Introduction
Aerobic glycolysis in cancer cells, also referred to
as the ‘Warburg effect’, was first observed and described by
Warburg 90 years ago (1,2). The Warburg effect suggests that tumor
cells produce excessive amount of lactate in the presence of oxygen
(3). This effect has been studied
extensively improving the understanding of various metabolic
characteristics of tumor cells (4–6).
Elucidating the mechanisms underlying the expression levels and/or
activity of key catalytic enzymes involved in tumor cell metabolic
pathways may improve cancer diagnosis and treatment.
Normal cells can metabolize glucose to produce
pyruvate by the process of glycolysis, which occurs in the
cytoplasm. The tricarboxylic acid cycle oxidizes the majority of
glycolysis substrates into pyruvate and carbon dioxide. Oxygen and
the mitochondrial electron transport chain are required to generate
an electrochemical gradient and facilitate adenosine triphosphate
(ATP) production (7). The metabolic
abnormalities of tumor cells are manifested when aerobic glycolysis
is severely altered (6). The
tumor-associated changes in glycolysis include weakened oxidative
phosphorylation, accelerated pentose phosphate metabolic pathway,
activated glutamine catabolism, fatty acid de novo synthesis
and β-oxidations (8). In order to
meet the increased demand of tumor cells for energy and substance
anabolism, metabolic reprogramming redefines and directs the flow
and flux of nutrients to the metabolic network of tumor cells
(3). Much of this reprogramming
depends on mitochondria as functional biosynthetic organelles. The
metabolic signatures of cancer cells are not restricted to passive
responses to damaged mitochondria, but result from
oncogene-directed metabolic reprogramming, which is required for
support of anabolic growth (8).
Current cancer research focuses on targeting
cancer-associated defects in apoptosis. Mitochondria are not only
the major center of cell respiration and oxidative phosphorylation,
but also the control center of apoptosis. Apoptosis is regulated by
two major mechanisms. The extrinsic pathway, or death-receptor
pathway, is mediated by the transduction of extracellular death
receptor ligand signaling (9). The
intrinsic pathway, also referred to as the mitochondrial pathway,
is governed by a specific mitochondrion-localized signaling
cascade. The activation of the mitochondrial death pathway includes
changes in mitochondrial outer membrane permeabilization, membrane
potential (∆Ψm) collapse, assembly of the permeability pore complex
and the activation of pro-apoptotic B-cell lymphoma 2 (Bcl-2)
family members apoptotic regulator BAX (Bax) and Bcl-2 homologous
antagonist/killer (10). The
anti-apoptotic Bcl-2 family members, including Bcl-2 and B-cell
lymphoma-extra large, mediate signaling in normal physiology to
prevent cell death (11).
Pro-apoptotic proteins oligomerize at the outer membrane of the
mitochondria to mediate mitochondrial outer membrane
permeabilization that works in tandem with the voltage dependent
anion channel (VDAC) and adenine nucleus translocator (ANT) protein
(10). This complex activation of
different target proteins results in pore formation and the release
of cytochrome C from mitochondria into the cytosol (12). Cytochrome C activates caspases, the
executors of programmed cell death which trigger a caspase cascade
reaction cleaving >100 substrates in cells and leading to cell
