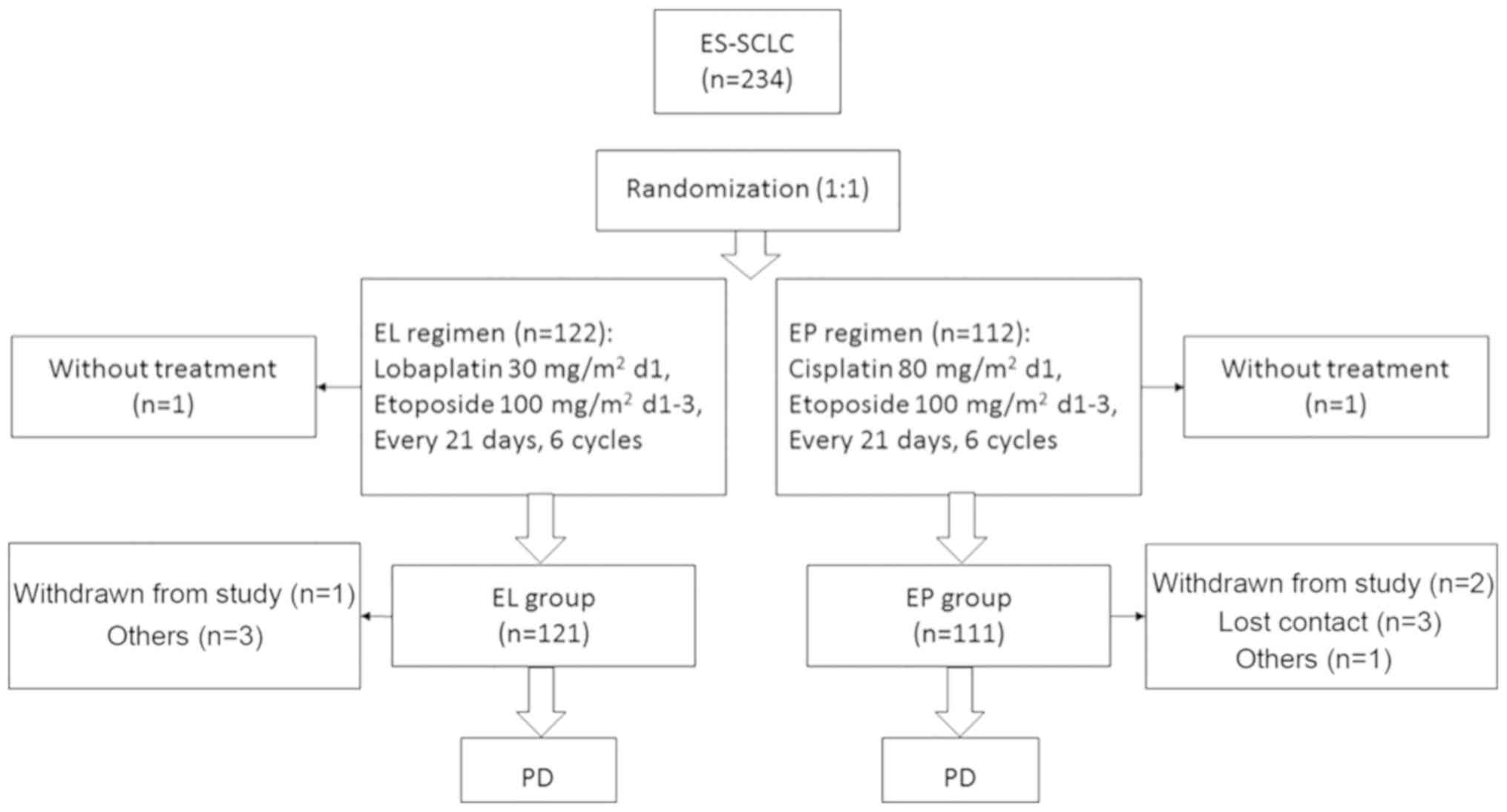
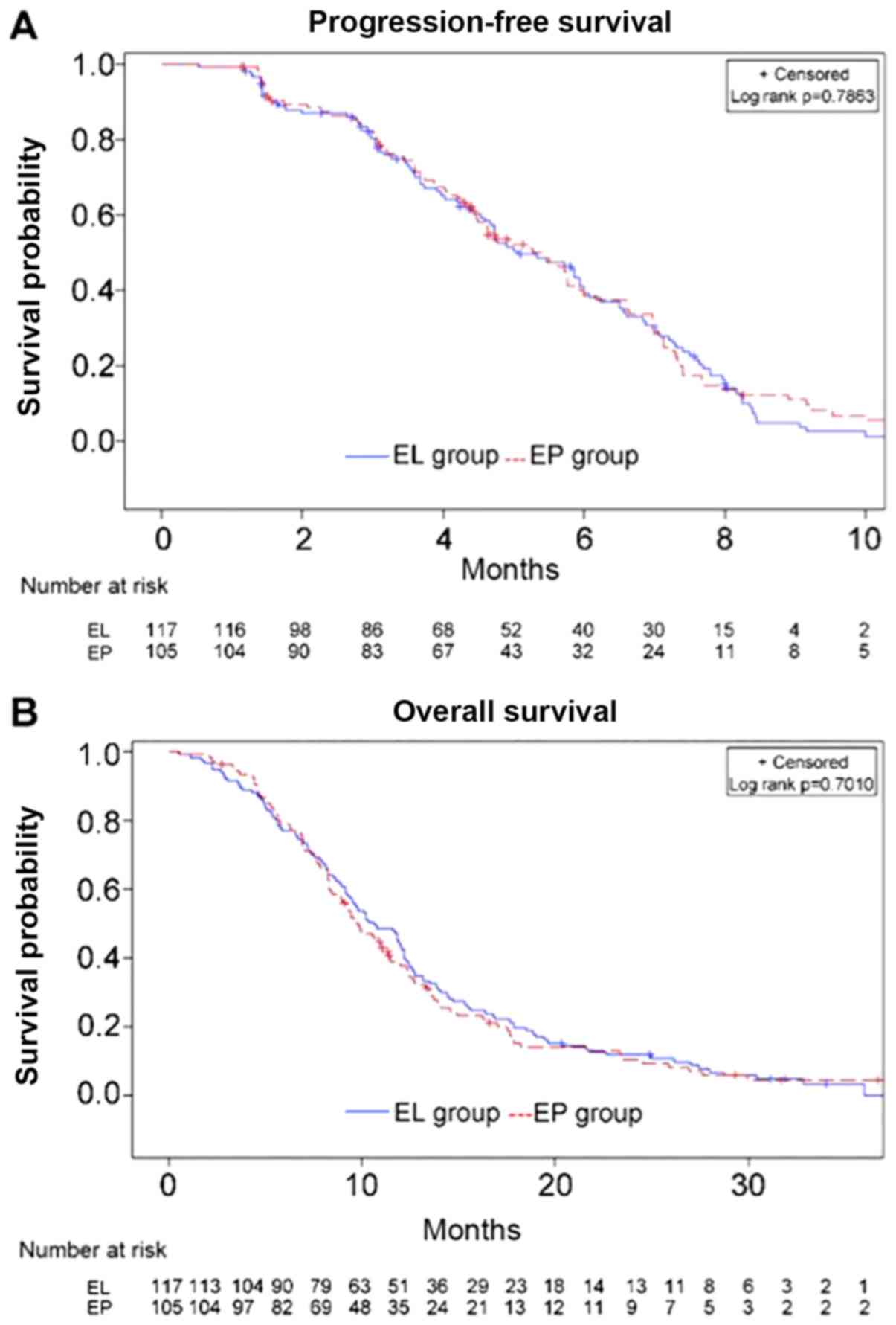
|
1
|
Howlader N, Noone A and Krapcho M: SEER
cancer statistics review, 1975–2013, based on November 2015 SEER
data submission, posted to the SEER web site, April 2016. Bethesda:
National Cancer Institute; 2016
|
|
2
|
Pietanza MC, Byers LA, Minna JD and Rudin
CM: Small cell lung cancer: Will recent progress lead to improved
outcomes? Clin Cancer Res. 21:2244–2255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seiter K: Toxicity of the topoisomerase II
inhibitors. Expert Opin Drug Saf. 4:219–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayati F, Hossainzadeh M, Shayanpour S,
Abedi-Gheshlaghi Z and Beladi Mousavi SS: Prevention of cisplatin
nephrotoxicity. J Nephropharmacol. 5:57–60. 2016.PubMed/NCBI
|
|
5
|
McKeage MJ: Lobaplatin: A new antitumour
platinum drug. Expert Opin Investig Drugs. 10:119–128. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harstrick A, Bokemeyer C, Scharnofkse M,
Hapke G, Reile D and Schmoll HJ: Preclinical activity of a new
platinum analogue, lobaplatin, in cisplatin-sensitive and
-resistant human testicular, ovarian, and gastric carcinoma cell
lines. Cancer Chemother Pharmacol. 33:43–47. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang Y, Sui X and Cheng Y: Clinical
research progress of the new antitumor drug Lobaplatin in the
treatment of small cell lung cancer. Chin J N Drugs. 23:184–188.
2014.
|
|
8
|
Jakupec MA, Galanski M and Keppler BK:
Tumour-inhibiting platinum complexes-state of the art and future
perspectives. Rev Physiol Biochem Pharmacol. 146:1–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monneret C: Platinum anticancer drugs.
From serendipity to rational design. Ann Pharm Fr. 69:286–295.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiebig HH, Henss H, von Pawel I,
Gatzemeier U, Manegold CH, Edler L and Berdel W: Phase II clinical
trial of lobaplatin (D-19466) in pretreated patients with
small-cell lung cancer. Onkologie. 19:328–332. 1996.
|
|
11
|
Yang L and Qin S: Progression of
Lobaplatin as the third generation platinum drug. Chin Clin Oncol.
