Introduction
Lung cancer remains the leading cause of
cancer-related mortality in the world. More than 180,000 men and
90,000 women succumb to lung cancer every year within the European
Union (1). Non-small-cell lung
cancer (NSCLC) accounts for >80% of all lung cancer cases
(2). Surgical resection represents
the treatment of choice for patients with NSCLC at stages I and II,
and can also be used for some patients with locally advanced
disease (stage IIIA and IIIB) as an important component of the
multimodal treatment approach. In general, only 20–25% of patients
with NSCLC are suitable for surgical resection at the time of
diagnosis (3,4). For those with advanced disease,
radiation therapy, chemotherapy and targeted therapies are commonly
used (2,5). However, despite the fact that the
multimodal treatment approach has improved outcomes over the last
20 years, the 5-year survival rate for stage III patients still
ranges between 13 and 36% (6).
In the last ten years, there has been a surge of
ablative treatments for solid tumors, which have been shown to be
effective in patients not suitable for surgery (7–14). In
particular, thermal ablation techniques, such as radiofrequency
ablation, microwave ablation (MWA), cryoablation and laser
ablation, have been used with the aim of treating primary and
secondary lung tumors in a minimally invasive manner (9,15–20).
Among them, MWA can be considered a relatively new method, in which
one or more microwave antennas are inserted percutaneously inside
the tumor mass. The electromagnetic microwaves produce thermal
energy that causes coagulative necrosis of neoplastic cells and the
surrounding parenchyma (10,14,18,21). MWA
offers some advantages when compared to radiofrequency ablation,
which has been the most used method for ablation of nonresectable
lung NSCLC (8,15,22). In
fact, radiofrequency is associated with some limitations and
disadvantages, such as reduced efficacy due to increased impedance
from a temperature >100°C, and the heat sink effect. By
contrast, the efficacy of MWA ablation is not affected by high
temperatures and seems less affected by the heat sink effect
(8,10,11,15).
Moreover, two or more antennae can be used when necessary to obtain
a larger ablation zone in a shorter time (4,8,10,11,22,23). A
multicenter study, including 52 patients with lung cancer,
comparing radiofrequency ablation and MWA reported that the latter
was associated with less intraprocedural pain and a significant
reduction in tumor mass (24).
Although evidence has emerged that MWA is a
promising treatment option for primary and metastatic lung
malignancies, long-term follow-up data are scant. This study was
performed with the main aim of evaluating the survival outcomes of
patients with large advanced NSCLC undergoing MWA under CT
guidance.
Patients and methods
Study population
The present study was conducted at an oncological
institution with expertise in interventional radiology, where
different ablative techniques have been used in the last decade for
thermal ablation of either primary or metastatic malignancies of
the liver, pancreas, bone, breast and lung. For the purposes of
this study, a retrospective review was conducted on NSCLC patients
receiving MWA between 2010 and 2013. The Institutional Review Board
of the Division of Interventional Radiology, Department of
Oncological Radiology, Oncological Hospital A. Businco, Cagliari
(Italy) approved the present study. All patients gave informed
written consent for the MWA treatment.
Indications for lung MWA included one or more of the
following: i) NSCLC at stage IIIb-IIIc; ii) NSCLC not suitable for
surgical treatment because of poor general status or concomitant
medical conditions; iii) advanced NSCLCs not responding to
chemotherapy and/or radiotherapy; iv) patients with operable
disease refusing surgical treatment. Exclusion criteria included:
Primary lung tumor different from the NSCLC (e.g., small-cell lung
cancer and neuroendocrine tumors); tumors infiltrating large
vascular structures, the trachea or esophagus; parietal pleural
transgression into the chest wall; severe pulmonary dysfunction
(maximum ventilation capacity <39%); platelet count of
<50,000 per microliter; and/or unmanageable coagulation
disorders.
The following data were extrapolated for the entire
study population: Age, sex and tumor characteristics (size, number
and localization of lesions, histotype and vicinity to relevant
anatomical structures), as well as ablation technique details and
MWA-related complications. Follow-up data included the occurrence
of local tumor recurrence, survival time and cause of mortality.
Patients with at least 3 months follow-up were eligible.
Preoperative work-up
Patients received a pre-procedural visit
approximately 2 weeks (10–20 days) before MWA. Staging of all
patients included: Histologic confirmation of NSCLC, chest
radiography, contrast-enhanced total body CT-scan, lung function
tests, cardiological assessment, complete blood counts and a
coagulation study. Only patients with a life expectancy of >3
months were considered for MWA. When present, anticoagulative and
antiplatelet medications were stopped 2 days and 1 week before the
procedure, respectively. Risks and benefits of the MWA procedure
were discussed with all patients, and informed consent was obtained
from all individual participants included in the study.
MWA procedure
All MWA treatments were carried out under CT
guidance (Somatom Sensation Unit; Siemens, Germany) by
board-certified interventional radiologists. The patients were
maintained in a state of conscious sedation by using intravenous
fentanyl citrate, and their vital signs (oximetry, blood pressure
and heart rate) were continuously monitored throughout the
procedure. After sterile preparation of the skin, 2–5 ml of 2%
lidocaine was injected into the deep subcutaneous tissue along the
expected course of the MWA antenna. Percutaneous MWA was performed
with patients in the prone or supine position by using a 2.45-GHz
microwave generator (AMICA-GEN; HS Hospital Service, Aprilia,
Italy) with energy delivered via 14- or 16-gauge mini-choked,
water-cooled interstitial antennae (HS AMICA; HS Hospital Service).