apoptosis (12,13).
Selective targeting of cancer metabolism and
apoptosis-associated signaling may provide an alternative strategy
for the development of anticancer drugs that have minimal adverse
effects on normal cells. The current review focuses on the role of
ursolic acid (UA) as a potential anticancer drug that influences
mitochondrial function.
Structure and function of ursolic acid
UA is a pentacyclic triterpenoid (14). UA-associated compounds include
oleanolic acid, betulinic acid, uvaol and α- and β-amyrin (14). Triterpenoids have been used as
ingredients of herb extracts employed in traditional medicine. The
presence of UA has been confirmed in numerous classes of medicinal
plants including the peels of the orchard apple (Malus
domestica), marjoram (Origanum majorana) leaves, oregano
(Origanum vulgare) leaves, rosemary (Rosmarinus
officinalis) leaves, sage (Salvia officinalis) leaves,
thyme (Thymus vulgaris) leaves, lavender (Lavandula
angustifolia) leaves and flowers, eucalyptus
(Eucalyptus) leaves and bark, black elder (Sambucus
nigra) leaves and bark, hawthorn (Crataegus spp.) leaves and
flowers, coffee (Coffea arabica) leaves as well as the wax
layer of numerous edible fruits (15–17). The
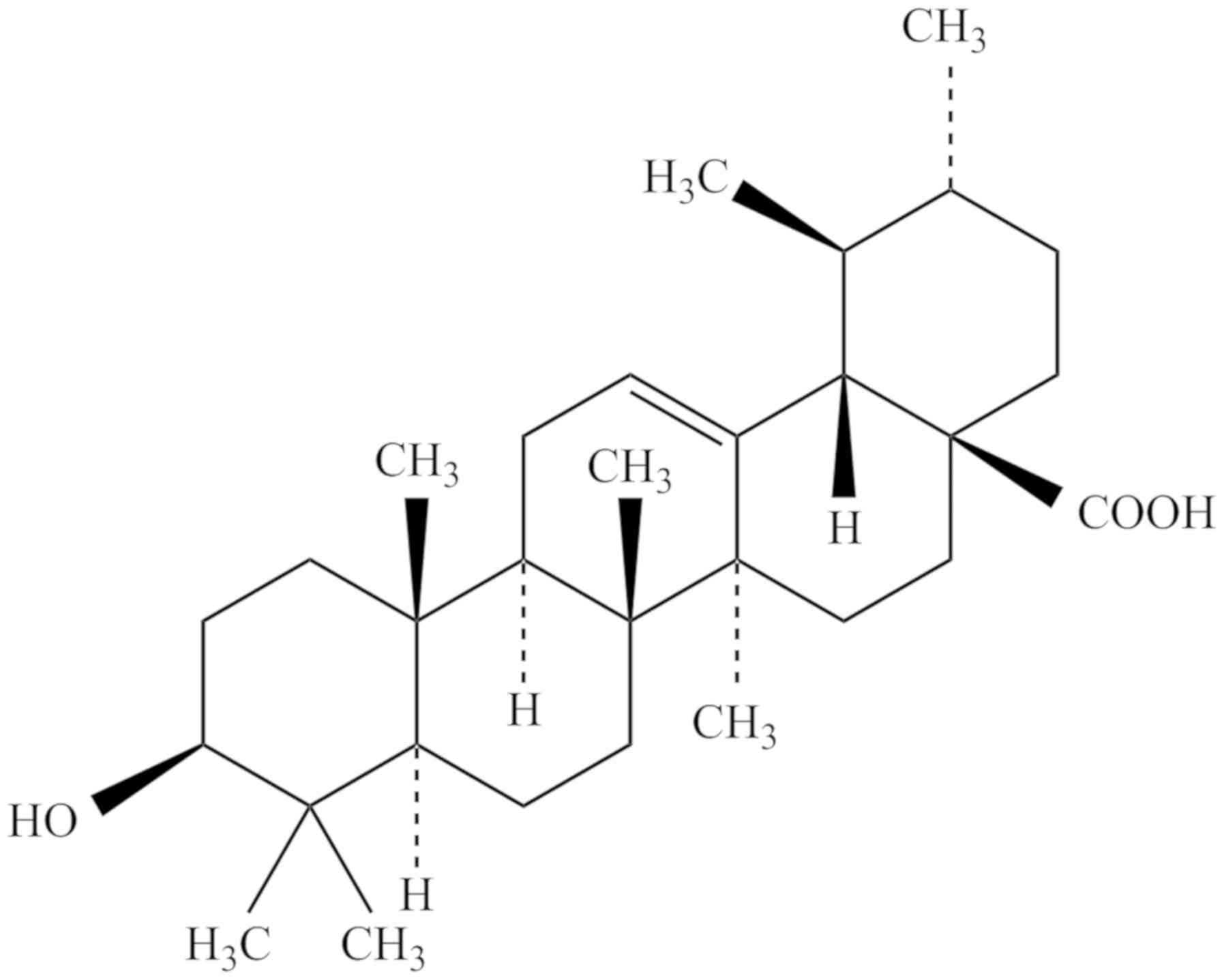
chemical structure of UA (3β-hydroxy-urs-12-en-28-oic-acid) is
presented in Fig. 1. UA has the
molecular formula C30H48O3, a
molecular weight of 456.70032 g/mol and a melting point of
283–285°C. UA can be dissolved in methanol, pyridine and acetone,
but is insoluble in water and petroleum ether (18).
UA and its derivatives exhibit potent biological and
pharmaceutical effects (18). The
anti-inflammatory effect of UA was linked to attenuation of
production of proinflammatory cytokines including tumor necrosis
factor α, interleukin (IL)-6 and/or IL-17 (19,20). UA
was associated with suppression of the nuclear factor-κB (NF-κβ)
pathway, inhibition of expression of cyclooxygenase-2 (COX-2) and
nitric oxide synthase and the reduction of perhydrides including
nitric oxide and hydrogen peroxide (21). Furthermore, UA demonstrated an
antidiabetic function and inhibited the activity of pancreatic
α-amylase, succinate dehydrogenas, and glucose-6-phosphatase and
aldose reductase (22). UA also
induced fatty acid synthetase activity and glucokinase activity,
and upregulated glucose transporter (GLUT) 2 mRNA levels, thereby
reducing the blood glucose levels of diabetic mice (22–25).
UA stimulated an increase in the level of plasma
total cholesterol, low-density lipoprotein cholesterol, very
low-density lipoprotein cholesterol, free fatty acids and
phospholipids in high fat diet-fed mice or rabbits (26). By contrast, UA reduced the expression
of sterol regulatory element binding protein 1c, Fas cell surface
death receptor, acetyl-coenzyme A carboxylase and carnitine
palmitoyltransferase-1 (27). UA
induced the phosphorylation of adenosine 5′-monophosphate
(AMP)-activated protein kinase (AMPK) and stimulated expression of
sirtuin 1, thus serving an antihyperlipidemic role (26). UA can provide hepatoprotective
activity against several liver diseases, including fatty liver,
liver fibrosis, hepatocellular carcinoma and other types of liver
cancer by influencing multiple metabolic factors (28). For instance, UA reduced the
serum/plasma levels of alanine transaminase and aspartate
transaminase, which are liver disease biomarkers (27,29,30).
