathological and immunohistochemical staining between January
2012 and December 2016 at the Department of Pathology, Colentina
University Hospital (Bucharest, Romania). Skin biopsies (incisional
or excisional), received for histopathological diagnosis, were also
included. All specimens were sampled according to the Pathology
Guidelines of Medical Practice (Ministry of Health Regulation no.
1217/September 16, 2010 published in Official Monitor no.
723/October 29, 2010, annex 1), routinely processed for
paraffin-embedding. The tissue was fixed in formaldehyde, solution
4%, buffered, pH 6.9, room temperature, 6–8 h. Then, 3 µm sections
were cut and routinely stained with hematoxylin and eosin
(H&E). All slides were examined by at least two senior
pathologists and, in selected cases, immunohistochemical assays
were performed for diagnosis. After establishing the diagnosis, 13
samples of mycosis fungoides, psoriasis, LE, spongiotic dermatitis
and T cell lymphoma were included in the research cohort.
This study was approved by the Ethics Committee of
Colentina University Hospital (Bucharest, Romania), and written
informed consent was obtained from all the patients for the use of
their tissue, after diagnosis for experimental purposes.
Mehods
From each case we performed manual tissue multiarray
(TMA) blocks. Tissue was extracted from the paraffin blocks and
then embedded into recipient blocks, each including 8 samples from
8 patients. From each TMA block 3 µm sections were performed using
a semi-automatic Leica RM2245 rotary microtome. One slide was used
for routine stain (H&E) and 10 immunohistochemical slides for
CD1a, CD11c and langerin (Table I).
As a detection system, we used Polymer Novolink (Leica Biosystems
Nussloch GmbH, Nussloch, Germany). Immunohistochemical staining was
analyzed using a microscope Olympus CX41 (Olympus, Tokyo,
Japan).
 | Table I.Antibodies used for the study. |
Table I.
Antibodies used for the study.
| Primary antibody | Clone | Host | Supplier | Dilution | Specificity |
|---|
| CD1a | MTB1 monoclonal | Mouse | Leica Biosystems | 1:50 | Human CD1a
molecule |
| CD11c | 5D11 monoclonal | Mouse | Leica Biosystems |
1:100 | Human CD11c
antigen |
| Langerin | 12D6 monoclonal | Mouse | Leica Biosystems | 1:50 | Human langerin |
Density and distribution of DCs were evaluated by
two independent pathologists with expertise in dermatopathology.
They estimated both the number of DCs and their pattern of
distribution.
All data were registered in a database and
statistically analyzed using Microsoft Excel (Microsoft, Redmond,
Washington, USA) and IBM SPSS Statistics (version 22.0; IBM Corp.,
Armonk, NY, USA).
Results
Histological analysis of DC
distribution
Histological examination of all cases identified
various patterns of the DC distribution in the examined conditions:
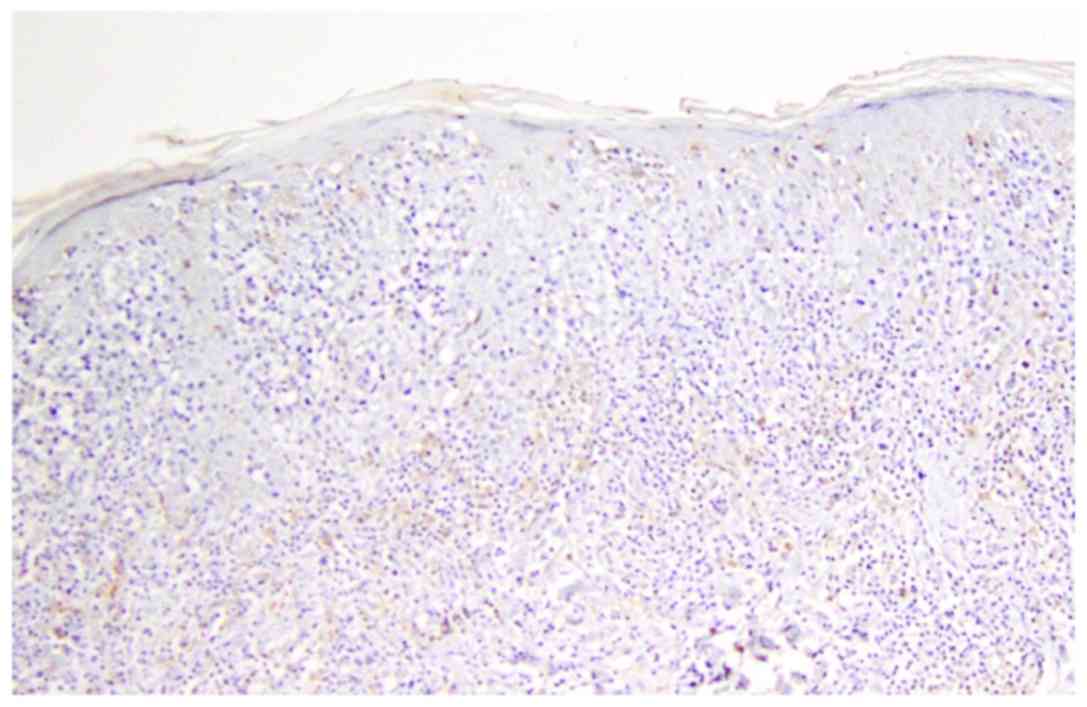
Clusters of DCs with arachnoid extension in mycosis fungoides,
nodular pattern (Fig. 1) in
inflammatory lesions and dispersed distribution with peripheric
accumulation and arachnoid in T-skin lymphomas (Fig. 2). In mycosis fungoides and T-cell
lymphoma, the number of DCs was increased both in epidermis and
malignant infiltrate (Fig. 3)
compared with inflammatory lesions, in which the number of DCs was
decreased in dermal inflammatory infiltrate while it remained in
moderate numbers in epidermis.