For treatment of synchronous tumor lesions, two generators were
used, each driving a single antenna. The duration of each MWA
application was based on the size and location of the lesion
measured by the pre-operative CT scan. The antenna was located into
the tumor and connected to the generator. In all cases, the
positioning of the antenna within the lesion was performed
carefully in order to avoid non-target thermal damage to other
anatomical structures (e.g., aorta or bronchus). The number and
type of antenna were chosen at the operator's discretion according
to several factors, such as the ‘access window’, tumor size,
morphology, tumor location and adjacent structures. CT-guided MWA
was performed with a single antenna for tumors of 3–4 cm in
diameter and with two antennae for tumors >4 cm. The time for
energy deposition ranged from 5–10 mins and the ablation power
varied between 40 and 80 W. When technically possible, the MWA
procedure was performed with the aim of encompassing the lung tumor
with an ablation zone at least 5 mm of safety margin.
Post-procedural imaging and follow-up
evaluation
Assessment of MWA efficacy was made by CT scan at 1,
3, 6 and 12 months for the first year after treatment, and every 6
months thereafter. Tumor ablation was deemed to be complete when no
contrast enhancement was observed in the arterial phase at the
1-month follow-up CT, whereas ablation was considered incomplete
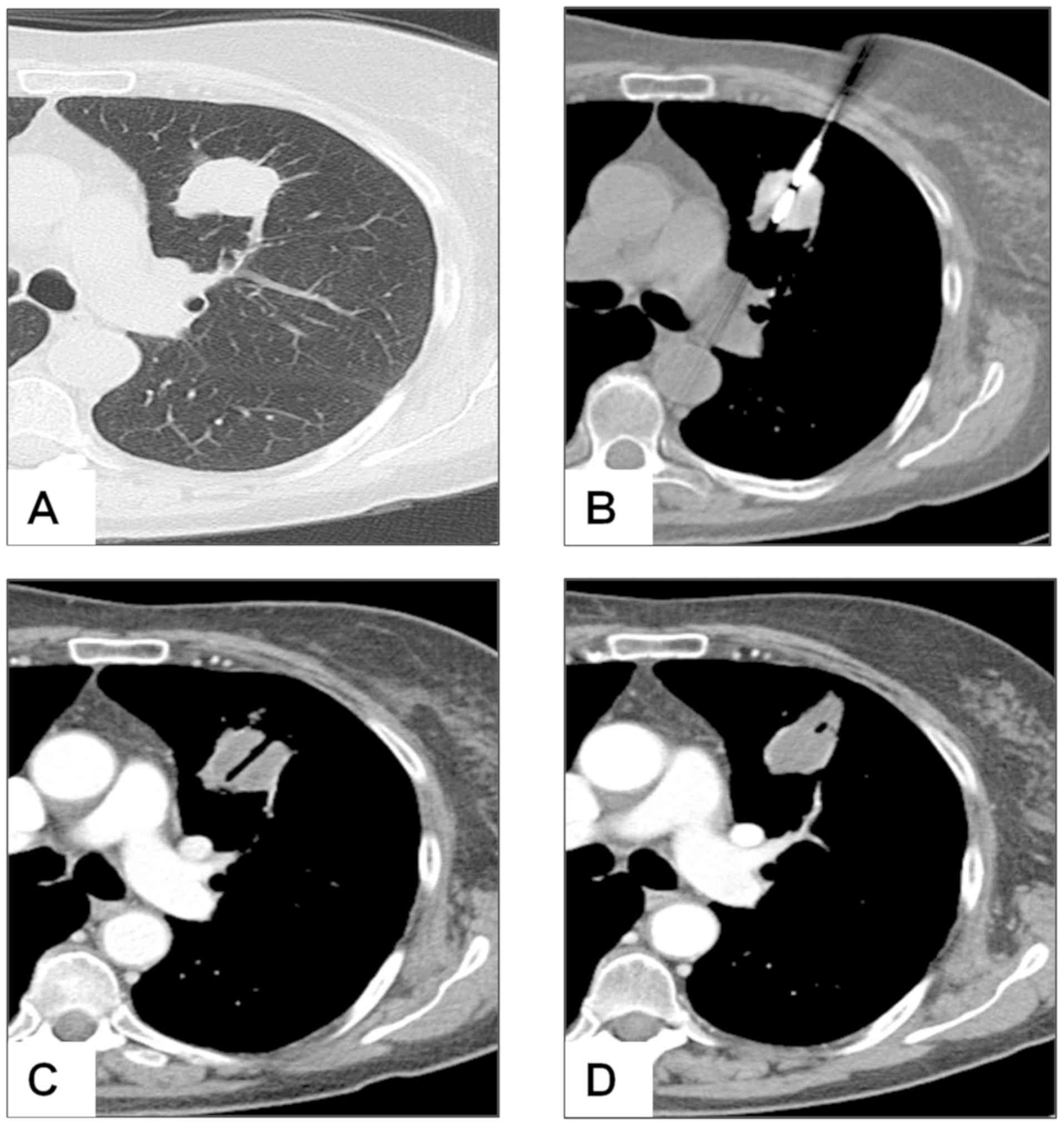
when a residual contrast enhancement was observed (Fig. 1). The size of the ablation zone
measured at the 1-month CT scan was used as the basal assessment to
which subsequent follow-up imaging was compared. Increases in the
diameter of the MWA ablation zone were considered as tumor
progression, while a plateau or decrease in the diameter of the
ablation zone was interpreted as successful ablation. In some cases
of residual ablation, a second MWA session was performed.