Numerous studies in rodents and humans have
investigated the beneficial effects of UA that targeted cancer cell
metabolism. Data demonstrated that UA inhibited tumorigenesis and
cancer cell proliferation, modulated apoptosis and cell cycle
progression and promoted autophagy (18,31–36). Luo
et al (37) reported that
treatment with UA inhibited the viability and migration of T47D,
MCF-7 and MDA-MB-231 breast cancer cells by targeting
phosphoinositide-3-kinase/protein kinase B (PI3K/Akt)-regulated
glycogen synthase kinase 3 β phosphorylation levels and caspase-3
activation via the NF-κβ signaling pathway. Lewinska et al
(38) analyzed the effects of low
doses of UA in breast cancer cell lines with different hormone and
growth factor receptor status. The authors revealed that UA
promoted autophagy, apoptosis and induced gap (G)0/G1 cell cycle
arrest. Additionally, UA affected glycolysis. The effect was
accompanied by decreased levels of ATP, lactate, hexokinase 2 and
pyruvate kinase. It was suggested that these effects were mediated
by Akt-5′-AMP-activated protein kinase signals, activation of
phospho-extracellular signal-regulated kinases1/2 and/or by the
oxidative stress pathway. Yeh et al (39) revealed that UA suppressed the
migration and metastasis of the MDA-MB-231 breast cancer cell line
by modulating c-Jun N-terminal kinase (JNK), Akt and mechanistic
target of rapamycin mTOR signaling. UA may down-regulate the
expression of COX-2 (40,41). The effect has been observed in
various types of cancer cells and was directly proportional to
tumor aggressiveness and metastasis in gastric cancer SGC7901 cells
(34) and hepatic cancer HepG2 cells
(42). UA upregulated COX-2 in
colorectal cancer HT-29 and prostate cancer DU145 cells (43). Furthermore, it was reported that
treatment with UA suppressed the metastasis of HeLa cells,
fibrosarcoma HT1080 cells and C6 glioma cells through the
downregulation of matrix metallopeptidase 9 (33,36,44).
Furthermore, it has been indicated that UA induced pro-apoptotic
signaling in human liver cancer cell lines as well as gastric
cancer cell lines, including HepG2, Hep3B, Huh7, AGS, BGC823 and
SGC7901 (39,45–48). UA
significantly enhanced proapoptotic effects and stimulated
mitochondrial dysfunction by activating caspases 3, 8 and 9, and
downregulated Bcl-2 expression in these cancer cells.
Anticancer effect of ursolic acid via
mitochondrial energy metabolism
Mitochondria are involved in oxidative
phosphorylation and ATP formation in cells; furthermore, 95% of the
energy required for cellular activity is generated in these
organelles (49). The regulation of
mitochondrial metabolism is associated with the activities of key
enzymes in different energy-linked metabolic pathways (50).
Hexokinases (HKs) are irreversible, rate-limiting
enzymes in the first step of glycolysis (7). HKs catalyze the conversion of glucose
to glucose-6-phosphate with concomitant dephosphorylation of ATP
(8,51). Among the four known HK isoforms, HK2
has the greatest association with tumors (52). It is strategically located on the
mitochondrial outer membrane, interacts with the VDAC and provides
preferential access to the mitochondrial ATP via ANT in the
mitochondrial inner membrane (53,54).
This interaction results in a shift in the susceptibility of
mitochondria to proapoptotic signals that are mediated through
Bcl-2-family proteins. The upregulation, or increased activity of
HK accelerate glycolysis in tumor cells and increase their energy
metabolism. The enhancement of glycolysis increases the production
of lactate, which acidifies the tumor microenvironment (53). The acidified extracellular fluid
decomposes and destroys the cell matrix, thus facilitating the
invasion of tumor cells into surrounding tissues (8).
Several studies indicated that UA may modulate the
expression and function of mitochondria-associated enzymes,
resulting in antiproliferative and apoptosis-promoting effects in
various experimental cancer models in vitro and in
vivo (55–59). Lewinska et al (38) demonstrated that UA downregulated the
Akt signaling in three breast cancer cell lines with different
phenotypes including MCF-7 estrogen receptor (ER) positive;
progesterone receptor (PR) positive or negative; human epidermal
growth factor receptor 2 (HER2) negative, MDA-MB-231 (ER negative;
PR negative; HER2 negative) and SK-BR-3 (ER negative; PR negative;
HER2 positive) cells. The Akt inhibition affected glycolysis and
markedly decreased levels of HK2, pyruvate kinase M2, ATP and
lactate.
Wang et al (60) synthesized a series of UA diamine
derivatives to evaluate their biological activities. The authors
demonstrated that the carbon chains of the modified UA derivatives
competed strongly with glucose for binding sites in glucokinase
(GK). Furthermore, the combination of 2-deoxy-D-glucose (2-DG) and
UA derivative US597 (UA-4) induced cell cycle arrest at the
G2/mitotic (M) phase, promoted caspase-dependent cell death,
reduced HK activity, aggravated depletion of intracellular ATP,
decreased lactate production and synergistically inhibited cancer
cell growth in vitro (HepG2) and in vivo (H22).
Another study indicated that the UA derivative UP12 could bind to
the active sites of GK, GLUT1 and ATPase in hepatocellular
carcinoma (61). Combined with 2-DG,
UP12 depleted intracellular ATP, decreased lactate production and
arrested an increased number of cancer cells at the synthesis and
G2/M cell cycle phases (61).
Anticancer effect of ursolic acid via
reduction of mitochondrial oxidative stress
In addition to energy production, mitochondria are
involved in the regulation of a number of processes including the
generation of reactive oxygen species (ROS), redox molecules and
metabolites (7). The majority of
these by-products are removed by free radical scavenger molecules,
including superoxide dismutase (SOD), in order to maintain balance
under normal physiological conditions and counteract initiation of
cell death (62). Oxidative stress
is induced when the functional domain of mitochondria is exposed to
high concentrations of ROS (63).
Mild oxidative stress often has protective effects including
promoting cell survival, proliferation and differentiation.