In inflammatory lesions, spongiotic dermatitis
(Fig. 4), psoriasis (Fig. 5) and LE (Fig. 6), the predominant pattern was the
nodular, followed by a dispersed pattern. The density of the DCs
was decreased in inflammatory infiltrate compared with neoplastic
conditions. The number of DCs in inflammatory lesions was higher in
epidermis.
In mycosis fungoides (Fig. 7) and cutaneous T-cell lymphoma
(Fig. 8), the predominant pattern
was the arachnoid one. In cutaneous T-cell lymphoma, peripheric
accumulation of DCs was observed, while an increased number of DCs
was displayed in epidermis.
Frequency of DCs
In neoplastic conditions, CD1a, CD11c and
langerin-positive DCs were present in a higher number both in
epidermis and neoplastic infiltrate (Table II).
 | Table II.Frequency of dendritic cells. |
Table II.
Frequency of dendritic cells.
| Markers | Mycosis
fungoides | Cutaneous T-cell
lymphoma | Spongiotic
dermatitis | Psoriasis | Lupus
erythematosus |
|---|
| CD1a | Numerous DCs
CD1a+ in neoplastic infiltrate and in epidermis | Numerous DCs
CD1a+ in neoplastic infiltrate and in epidermis | Rare DCs
CD1a+ in inflammatory infiltrate and in epidermis | Numerous DCs
CD1a+ in inflammatory infiltrate and rare in
epidermis | Rare DCs
CD1a+ in inflammatory infiltrate and in epidermis |
| CD11c | Numerous DCs
CD11c-positive neoplastic infiltrate and rare in epidermis | Numerous DCs
CD11c-positive in neoplastic infiltrate and in epidermis | Numerous DCs
CD1a-positive in inflammatory infiltrate and rare in epidermis | Numerous DCs
CD1a-positive in inflammatory infiltrate and in epidermis | Numerous CD1a
DC-positive in inflammatory infiltrate and in epidermis |
| Langerin | Numerous DCs
langerin-positive in neoplastic infiltrate and in epidermis | Rare DCs
langerin-positive in neoplastic infiltrate and numerous in
epidermis | Rare DCs
langerin-positive in inflammatory infiltrate and numerous in
epidermis | Numerous DCs
langerin-positive in inflammatory infiltrate and rare in
epidermis | Rare DCs
langerin-positive in inflammatory infiltrate and in epidermis |
In inflammatory lesions, the frequency of CD1a,
CD11c and langerin-positive DCs was different. CD1a-positive DCs
were rare in epidermis of the spongiotic dermatitis, psoriasis and
frequently in LE. In inflammatory infiltrate, CD1a-positive DCs
were rare in spongiotic dermatitis and LE and numerous in
psoriasis. CD11c-positive DCs was rare in epidermis of the
spongiotic dermatitis and frequent in psoriasis and LE. In
inflammatory infiltrate, CD11c-positive DCs were numerous in
psoriasis, LE and psoriasis. Langerin-positive DCs were rare in
epidermis of the psoriasis, and LE, but they were frequent in
spongiotic dermatitis. In inflammatory infiltrate,
langerin-positive DCs were rare in spongiotic dermatitis and LE,
but they were numerous in psoriasis.
Discussion
Although the role of DCs in various skin diseases
has been described by many studies, there are still very few recent
studies concerning DC distribution patterns in skin inflammatory
conditions and cutaneous malignant T-cell disorders. In our clinic,
this is the second study analyzing the distribution patterns
classified as arachnoid, diffuse and nodular in inflammatory
cutaneous diseases and malignant T-cell conditions and identifying
significant differences between distribution of DCs in benign and
malignant skin lesions (1,9).