Statistical analysis
Continuous patient and tumor data are presented as
the mean, range and standard deviation. The three patients with
early NSCLC who underwent MWA because of surgery refusal were
excluded from the survival analysis. Kaplan-Meier analysis was used
to evaluate overall survival (OS) and cancer-specific survival
(CSS), which were calculated from the date of treatment to the date
of mortality or final follow-up. The log-rank test was used to
compare survival data between patients having an NSCLC size greater
or smaller than 40 mm in maximum diameter. The likelihood of local
tumor progression (LTP) was analyzed through a multivariable
logistic regression model. Tumor size was dichotomized to a binary
variable (<4 cm vs. ≥4 cm) as well as the remaining covariates
(proximity to relevant structures, yes vs. no; and complete vs.
incomplete tumor necrosis after first MWA treatment). Covariates
were chosen based on clinical significance. For each variable, a
reference category was chosen, generally the no-exposure or
majority category, and the other category was compared with the
reference category. The odds ratios (ORs) in each category vs. the
odds in the reference category were estimated. The goodness of fit
of the model was assessed by the Hosmer and Lemeshow test, and
P<0.05 was considered to indicate a statistically significant
difference. Analysis was conducted using IBM SPSS Statistics v.20
(IBM Corp.).
Results
Patients and tumours
characteristics
From January 2010 to December 2013, 53 patients with
primary NSCLC lesions consecutively underwent percutaneous MWA
treatment under CT guidance. Patients and tumor characteristics are
summarized in Table I. The mean age
was 70.3 years (median 70.5 years), and 86% of patients were male.
A total of 50 patients (94.3%) were considered not suitable for
surgery after a multidisciplinary team discussion, and 3 patients
(5.7%) with early-stage disease refused surgical treatment. Prior
to MWA treatment, among the 50 patients unsuitable for surgery, 16
(32.0%) patients received chemotherapy as primary treatment. Two
patients (4%) refused pharmacologic treatment, and 32 (64%) were
excluded from chemotherapy due to advanced age or coexisting
comormibidities. MWA was used as a further treatment in patients in
whom chemotherapy failed to significantly reduce the tumor
size.
 | Table I.Patient and tumor
characteristics. |
Table I.
Patient and tumor
characteristics.
|
Characteristics | No. (%) |
|---|
| Patients submitted
to MWA for NSCLC | 53 |
| Age, mean ± SD
(range) | 70.3±10.0
(43–84) |
| Sex |
|
Male | 43 (86%) |
|
Female | 7 (14%) |
| MWA treatment
intention |
|
Palliative | 50 (94.3) |
|
Curative | 3 (5.7) |
| Chemotherapy as
primary treatment in patients at IIIa/IIIb stages | 16 (32%) |
| Total no. of NSCLC
nodules treated with MWA | 65 (100%) |
| Single NSCLC | 53 (81.5%) |
| 2 nodules of
NSCLC | 12 (18.5%) |
| Tumor location |
|
Central | 16 (24.6%) |
|
Peripheral | 49 (75.4%) |
| Tumors in close
proximity to relevant anatomical structuresa | 11 (16.9) |
| Tumor size, mean ±
SD (range) | 5.0±1.8
(3.0–11.0) |
| 3–4
cm | 26 (40.0%) |
| >4
cm | 39 (60.0%) |
| T stage |
|
T2a | 26 (40.0%) |
|
T2b | 14 (21.5%) |
| T3 | 17 (26.2%) |
| T4 | 8 (12.3%) |
| Histotype |
|
Squamous cell carcinoma | 13 (20.0%) |
|
Adenocarcinoma | 51 (78.4%) |
| Large
cell carcinoma | 1 (1.6%) |
| Tumor location |
| Right
upper lobe | 22 (33.8%) |
| Right
lower lobe | 14 (21.6%) |
| Left
upper lobe | 16 (24.6%) |
| Left
lower lobe | 13 (20.0%) |
MWA treatment
A total of 12 patients had 2 NSCLC lesions, thus
there were a total of 65 MWA treatment sessions. Most of the tumors
(75.4%) had a peripheral lung location. At baseline, mean tumor
size was 5.0±1.8 cm (median size 4.6 cm), being the 60% of cases
>4 cm in major diameter (Fig. 2).