However, severe oxidative stress causes irreversible damage,
decreases proliferation, and induces aging, apoptosis and necrosis
(64). In cancer cells,
mitochondrial ROS amplify the tumorigenic phenotype and accelerate
the accumulation of additional mutations that lead to metastatic
behavior (65). Targeting of
mitochondrial metabolism that contributes to redox regulation
presents a promising avenue for future anticancer therapy (66,67).
Synthesized UA derivatives 5, 17 and 23 inhibited
cell growth in the human bladder cancer cell line NTUB1 (68). Derivative 17 significantly increased
the production of ROS for 24 h, while 5 and 23 did so for 48 h. Wu
et al (32) evaluated ROS
generation in osteosarcoma U-2 and MG-63 cells exposed to a
combination of UA and zoledronic acid (ZOL) using the fluorescent
dye hydroethidine. The results indicated that the UA/ZOL
combination increased intracellular ROS levels more effectively
than either of the compounds alone. Furthermore,
N-acetyl-L-cysteine, a ROS scavenger, suppressed this effect, and
significantly reduced the apoptosis induced by the UA/ZOL
combination. Mitochondrial oxidative damage is further aggravated
by accumulation of high ROS level. A high level of ROS inhibited
the activity of respiratory enzymes and electron transport through
the respiratory chain (67). ROS
adversely impacted oxidase and antioxidant enzyme activity,
stimulated depolarization of mitochondrial membrane potential and
facilitated1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine pore
opening (66). These actions
increased mitochondrial membrane permeability and resulted in
changes in calcium metabolism, leading to mitochondrial
morphological destruction, loss of mitochondrial function and cell
death (66).
In the human breast cancer cell line MDA-MB-231, UA
decreased the mitochondrial ∆Ψm, demonstrated by affecting the
level of
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanine
iodide (JC-1) which gathered in the mitochondrial matrix to form
aggregates (69). Another study also
revealed that after 24 h of treatment with 15 µM of UA, human
melanoma M4Beu cells had a low mitochondrial membrane potential,
identified by staining with JC-1/TOTO-3 iodide. Therefore, UA may
participate in ROS-mediated oxidative stress, activate metabolic
disturbances in mitochondria in cancer cells and induce
proapoptotic cascade reactions (55). The detailed mechanisms of the
anticancer effects of UA require further research.
Anticancer effect of ursolic acid mediated
by the p53-modulated mitochondrial pathway
Inactivation of tumor suppressor genes is an
important initiating factor for tumor reprogramming. One of the
established tumor suppressors, p53 protein, encoded by human tumor
protein p53 (TP53) gene, controls tumorigenesis through various
cellular mechanisms including DNA damage repair, cell cycle
regulation and cell death (70).
According to the Cancer Gene Census mutation database, TP53 is the
most frequently mutated gene (36.1%) in different types of cancer
(71). p53 serves an important role
in modulating the balance between mitochondrial respiration and
glycolysis (70). Matoba et
al (72) reported that p53
regulated the expression of the synthesis of cytochrome C oxidase 2
(SCO2) protein, which is required for regulating the cytochrome C
oxidase complex. The authors revealed that oxygen consumption was
reduced in the liver mitochondria of p53−/− knockout
mice, as well as in p53−/−HCT116 deficient cells
compared with the wild type. In addition, lactate production was
increased in p53−/−knockout cells. Further study
verified that SCO2links p53 to mitochondrial respiration, providing
a possible explanation for the Warburg effect (73). In addition to regulating SCO2
transactivation and expression, p53 regulates mitochondrial
biogenesis genes, including mitochondrial transcription factor A
and apoptosis-inducing factor which encode for major
apoptosis-affecting proteins involved in complex 1 assembly
(72). p53 regulates expression of
ferredoxin reductase protein responsible for the maturation of
iron-sulfur proteins essential in electron transfer reactions. p53
also modulates fatty acid metabolism through sphingosine kinase 1
and lipin 1, which can promote cell growth and mediate nutritional
and genotoxic stress signals (74).
p53 may induce growth arrest or programmed cell
death through transcriptional activity. Nam and Kim (75) reported that treatment with UA in
human colon adenocarcinoma SW480 cells significantly increased the
expression level and transcriptional activity of p53, as well as
that of NF-κβ and Bax. UA also enhanced p21 transcriptional
activity and induced caspase3-dependent apoptosis (76). Manu and Kuttan (77) suggested that the activation of p53 by
treatment with UA mediated activation of proapoptotic pathways in
B16F-10 melanoma cells. Yu et al (78) demonstrated that treatment with UA
induced cycle arrest and apoptosis in the human hepatoma cell line
SMMC-7721. The authors also verified that the proapoptotic and
regulatory proteins p53 and Bax were upregulated while the
antiapoptotic protein Bcl-2 was downregulated following treatment
with UA. Furthermore, the mRNA level of growth differentiation
factor 15, SOD2 and activating transcription factor 3 were
increased, while Fos levels were decreased. The p53 inhibitor
pifithrin-α blocked these effects, supporting the role of p53. The
aforementioned studies provide a preliminary interpretation of UA
signaling via activation of the p53 pathway and the induction of
tumor cell apoptosis. However, p53 can regulate the expression of a
variety of metabolism-associated enzymes in mitochondria and, thus,
its role may not be limited to the induction of apoptosis (78). The metabolic mechanism of the
anticancer effect of UA via the p53 pathway requires further
investigation.