14:1134–1139. 2009.
|
|
12
|
De Vore RF: Chemotherapy for small cell
lung cancer. Pass HI: Lung Cancer Principles and Practice. 2nd.
Philadelphia: Lippincott Williams & Wilkins; 2000
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Satouchi M, Kotani Y, Shibata T, Ando M,
Nakagawa K, Yamamoto N, Ichinose Y, Ohe Y, Nishio M, Hida T, et al:
Phase III study comparing amrubicin plus cisplatin with irinotecan
plus cisplatin in the treatment of extensive-disease small-cell
lung cancer: JCOG 0509. J Clin Oncol. 32:1262–1268. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Russell SD, Saval MA, Robbins JL, Ellestad
MH, Gottlieb SS, Handberg EM, Zhou Y and Chandler B: HF-ACTION
Investigators: New York Heart Association functional class predicts
exercise parameters in the current era. Am Heart J. 158 (4
Suppl):S24–S30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gietema JA, de Vries EG, Sleijfer DT,
Willemse PH, Guchelaar HJ, Uges DR, Aulenbacher P, Voegeli R and
Mulder NH: A phase I study of
1,2-diamminomethyl-cyclobutane-platinum (II)-lactate (D-19466;
lobaplatin) administered daily for 5 days. Br J Cancer. 67:396–401.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fiebig HH, Henss H, Mross K, Meyberg F,
Aulenbacher P, Burk K and Queißer W: Phase I clinical trial of
lobaplatin (D-19466) after intravenous bolus injection. Onkologie.
17:142–148. 1994.
|
|
18
|
Basch E, Iasonos A, McDonough T, Barz A,
Culkin A, Kris MG, Scher HI and Schrag D: Patient versus clinician
symptom reporting using the national cancer institute common
terminology criteria for adverse events: Results of a
questionnaire-based study. Lancet Oncol. 7:903–909. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Osoba D, Rodrigues G, Myles J, Zee B and
Pater J: Interpreting the significance of changes in health-related
quality-of-life scores. J Clin Oncol. 16:139–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anderson H, Hopwood P, Stephens RJ,
Thatcher N, Cottier B, Nicholson M, Milroy R, Maughan TS, Falk SJ,
Bond MG, et al: Gemcitabine plus best supportive care (BSC) vs BSC
in inoperable non-small cell lung cancer-a randomized trial with
quality of life as the primary outcome. UK NSCLC Gemcitabine Group.
Non-small cell lung cancer. Br J Cancer. 83:447–453. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noda K, Nishiwaki Y, Kawahara M, Negoro S,
Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, et
al: Irinotecan plus cisplatin compared with etoposide plus
cisplatin for extensive small-cell lung cancer. N Engl J Med.
346:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lara PN Jr, Natale R, Crowley J, Lenz HJ,
Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR,
et al: Phase III trial of irinotecan/cisplatin compared with
etoposide/cisplatin in extensive-stage small-cell lung cancer:
Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol.
27:2530–2535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung SH, Kang SJ, McCall LM and
Blumenstein B: Sample size computation for two-sample
noninferiority log-rank test. J Biopharm Stat. 15:969–979. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lakatos E: Sample sizes based on the
log-rank statistic in complex clinical trials. Biometrics.
44:229–241. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller RP, Tadagavadi RK, Ramesh G and
Reeves WB: Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel).
2:2490–2518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen MQ, Chen C, Lu HJ and Xu BH: The
efficacy and toxicities of combined lobaplatin with paclitaxel as a
first-line chemotherapy for advanced esophageal squamous cell
carcinoma. J Thorac Dis. 7:1749–1755. 2015.PubMed/NCBI
|
|
27
|
Yang JS, Wang T, Qiu MQ and Li QL:
Comparison of efficacy and toxicity profiles between
paclitaxel/lobapoatin- and cisplatin/5-fluorouracil-based
concurrent chemoradiotherapy of advanced inoperable oesophageal
cancer. Intern Med J. 45:757–761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li WP, Liu H, Chen L, Yao YQ and Zhao EF:
A clinical comparison of lobaplatin or cisplatin with mitomycine
and vincristine in treating patients with cervical squamous
carcinoma. Asian Pac J Cancer Prev. 16:4629–4631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang JQ, Wang T, Shi F, Yang YY, Su J,
Chai YL and Liu Z: A randomized controlled trial comparing clinical
outcomes and toxicity of lobaplatin-versus cisplatin-based
concurrent chemotherapy plus radiotherapy and high-dose-rate
brachytherapy for FIGO Stage II and III cervical cancer. Asian Pac
J Cancer Prev. 16:5957–5961. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mirsadraee S, Oswal D, Alizadeh Y, Caulo A
and van Beek E Jr: The 7th lung cancer TNM classification and
staging system: Review of the changes and implications. World J
Radiol. 4:128–134. 2012. View Article : Google Scholar : PubMed/NCBI
|