As for histological type, 51 (78.4%) of the tumors were
adenocarcinomas, 13 (20%) squamous cell carcinomas, and 1 (1.6%)
large cell carcinoma. More than one-half of the NSCLC tumors were
located in the right lung. Out of 65 tumors, 11 (19.6%) were in
proximity to relevant structures such as the aorta, mediastinal
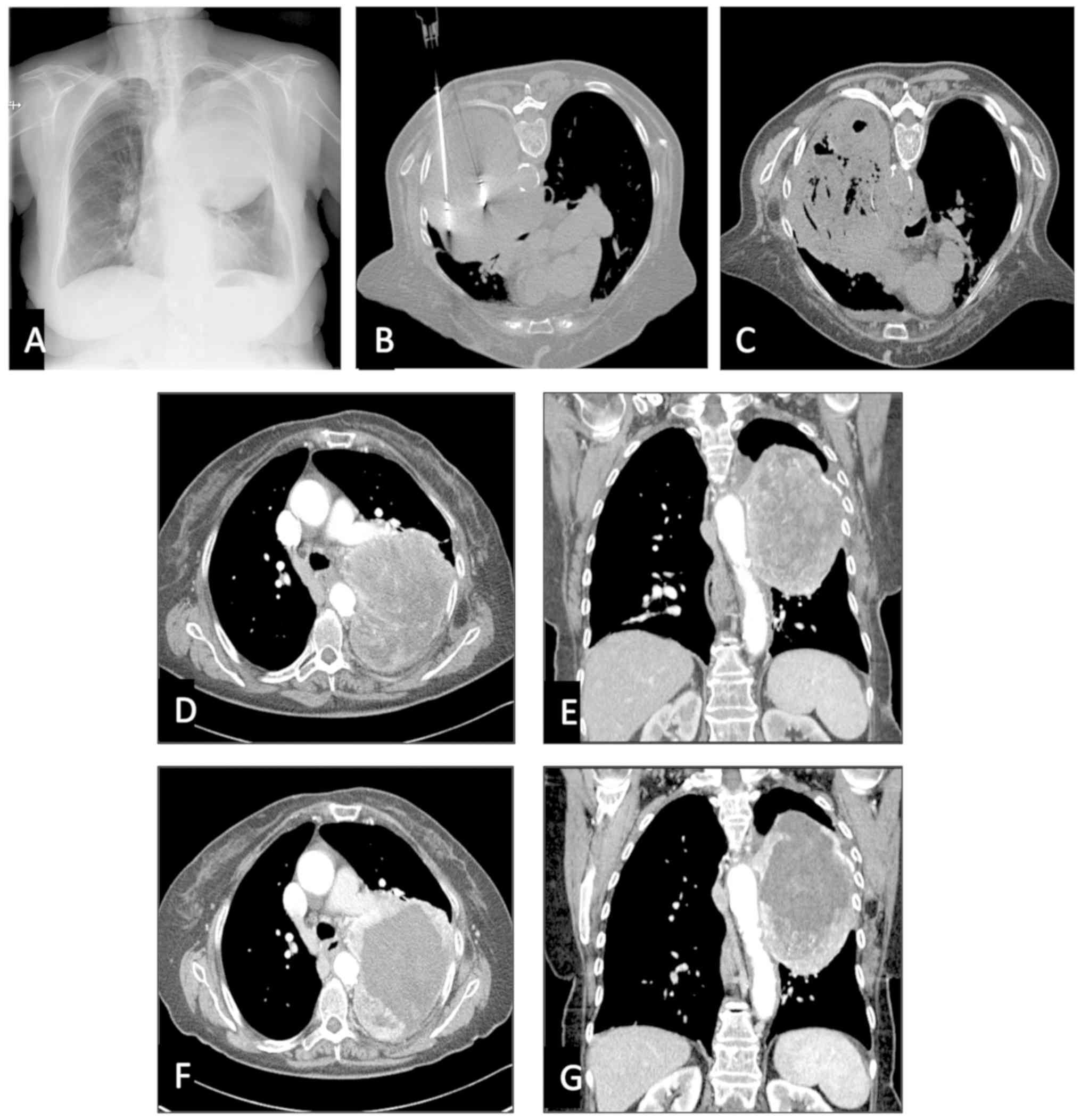
pleura, main stem bronchus, pericardium or diaphragm (Fig. 3).
All procedures were successfully completed.
Complications occurred after 18 (27.7%) procedures, all of which
were resolved conservatively except in one patient, who developed a
pneumothorax needing tube thoracostomy. The overall 30-day
mortality rate was 0%. At the 1-month CT scan, a complete tumor
ablation was observed after 29 MWA procedures (44.6%), and a
partial tumor ablation after 36 (55.4%). In 12 cases (18.5%) a
redo-MWA session was carried out to obtain complete necrosis, while
in 3 cases (4.6%) a third MWA was necessary (Table II). Combinatorial treatment used
along with MWA involved 17 patients (32.1%), of whom 11 received
second-line chemotherapy, 4 radiation therapy and 2 chemotherapy
plus radiation therapy.
 | Table II.Summary of MWA procedures and
combinatorial treatment. |
Table II.
Summary of MWA procedures and
combinatorial treatment.
|
Characteristics | No (%) |
|---|
| No. of antennas
used per single procedure |
| 1 | 39 (60.0) |
| 2 | 26 (40.0) |
| Initial response to
MWA |
|
Complete tumor ablation | 29 (44.6) |
| Partial
tumor ablation | 36 (55.4) |
| Second
session of MWA | 12 (18.5) |
| Third
session of MWA | 3 (4.6) |
| Total
procedure-related complication | 18 (27.7) |
|
Pneumothorax treated
conservatively | 11 (16.9) |
|
Pneumothorax treated with tube
thoracostomy | 1 (1.5) |
| Pleural
effusion | 2 (3.1) |
|
Cavitation and infection | 3 (4.6) |
|
Bronchopleural fistula | 1 (1.5) |
|
Combinatorial treatment used
along with MWA | 17 (32.1) |
|
Chemotherapy | 11 (20.7) |
|
Radiation therapy | 4 (7.5) |
|
Chemotherapy + Radiation
therapy | 2 (83.8) |
Follow-up
The mean follow-up time was 28.10±20.6 months with a
median duration of 21.5 months (range, 3–84 months). A total of 35
patients succumbed during the considered follow-up period. Median