Conclusions and perspectives
Reprogrammed energy metabolism is an emerging
hallmark of cancer phenotyping. In the search for new anticancer
agents, phytochemicals have drawn an increasing amount of
attention. Clinicians are searching for natural drugs with a high
efficiency, which are less toxic to normal cells compared with
conventional treatments. Mitochondrial metabolism was investigated
as a target for cancer therapy, owing to the reemergence of
mitochondria as central metabolic organelles adversely affected by
tumorigenesis. Numerous studies suggest that UA regulates
mitochondrial function through the activation of multiple pathways.
UA regulates the expression of associated metabolic enzymes,
decreases tumor proliferation, promotes ROS production and
accumulation in mitochondria under stress conditions, destabilizes
mitochondrial membrane potential, activates the p53 pathway and
promotes apoptosis in various types of cancer cells (Fig. 2). Further research investigating the
cellular and molecular mechanisms underlying the effects of UA is
required for the development of new therapeutic agents.
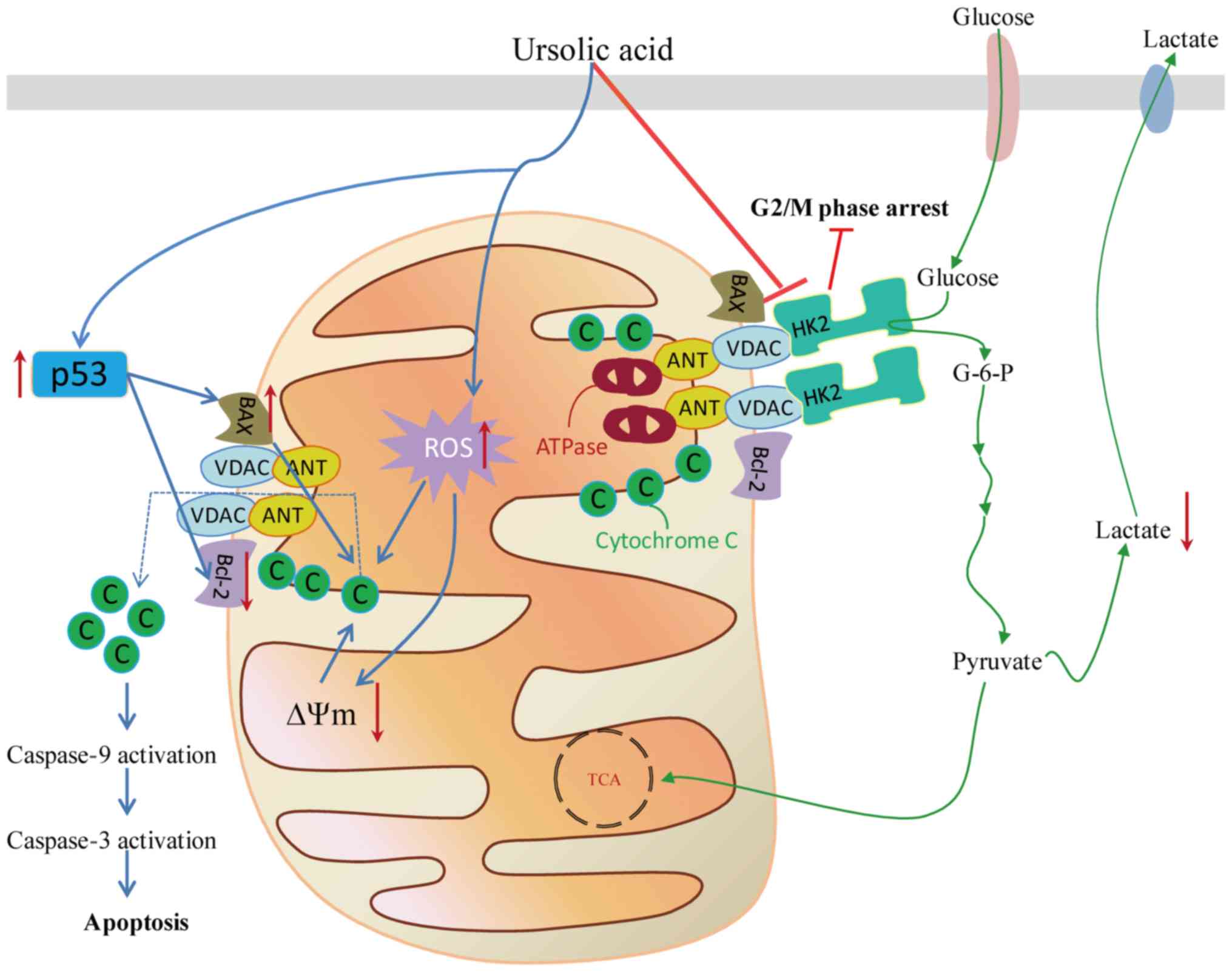 | Figure 2.Schematic presentation of the
antiproliferative effect of the UA targeting the mitochondrial
pathway in cancer cells, including the associated metabolic
enzymes, the promotion of ROS production and accumulation in
mitochondria under stress conditions, destabilization of
mitochondrial membrane potential and activation of the p53 pathway
to decrease proliferation or promote apoptosis. G2, gap 2; M,
mitosis; G-6-P, glucose 6-phosphate; HK2, hexokinase 2; Bcl-2,
B-cell lymphoma 2; VDAC, voltage dependent anion channel; ANT,
adenine nucleus translocator; ATP, adenosine triphosphate; ROS,
reactive oxygen species; C, cytochrome C; TCA, tricarboxylic acid;
∆Ψm, membrane potential. |
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660468).
Availability of data and materials
Not applicable.