These data are important, in the first place, for
diagnosis since sometimes, because of the marked inflammatory
infiltrate, differential diagnosis between malignant lymph cell
proliferation and inflammatory dermatosis can be difficult. The
pattern of distribution of DCs can help to differentiate these
disorders, as a supplementary tool to usual histopathological and
immunohistochemical features.
In addition, identification of significant
differences between DC distribution in these two types of diseases
is an indicator of different levels of involvement of DCs in the
pathophysio-logical mechanisms of the disease. In inflammatory
dermatoses, DCs have mostly a nodular distribution pattern, while
in malignant lymph cell infiltrates, the most frequent pattern is
an arachnoid distribution, intermingled with tumoral cells. These
data are consistent with the fact that in inflammatory dermatoses,
DCs most important role is activation by various antigens with
subsequent trigger and modulation of inflammation, while in
malignant lymph cell infiltrates, DCs have a more complex role as
they are involved not only in triggering the disease, but also in
tumor progression and regression.
In conclusion, immunohistochemical characterization
of DC distribution can be an adjuvant tool in differential
diagnosis in inflammatory dermatosis and skin lymphomas. At the
same time, it can be correlated with functional studies explaining
the role of skin DCs (local and migrated) in all phases of the
disease.
This study identifies significant differences
between benign and malignant skin conditions concerning DC
distribution and can be integrated in a larger framework of immune
interactions between the host and immune as well as tumoral factors
involved in skin lesions.
Acknowledgements
Not applicable.
Funding
This study is partially supported by Executive
Agency for Higher Education, Research, Development and Innovation
(UEFISCDI; Bucharest, Romania) under the contract no.
PN-III-P1-1.2-PCCDI-2017-0341.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC, CC and SZ contributed to the conception of this
study and performed the preliminary documentation. All authors
participated in the design of the study and implemented the
research. MC, SZ, CP, LN, LS, AB and AC examined the archives and
identified the cases included in the study, examined the slides and
collected the pathological information. CC, DB and GJ enrolled
patients in the study, performed clinical diagnosis and collected
clinical data. All authors participated in the statistical analysis
and contributed to the interpretion of the results as well as the
writing of the study. All authors reviewed all data and approved
the final manuscript.
Ethics approval and consent to
participate
This research abides by the International and
National regulations in accordance with the Declaration of
Helsinki. It was approved by the Ethics Committee of the Colentina
University Hospital (Bucharest, Romania). All patients signed an
informed consent before being included in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neagu M, Caruntu C, Constantin C, Boda D,
Zurac S, Spandidos DA and Tsatsakis AM: Chemically induced skin
carcinogenesis: Updates in experimental models (Review). Oncol Rep.
35:2516–2528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nedelcu RI, Ion DA, Holeab CA, Cioplea MD,
Brînzea A and Zurac SA: Dendritic cells in melanoma -
immunohistochemical study and research trends. Rom J Morphol
Embryol. 56:997–1002. 2015.PubMed/NCBI
|
|
3
|
West HC and Bennett CL: Redefining the
role of langerhans cells as immune regulators within the skin.
Front Immunol. 8:19412018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Serafim A, Petre DG, Moraru L, Cioflan HE,
Vasile E, Mastalier-Manolescu B, Petrutescu M and Stancu IC:
Gelatin-PVP hydrogels with potential skin grafts applications. Key
Eng Mater. 638:38–46. 2015. View Article : Google Scholar
|
|
5
|
Deckers J, Hammad H and Hoste E:
Langerhans cells: Sensing the environment in health and disease.
Front Immunol. 9:932018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nichita L, Zurac S, Popp C, Micu G,
Bastian A, Stăniceanu F and Streinu-Cercel A: Dendritic cells -
immunodeficiency virus (HIV): Early interactions. Rom J Intern Med.
49:251–255. 2011.PubMed/NCBI
|
|
7
|
Malissen B, Tamoutounour S and Henri S:
The origins and functions of dendritic cells and macrophages in the
skin. Nat Rev Immunol. 14:417–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013. View Article : Google Scholar
|
|
9
|
Petre M, Zurac S, Andrei R, Tebeica T,
Birceanu A, Chirculescu R, Popp C, Evsei A, Staniceanu F and
Bastian A: Langerhans cells distributions may discriminate early
stage mycosis fungoides and inflammatory dermatoses. Virchows Arch.