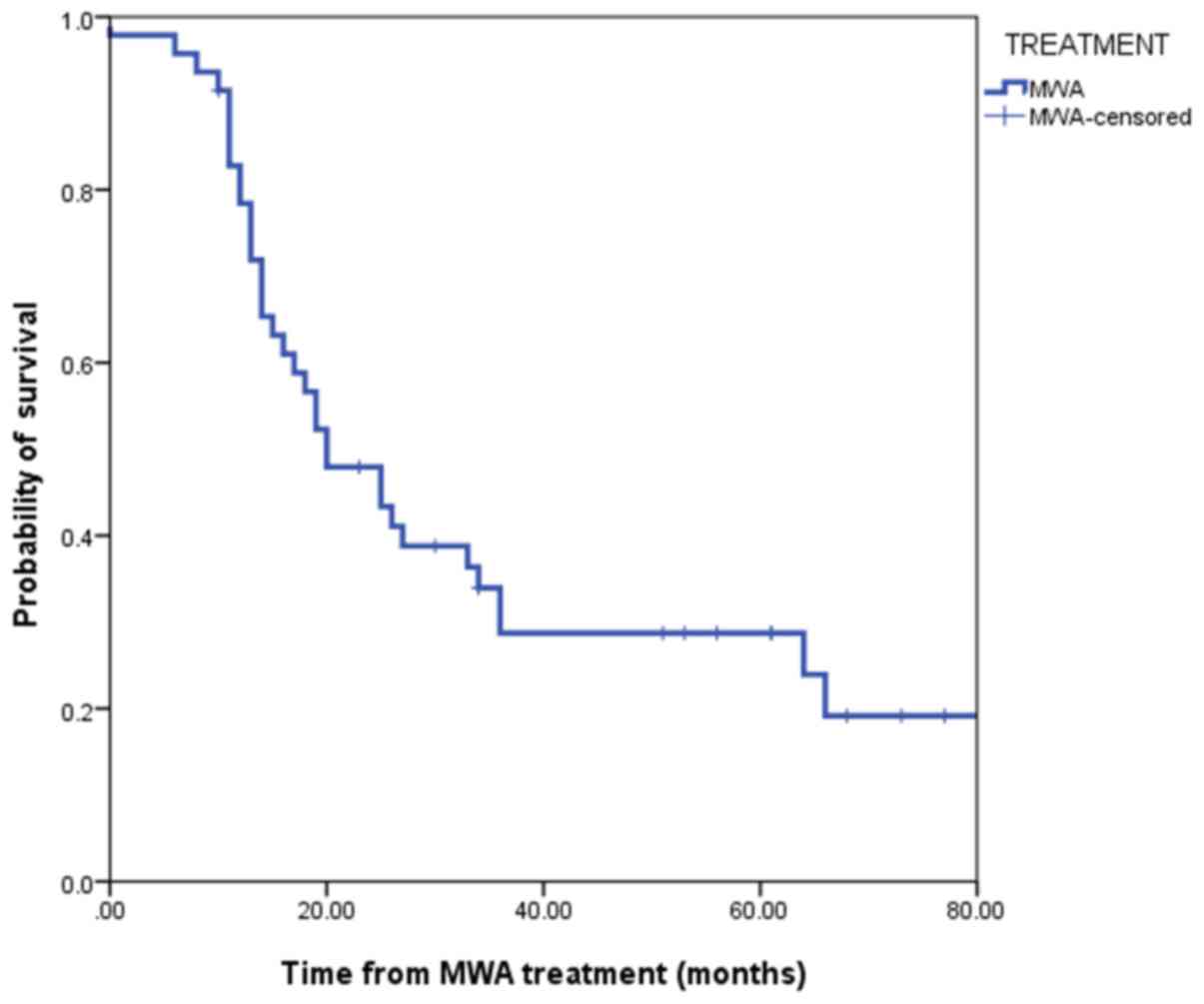
OS was 20.0 months (95% confidence interval, 12.38–27.61). The
1-year, 2-year, 3-year and 5-year OS rates were 78.2, 48.3, 34.8
and 18.3%, respectively (Fig. 4).
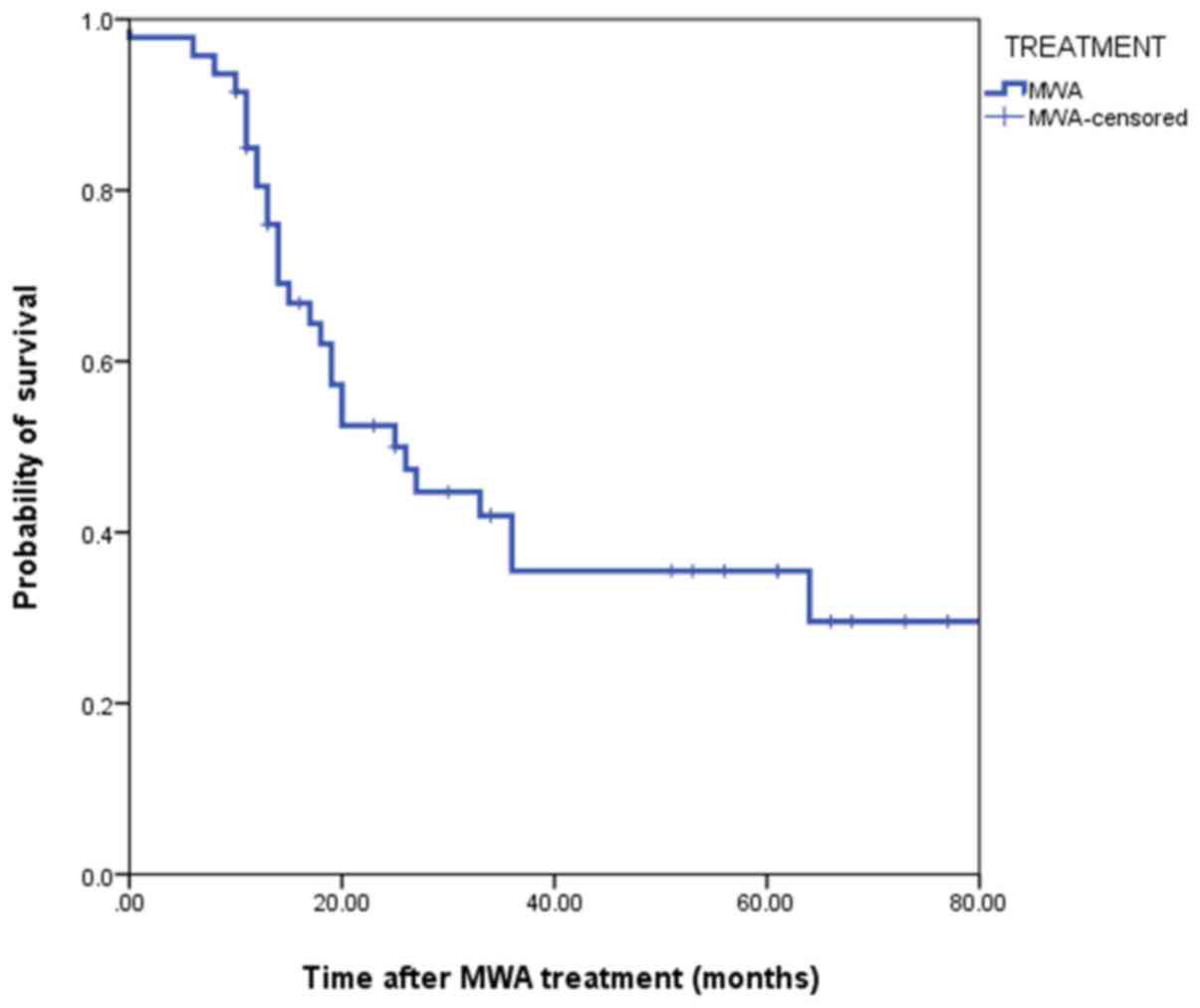
Overall CSS was 25 months (95% confidence interval, 15.47–34.52).
The 1-year, 2-year, 3-year and 5-year CSS rates were 84.3, 53.7,
42.1 and 30.0%, respectively (Fig.
5). Overall survival in patients with a tumor size ≥4 cm was
significantly lower when compared with patients with smaller tumors
(P=0.03; Fig. 6). LTP was observed
in 19 patients (35.8%). According to the multivariable analysis,
incomplete tumor ablation [odds ratio (OR), 6.57; P<0.05] and a
tumor size ≥4 cm (OR, 0.18; P<0.05) were significant predictors
of LTP (Table III).
 | Table III.Multivariate logistic regression for
factors associated with LTP in patients who received microwave
ablation for NSCLC. |
Table III.
Multivariate logistic regression for
factors associated with LTP in patients who received microwave
ablation for NSCLC.
| Variables | Odds ratio | Standard error | Z score | P-value | 95% CI |
|---|
| Complete tumor
ablation after the 1st MWA session |
| No | 6.57 | 0.95 | 1.88 | 0.048a | 1.02–42.34 |
|
Yes | Ref |
| Proximity to
relevant structures |
|
Yes | 2.09 | 0.73 | 0.73 | 0.313 | 0.50–8.74 |
| No | Ref |
| Tumor size |
| ≥4
cm | 0.18 | 0.70 | −1.68 | 0.017a | 0.04–0.74 |
| <4
cm | Ref |
Discussion
Most patients with NSCLC are commonly diagnosed at
an advanced stage, and are not candidates for surgical treatment
(8,9,24,25).
These patients are usually treated in a multidisciplinary fashion,
with systemic therapies and radiation therapy being the most
commonly used modalities. However, all these treatments rarely
provide a cure or good long term survival outcomes (8). In this scenario, MWA has been shown to
be effective in reducing tumor size in patients with inoperable
lung cancer. However, many studies in the literature include
patients with both primary and metastatic tumors, thus resulting in
limited validity in evaluating survival outcomes in those affected
by primary lung cancer (9,11,15,18,22).