Authors' contributions
XMF conducted the literature review and wrote the
manuscript. XLS was involved in drafting and revising the
manuscript. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vlassenko AG, McConathy J, Couture LE, Su
Y, Massoumzadeh P, Leeds HS, Chicoine MR, Tran DD, Huang J, Dahiya
S, et al: Aerobic glycolysis as a marker of tumor aggressiveness:
Preliminary data in high grade human brain tumors. Dis Markers.
2015:8749042015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lunt SY and Vander Heiden MG: Aerobic
glycolysis: Meeting the metabolic requirements of cell
proliferation. Annu Rev Cell Dev Biol. 27:441–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alberts B: Molecular biology of the cell.
4th. New York: Garland Science; 2002
|
|
8
|
Mathupala SP, Ko YH and Pedersen PL: The
pivotal roles of mitochondria in cancer: Warburg and beyond and
encouraging prospects for effective therapies. Biochim Biophys
Acta. 1797:1225–1230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wajant H: The Fas signaling pathway: More
than a paradigm. Science. 296:1635–1636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Desagher S and Martinou JC: Mitochondria
as the central control point of apoptosis. Trends Cell Biol.
10:369–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang X and Wang X: Cytochrome C-mediated
apoptosis. Annu Rev Biochem. 73:87–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ernster L and Schatz G: Mitochondria: A
historical review. J Cell Biol. 91:227s–255s. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hill RA and Connolly JD: Triterpenoids.
Nat Prod Rep. 30:1028–1065. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jager S, Trojan H, Kopp T, Laszczyk MN and
Scheffler A: Pentacyclic triterpene distribution in various
plants-rich sources for a new group of multi-potent plant extracts.
Molecules. 14:2016–2031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szakiel A, Paczkowski C, Pensec F and
Bertsch C: Fruit cuticular waxes as a source of biologically active
triterpenoids. Phytochem Rev. 11:263–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wozniak L, Skapska S and Marszalek K:
Ursolic Acid-A pentacyclic triterpenoid with a wide spectrum of
pharmacological activities. Molecules. 20:20614–20641. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kashyap D, Tuli HS and Sharma AK: Ursolic
acid (UA): A metabolite with promising therapeutic potential. Life
Sci. 146:201–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santos Rosa C, Garcia Gimenez MD, Saenz
Rodriguez MT and De la Puerta Vazquez R: Antihistaminic and
antieicosanoid effects of oleanolic and ursolic acid fraction from
Helichrysum picardii. Pharmazie. 62:459–462. 2007.PubMed/NCBI
|
|
20
|
Xu T, Wang X, Zhong B, Nurieva RI, Ding S
and Dong C: Ursolic acid suppresses interleukin-17 (IL-17)
production by selectively antagonizing the function of RORgamma t
protein. J Biol Chem. 286:22707–22710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei ZY, Chi KQ, Wang KS, Wu J, Liu LP and
Piao HR: Design, synthesis, evaluation, and molecular docking of
ursolic acid derivatives containing a nitrogen heterocycle as
anti-inflammatory agents. Bioorg Med Chem Lett. 28:1797–1803. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee J, Lee HI, Seo KI, Cho HW, Kim MJ,
Park EM and Lee MK: Effects of ursolic acid on glucose metabolism,
the polyol pathway and dyslipidemia in non-obese type 2 diabetic
mice. Indian J Exp Biol. 52:683–691. 2014.PubMed/NCBI
|
|
23
|
Poongunran J, Perera HK, Jayasinghe L,
Fernando IT, Sivakanesan R, Araya H and Fujimoto Y: Bioassay-guided
fractionation and identification of α-amylase inhibitors from
Syzygium cumini leaves. Pharm Biol. 55:206–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee J, Yee ST, Kim JJ, Choi MS, Kwon EY,
Seo KI and Lee MK: Ursolic acid ameliorates thymic atrophy and
hyperglycemia in streptozotocin-nicotinamide-induced diabetic mice.
Chem Biol Interact. 188:635–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kazmi I, Rahman M, Afzal M, Gupta G,
Saleem S, Afzal O, Shaharyar MA, Nautiyal U, Ahmed S and Anwar F:
Anti-diabetic potential of ursolic acid stearoyl glucoside: A new
triterpenic gycosidic ester from Lantana camara. Fitoterapia.
83:142–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang YL, Wang ZJ, Shen HL, Yin M and Tang
KX: Effects of artesunate and ursolic acid on hyperlipidemia and
its complications in rabbit. Eur J Pharm Sci. 50:366–371. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sundaresan A, Radhiga T and Pugalendi KV:
Effect of ursolic acid and Rosiglitazone combination on hepatic
lipid accumulation in high fat diet-fed C57BL/6J mice. Eur J
Pharmacol. 741:297–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seo DY, Lee SR, Heo JW, No MH, Rhee BD, Ko
KS, Kwak HB and Han J: Ursolic acid in health and disease. Korean J
Physiol Pharmacol. 22:235–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kunkel SD, Elmore CJ, Bongers KS, Ebert
SM, Fox DK, Dyle MC, Bullard SA and Adams CM: Ursolic acid
increases skeletal muscle and brown fat and decreases diet-induced
obesity, glucose intolerance and fatty liver disease. PLoS One.
7:e393322012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma JQ, Ding J, Zhang L and Liu CM:
Protective effects of ursolic acid in an experimental model of
liver fibrosis through Nrf2/ARE pathway. Clin Res Hepatol
Gastroenterol. 39:188–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hollosy F, Idei M, Csorba G, Szabó E,
Bökönyi G, Seprödi A, Mészáros G, Szende B and Kéri G: Activation
of caspase-3 protease during the process of ursolic acid and its
derivative-induced apoptosis. Anticancer Res. 21:3485–3491.