463:101–352. 2772013.PubMed/NCBI
|
|
10
|
Tebeică T, Andrei R, Zurac S and
Stăniceanu F: Practical aspects regarding the histopathological
diagnosis of early mycosis fungoides. Rom J Intern Med. 54:3–10.
2016.PubMed/NCBI
|
|
11
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neagu M, Constantin C, Dumitrascu GR, Lupu
AR, Caruntu C, Boda D and Zurac S: Inflammation markers in
cutaneous melanoma - edgy biomarkers for prognosis. Discoveries
(Craiova). 3:e382015. View Article : Google Scholar
|
|
13
|
Zaba LC, Fuentes-Duculan J, Eungdamrong
NJ, Abello MV, Novitskaya I, Pierson KC, Gonzalez J, Krueger JG and
Lowe NA: Psoriasis is characterized by accumulation of
immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic
cells. J Invest Dermatol. 129:79–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lowes MA, Chamian F, Abello MV,
Fuentes-Duculan J, Lin SL, Nussbaum R, Novitskaya I, Carbonaro H,
Cardinale I, Kikuchi T, et al: Increase in TNF-alpha and inducible
nitric oxide synthase-expressing dendritic cells in psoriasis and
reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci USA.
102:19057–19062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55 (Suppl 3):1191–1196.
2014.PubMed/NCBI
|
|
16
|
Farkas L, Beiske K, Lund-Johansen F,
Brandtzaeg P and Jahnsen FL: Plasmacytoid dendritic cells (natural
interferon-alpha/beta-producing cells) accumulate in cutaneous
lupus erythematosus lesions. Am J Pathol. 159:237–243. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blanco P, Palucka AK, Gill M, Pascual V
and Banchereau J: Induction of dendritic cell differentiation by
IFN-α in systemic lupus erythematosus. Science. 294:1540–1543.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vermi W, Lonardi S, Morassi M, Rossini C,
Tardanico R, Venturini M, Sala R, Tincani A, Poliani PL,
Calzavara-Pinton PG, et al: Cutaneous distribution of plasmacytoid
dendritic cells in lupus erythematosus. Selective tropism at the
site of epithelial apoptotic damage. Immunobiology. 214:877–886.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phung TI, Wright TS, Pourciau KY and
Smoller BR: Spongiotic dermatitis. Pediatr Dermatol. Jun
21–2017.(Epub ahead of print). doi:
10.1007/978-3-319-44824-4_1.
|
|
20
|
Colmenero I, Torrelo A and Reyes-Múgica M:
Skin. In: Essentials of Surgical Pediatric Pathology. Cohen MC and
Scheimberg I: Cambridge University Press; Cambridge: pp. 1–2. 2014,
PubMed/NCBI
|
|
21
|
Abreu Velez AM, Loebel AM and Howard MS:
Spongiotic dermatitis with a mixed inflammatory infiltrate of
lymphocytes, antigen presenting cells, immunoglobulins and
complement. Dermatol Online. 2:52–57. 2011.
|
|
22
|
Deguchi M, Aiba S, Ohtani H, Nagura H and
Tagami H: Comparison of the distribution and numbers of
antigen-presenting cells among T-lymphocyte-mediated dermatoses:
CD1a+, factor XIIIa+, and CD68+
cells in eczematous dermatitis, psoriasis, lichen planus and
graft-versus-host disease. Arch Dermatol Res. 294:297–302. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin TC, Wu PY, Lin TY, Yeh SP, Chen SC and
Lee TL: Langerhans cell hyperplasia in the tumor stage of mycosis
fungoides: A mimic of Langerhans cell histiocytosis. Dermatol Sin.
29:101–105. 2011. View Article : Google Scholar
|
|
24
|
Goteri G, Filosa A, Mannello B,
Stramazzotti D, Rupoli S, Leoni P and Fabris G: Density of
neoplastic lymphoid infiltrate, CD8+ T cells, and
CD1a+ dendritic cells in mycosis fungoides. J Clin
Pathol. 56:453–458. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berger CL, Hanlon D, Kanada D, Dhodapkar
M, Lombillo V, Wang N, Christensen I, Howe G, Crouch J, El-Fishawy
P, et al: The growth of cutaneous T-cell lymphoma is stimulated by
immature dendritic cells. Blood. 99:2929–2939. 2002.PubMed/NCBI
|
|
26
|
Ion A, Popa IM, Laura ML, Lisievici C,
Lupu M, Voiculescu V, Caruntu C and Boda D: Proteomic approaches to
biomarker discovery in cutaneous T-cell lymphoma. Dis Markers.
2016:96024722016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ni X and Duvic M: Dendritic cells and
cutaneous T-cell lymphomas. G Ital Dermatol Venereol. 146:103–113.
2011.PubMed/NCBI
|