Moreover, the few studies focusing on MWA of NSCLC mostly include
patients with inoperable lesions at an early stage (4,15,23,26).
The present study addressed the role of MWA in patients with large
advanced NSCLCs, mostly at stage III, who were unsuitable for
surgery. In fact, only three patients in this series received MWA
because they refused to undergo surgery.
In the present study, MWA was used in patients with
NSCLC lesions measuring between 3 and 14 cm. Zhong et al
(8), who published one of the
largest series of NSCLC patients treated with MWA, included tumor
lesions up to 6 cm in maximum diameter. Likewise, other authors
have included only tumors smaller than 4–5 cm for MWA (4,9,18). Notably, more than one-half of the
lesions in the present study were larger than 4 cm, with a mean
tumor size of 5 cm. According to the recent literature (4,18,27), no
NSCLC lesions >6 cm have been treated with MWA. This may be due
to the notion that ablation of very large lesions may not be able
to obtain complete tumor necrosis. However, the authors of the
present study hypothesize that cytoreduction may be of benefit in
such situations. Moreover, one of the advantages of MWA is the
possibility of treating large tumors by using two or more antennae
simultaneously. In these situations partial tumor necrosis is
usually achieved after the first session of treatment, and in
selected cases a redo-MWA can be carried out to obtain better
control of the disease. In the present series, 18.5% of patients
received a second MWA treatment.
A common problem in the application of percutaneous
ablation techniques is the proximity of the lesions to relevant
anatomical structures, because of the possible heat damage to the
surrounding tissues and organs (15,18).
Approximately 17% of patients included in the present study had
NSCLCs in the vicinity of structures such as the aorta, mediastinal
pleura, main stem bronchus, pericardium or diaphragm. Notably, in
those patients, MWA treatment was completed without any specific
consequences. Other recent papers have highlighted a progressive
broadening of indications for MWA treatment of lung malignancies
located near these structures (19,28).