2001.PubMed/NCBI
|
|
32
|
Wu CC, Huang YF, Hsieh CP, Chueh PJ and
Chen YL: Combined use of zoledronic acid augments ursolic
Acid-induced apoptosis in human osteosarcoma cells through enhanced
oxidative stress and autophagy. Molecules. 21(pii): E16402016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang K, Chi T, Li T, Zheng G, Fan L, Liu
Y, Chen X, Chen S, Jia L and Shao JW: Correction: A smart
pH-responsive nano-carrier as a drug delivery system for the
targeted delivery of ursolic acid: Suppresses cancer growth and
metastasis by modulating P53/MMP-9/PTEN/CD44 mediated multiple
signaling pathways. Nanoscale. 10:6212–6213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang H, Li X, Ding J, Xu H, Dai X, Hou Z,
Zhang K, Sun K and Sun W: Delivery of ursolic acid (UA) in
polymeric nanoparticles effectively promotes the apoptosis of
gastric cancer cells through enhanced inhibition of cyclooxygenase
2 (COX-2). Int J Pharm. 441:261–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harmand PO, Duval R, Liagre B,
Jayat-Vignoles C, Beneytout JL, Delage C and Simon A: Ursolic acid
induces apoptosis through caspase-3 activation and cell cycle
arrest in HaCat cells. Int J Oncol. 23:105–112. 2003.PubMed/NCBI
|
|
36
|
Cha HJ, Park MT, Chung HY, Kim ND, Sato H,
Seiki M and Kim KW: Ursolic acid-induced down-regulation of MMP-9
gene is mediated through the nuclear translocation of
glucocorticoid receptor in HT1080 human fibrosarcoma cells.
Oncogene. 16:771–778. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo J, Hu YL and Wang H: Ursolic acid
inhibits breast cancer growth by inhibiting proliferation, inducing
autophagy, and apoptosis and suppressing inflammatory responses via
the PI3K/AKT and NF-κB signaling pathways in vitro. Exp Ther Med.
14:3623–3631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lewinska A, Adamczyk-Grochala J,
Kwasniewicz E, Deregowska A and Wnuk M: Ursolic acid-mediated
changes in glycolytic pathway promote cytotoxic autophagy and
apoptosis in phenotypically different breast cancer cells.
Apoptosis. 22:800–815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yeh CT, Wu CH and Yen GC: Ursolic acid, a
naturally occurring triterpenoid, suppresses migration and invasion
of human breast cancer cells by modulating c-Jun N-terminal kinase,
Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res.
54:1285–1295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Subbaramaiah K, Michaluart P, Sporn MB and
Dannenberg AJ: Ursolic acid inhibits cyclooxygenase-2 transcription
in human mammary epithelial cells. Cancer Res. 60:2399–2404.
2000.PubMed/NCBI
|
|
41
|
Liu L, Zhang J, Li M, Zhang X, Li Z, Wang
L, Wu J and Luo C: Inhibition of HepG2 cell proliferation by
ursolic acid and polysaccharides via the downregulation of
cyclooxygenase-2. Mol Med Rep. 9:2505–2511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tian Z, Lin G, Zheng RX, Huang F, Yang MS
and Xiao PG: Anti-hepatoma activity and mechanism of ursolic acid
and its derivatives isolated from Aralia decaisneana. World J
Gastroenterol. 12:874–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Limami Y, Pinon A, Leger DY, Pinault E,
Delage C, Beneytout JL, Simon A and Liagre B: The
P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic
acid-induced apoptosis in colorectal and prostate cancer cells.
Biochimie. 94:1754–1763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang HC, Huang CY, Lin-Shiau SY and Lin
JK: Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma
invasion through suppressing the association ZIP/p62 with PKC-zeta
and downregulating the MMP-9 expression. Mol Carcinog. 48:517–531.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang X, Zhang F, Yang L, Mei Y, Long H,
Zhang X, Zhang J, Qimuge S and Su X: Ursolic acid inhibits
proliferation and induces apoptosis of cancer cells in vitro and in
vivo. J Biomed Biotechnol. 2011:4193432011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li R, Wang X, Zhang XH, Chen HH and Liu
YD: Ursolic acid promotes apoptosis of SGC-7901 gastric cancer
cells through ROCK/PTEN mediated mitochondrial translocation of
cofilin-1. Asian Pac J Cancer Prev. 15:9593–9597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tang Q, Ji Q, Tang Y, Chen T, Pan G, Hu S,
Bao Y, Peng W and Yin P: Mitochondrial translocation of cofilin-1
promotes apoptosis of gastric cancer BGC-823 cells induced by
ursolic acid. Tumour Biol. 35:2451–2459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tang C, Lu YH, Xie JH, Wang F, Zou JN,
Yang JS, Xing YY and Xi T: Downregulation of survivin and
activation of caspase-3 through the PI3K/Akt pathway in ursolic
acid-induced HepG2 cell apoptosis. Anticancer Drugs. 20:249–258.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wallace DC: A mitochondrial paradigm of
metabolic and degenerative diseases, aging, and cancer: A dawn for
evolutionary medicine. Annu Rev Genet. 39:359–407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cheng TL, Liao CC, Tsai WH, Lin CC, Yeh
CW, Teng CF and Chang WT: Identification and characterization of
the mitochondrial targeting sequence and mechanism in human citrate
synthase. J Cell Biochem. 107:1002–1015. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wilson JE: Isozymes of mammalian
hexokinase: Structure, subcellular localization and metabolic
function. J Exp Biol. 206:2049–2057. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pedersen PL, Mathupala S, Rempel A,
Geschwind JF and Ko YH: Mitochondrial bound type II hexokinase: A
key player in the growth and survival of many cancers and an ideal
prospect for therapeutic intervention. Biochim Biophys Acta.