Although side effects and serious complications
related to percutaneous thermoablation can occur (20), in the present study MWA-related
complications were observed in 27.7% of cases, none of which were
considered life-threatening for the patients. This data reinforce
the concept that MWA of lung tumors is a safe procedure when
performed by trained experts (4,20).
Local progression was observed in 35.8% of patients
in the present study. This figure is high compared with other
reports. For example, Zhong et al (8), reported a local progression or relapse
in 20.5% of 78 patients undergoing MWA for advanced NSCLC. In
general, rates of tumor progression after pulmonary MWA range
between 0 and 34% in the literature (10). The reason for this finding may be due
to the large tumor sizes in the present study. In fact the larger
the tumor mass, the lower the possibility of obtaining complete
necrosis after MWA. It was observed that incomplete tumor ablation
after the first MWA session was a significant independent predictor
of LTP, according to the multivariate analysis (P<0.05).
Nonetheless, thermal ablation can be repeated after tumor
progression (27) and can also be
considered as a salvage therapy in cases of local relapse after a
previous treatment (15).
Studies on IIIa/IIIb NSCLC cases not receiving MWA
showed a 5-year OS range between 5 and 25%, and a CSS range between
10 and 36% (10,29–32). In
the present report, the 5-year OS was 18.3%, while the 5-year CCS
was 30.0%. These data seem to compare favorably with previous
published data on survival in patients with locally advanced NSCLC,
especially if one takes into account that this study focused on
patients with large lesions. To date, no trials have been conducted
to compare MWA and non-ablative techniques, and few studies have
reported on long term outcomes. As expected, OS in patients with
NSCLC >4 cm at baseline CT scan was significantly lower when
compared to those with smaller-size tumors (P=0.03). The reasons
for this finding may involve both the more advanced stage of the
disease and the lower probability of obtaining complete tumor
necrosis with MWA in the group with larger tumors. Yang et
al (23), similarly reported
that NSCLC ≤3.5 cm was associated with better survival than tumors
>3.5 cm.
In 12 cases of the present series in which tumor
necrosis was incomplete after the first MWA session, the treatment
was repeated in order to obtain complete necrosis, and in 3 cases a
third session was needed. This confirms that MWA is a versatile
ablative method that can be safely repeated in the same patient in
cases of incomplete ablation or tumor progression (27).
It was observed that, along with incomplete tumor
ablation, a tumor size ≥4 cm was a significant independent
predictor of LTP (P<0.05). Similarly, Zheng et al
(11) reported a significant
difference in LTP in patients with lung tumors with a mean diameter
of 3.1 cm compared with those with a mean diameter of 4.9 cm. Also,
in the study of Lu et al (33) on a group of 69 patients, a higher
local progression rate was observed when tumors were >4 cm at
the baseline CT scan.
The present study had some limitations; firstly, its
non-comparative design and the small simple size. Nonetheless, the
preliminary results support the utility of MWA as a form of
palliative tumor ablation in patients with advanced NSCLC. In
particular, to the best of our knowledge this is the first study to
report long term follow-up data of MWA in patients with large
NSCLC. Although current guidelines comprise the use of
interventional radiological ablation as an option for selected
patients with stage I NSCLC who are medically inoperable (34–36), the
role of MWA thermoablation remains ill-defined. It is considered
that further multicenter studies are needed to better assess the
role of MWA in local tumor control and to evaluate survival
outcomes, as well as to improve its use in integrated multimodality
treatment. In addition, promising interactions between MWA and
targeted agents have been reported, and this merits further
investigation (15,37,38).
In summary, CT-guided MWA may represent an useful
tool in the multimodality treatment of patients with large advanced
NSCLC. MWA was successfully applied in large NSCLCs in close
proximity to relevant anatomical structures.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CP and AF contributed to the study conception and
design, wrote the manuscript, and analyzed the data. LM and BS were
involved in data collection and processing. DG acquired, analyzed
and interpreted the oncological data, and drafted the manuscript.
AP critically revised the manuscript for important intellectual
content and interpreted the oncological data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of the Division of
Interventional Radiology, Department of Oncological Radiology,
Oncological Hospital A. Businco, Cagliari (Italy) approved this
study. All patients gave informed written consent for the MWA
treatment.
Patient consent for publication
All patients or their immediate relatives provided
consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malvezzi M, Carioli G, Bertuccio P,
Boffetta P, Levi F, La Vecchia C and Negri E: European cancer
mortality predictions for the year 2017, with focus on lung cancer.
Ann Oncol. 28:1117–1123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lewis J, Gillaspie EA, Osmundson EC and
Horn L: Neoadjuvant approaches to locally advanced non-small cell
lung cancer. Front Oncol. 8:52018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ou W, Li N, Wang SY, Li J, Liu QW, Huang
QA and Wang BX: Phase 2 trial of neoadjuvant bevacizumab plus
pemetrexed and carboplatin in patients with unresectable stage III
lung adenocarcinoma (GASTO 1001). Cancer. 122:740–747. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu H and Steinke K: High-powered
percutaneous microwave ablation of stage I medically inoperable
non-small cell lung cancer: A preliminary study. J Med Imaging
Radiat Oncol. 57:466–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moya-Horno I, Viteri S, Karachaliou N and
Rosell R: Combination of immunotherapy with targeted therapies in
advanced non-small cell lung cancer (NSCLC). Ther Adv Med Oncol.