1555:14–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: Cancer's stygian link to the
‘Warburg Effect’ and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shoshan-Barmatz V, Zakar M, Rosenthal K
and Abu-Hamad S: Key regions of VDAC1 functioning in apoptosis
induction and regulation by hexokinase. Biochim Biophys Acta.
1787:421–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Duval RE, Harmand PO, Jayat-Vignoles C,
Cook-Moreau J, Pinon A, Delage C and Simon A: Differential
involvement of mitochondria during ursolic acid-induced apoptotic
process in HaCaT and M4Beu cells. Oncol Rep. 19:145–149.
2008.PubMed/NCBI
|
|
56
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Ursolic acid in cancer prevention and
treatment: Molecular targets, pharmacokinetics and clinical
studies. Biochem Pharmacol. 85:1579–1587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tang X, Gao J, Chen J, Fang F, Wang Y, Dou
H, Xu Q and Qian Z: Inhibition by [corrected] ursolic acid of
[corrected] calcium-induced mitochondrial permeability transition
and release of two proapoptotic proteins. Biochem Biophys Res
Commun. 337:320–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shyu MH, Kao TC and Yen GC: Oleanolic acid
and ursolic acid induce apoptosis in HuH7 human hepatocellular
carcinoma cells through a mitochondrial-dependent pathway and
downregulation of XIAP. J Agric Food Chem. 58:6110–6118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Saraswati S, Agrawal SS and Alhaider AA:
Ursolic acid inhibits tumor angiogenesis and induces apoptosis
through mitochondrial-dependent pathway in Ehrlich ascites
carcinoma tumor. Chem Biol Interact. 206:153–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang J, Jiang Z, Xiang L, Li Y, Ou M, Yang
X, Shao J, Lu Y, Lin L, Chen J, et al: Synergism of ursolic acid
derivative US597 with 2-deoxy-D-glucose to preferentially induce
tumor cell death by dual-targeting of apoptosis and glycolysis. Sci
Rep. 4:50062014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dong H, Yang X, Xie J, Xiang L, Li Y, Ou
M, Chi T, Liu Z, Yu S, Gao Y, et al: UP12, a novel ursolic acid
derivative with potential for targeting multiple signaling pathways
in hepatocellular carcinoma. Biochem Pharmacol. 93:151–162. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang Y, Branicky R, Noe A and Hekimi S:
Superoxide dismutases: Dual roles in controlling ROS damage and
regulating ROS signaling. J Cell Biol. 217:1915–1928. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sies H: Oxidative stress: A concept in
redox biology and medicine. Redox Biol. 4:180–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sabharwal SS and Schumacker PT:
Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles'
heel? Nat Rev Cancer. 14:709–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shadel GS and Horvath TL: Mitochondrial
ROS signaling in organismal homeostasis. Cell. 163:560–569. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tu HY, Huang AM, Wei BL, Gan KH, Hour TC,
Yang SC, Pu YS and Lin CN: Ursolic acid derivatives induce cell
cycle arrest and apoptosis in NTUB1 cells associated with reactive
oxygen species. Bioorg Med Chem. 17:7265–7274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim KH, Seo HS, Choi HS, Choi I, Shin YC
and Ko SG: Induction of apoptotic cell death by ursolic acid
through mitochondrial death pathway and extrinsic death receptor
pathway in MDA-MB-231 cells. Arch Pharm Res. 34:1363–1372. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ventura A, Kirsch DG, McLaughlin ME,
Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R
and Jacks T: Restoration of p53 function leads to tumour regression
in vivo. Nature. 445:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Matoba S, Kang JG, Patino WD, Wragg A,
Boehm M, Gavrilova O, Hurley PJ, Bunz F and Hwang PM: p53 regulates
mitochondrial respiration. Science. 312:1650–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kruse JP and Gu W: p53 aerobics: The major
tumor suppressor fuels your workout. Cell Metab. 4:1–3. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Heffernan-Stroud LA, Helke KL, Jenkins RW,
De Costa AM, Hannun YA and Obeid LM: Defining a role for
sphingosine kinase 1 in p53-dependent tumors. Oncogene.
31:1166–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nam H and Kim MM: Ursolic acid induces
apoptosis of SW480 cells via p53 activation. Food Chem Toxicol.
62:579–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang X, Song X, Yin S, Zhao C, Fan L and
Hu H: p21 induction plays a dual role in anti-cancer activity of
ursolic acid. Exp Biol Med (Maywood). 241:501–508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Manu KA and Kuttan G: Ursolic acid induces
apoptosis by activating p53 and caspase-3 gene expressions and
suppressing NF-kappaB mediated activation of bcl-2 in B16F-10
melanoma cells. Int Immunopharmacol. 8:974–981. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yu YX, Gu ZL, Yin JL, Chou WH, Kwok CY,
Qin ZH and Liang ZQ: Ursolic acid induces human hepatoma cell line
SMMC-7721 apoptosis via p53-dependent pathway. Chin Med J (Engl).
123:1915–1923. 2010.PubMed/NCBI
|