10:17588340177450122018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
AmericanCancer Society: Cancer Facts &
Figures. 2017, https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosisstaging/survival-rates.html
|
|
7
|
Nour-Eldin NA, Exner S, Al-Subhi M, Naguib
NNN, Kaltenbach B, Roman A and Vogl TJ: Ablation therapy of
non-colorectal cancer lung metastases: Retrospective analysis of
tumour response post-laser-induced interstitial thermotherapy
(LITT), radiofrequency ablation (RFA) and microwave ablation (MWA).
Int J Hyperthermia. 33:820–229. 2017.PubMed/NCBI
|
|
8
|
Zhong L, Sun S, Shi J, Cao F, Han X, Bao X
and You Q: Clinical analysis on 113 patients with lung cancer
treated by percutaneous CT-guided microwave ablation. J Thorac Dis.
9:590–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wolf FJ, Grand DJ, Machan JT, Dipetrillo
TA, Mayo-Smith WW and Dupuy DE: Microwave ablation of lung
malignancies: Effectiveness, CT findings, and safety in 50
patients. Radiology. 247:871–879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vogl TJ, Naguib NN, Gruber-Rouh T, Koitka
K, Lehnert T and Nour-Eldin NE: Microwave ablation therapy:
Clinical utility in treatment of pulmonary metastases. Radiology.
261:643–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng A, Ye X, Yang X, Huang G and Gai Y:
Local efficacy and survival after microwave ablation of lung
tumors: A retrospective study in 183 patients. J Vasc Interv
Radiol. 27:1806–1814. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pusceddu C, Melis L, Ballicu N, Meloni P,
Sanna V, Porcu A and Fancellu A: Cryoablation of primary breast
cancer in patients with metastatic disease: considerations arising
from a single-centre data analysis. Biomed Res Int.
2017:38390122017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pusceddu C, Melis L, Sotgia B, Fancellu A
and Meloni GB: Computed tomography-guided cryoablation of local
recurrence after primary resection of pancreatic adenocarcinoma.
Clin Pract. 5:7412015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pusceddu C, Sotgia B, Fele RM and Melis L:
Treatment of bone metastases with microwave thermal ablation. J
Vasc Interv Radiol. 24:229–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palussière J, Catena V and Buy X:
Percutaneous thermal ablation of lung tumors-Radiofrequency,
microwave and cryotherapy: Where are we going? Diagn Interv
Imaging. 98:619–625. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Littrup PJ, Duan Y, Zhang Y, Feng
H and Nie Z: Thoracic masses treated with percutaneous cryotherapy:
Initial experience with more than 200 procedures. Radiology.
235:289–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hegenscheid K, Behrendt N, Rosenberg C,
Kuehn JP, Ewert R, Hosten N and Puls R: Assessing early vascular
changes and treatment response after laser-induced ther- motherapy
of pulmonary metastases with perfusion CT: Initial experience. AJR
Am J Roentgenol. 194:1116–1123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belfiore G, Ronza F, Belfiore MP, Serao N,
di Ronza G, Grassi R and Rotondo A: Patients' survival in lung
malignancies treated by microwave ablation: Our experience on 56
patients. Eur J Radiol. 82:177–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pusceddu C, Melis L, Fancellu A, Melis M
and Meloni GB: Feasibility and safety of percutaneous
radiofrequency, microwave or cryoablation for unresectable thoracic
malignancies in close proximity to heart and large vessels. Ann
Surg Oncol. 20:S1072013.
|
|
20
|
Zheng A, Wang X, Yang X, Wang W, Huang G,
Gai Y and Ye X: Major complications after lung microwave ablation:
A single-center experience on 204 sessions. Ann Thorac Surg.
98:243–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pusceddu C, Melis L, Ballicu N, Sotgia B,
Melis M, Sanna V, Meloni GB, Porcu A and Fancellu A: Percutaneous
microwave ablation under CT guidance for hepatocellular carcinoma:
A single institutional experience. J Gastrointest Cancer.
49:295–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carrafiello G, Mangini M, Fontana F,
Ierardi AM, De Marchi G, Rotolo N, Chini C, Cuffari S and Fugazzola
C: Microwave ablation of lung tumors: Single-centre preliminary
experience. Radiol Med. 119:75–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X, Ye X, Zheng A, Huang G, Ni X, Wang
J, Han X, Li W and Wei Z: Percutaneous microwave ablation of stage
I medically inoperable non-small cell lung cancer: Clinical
evaluation of 47 cases. J Surg Oncol. 110:758–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Macchi M, Belfiore MP, Floridi C, Serra N,
Belfiore G, Carmignani L, Grasso RF, Mazza E, Pusceddu C, Brunese L
and Carrafiello G: Radiofrequency versusmicrowave ablation for
treatment of the lung tumors: LUMIRA (lung microwave
radiofrequency) randomized trial. Med Oncol. 34:962014. View Article : Google Scholar
|
|
25
|
Lackey A and Donington JS: Surgical
management of lung cancer. Semin Intervent Radiol. 30:133–140.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Narsule CK, Sridhar P, Nair D, Gupta A,
Oommen RG, Ebright MI, Litle VR and Fernando HC: Percutaneous
thermal ablation for stage IA non-small cell lung cancer: Long-term
follow-up. J Thorac Dis. 9:4039–4045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Ye X, Huang G, Han X, Wang J, Li
W, Wei Z and Meng M: Repeated percutaneous microwave ablation for
local recurrence of inoperable Stage I nonsmall cell lung cancer. J
Cancer Res Ther. 13:683–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maxwell AWP, Healey TT and Dupuy DE:
Microwave ablation of lung tumors near the heart: A retrospective
review of short-term procedural safety in ten patients. Cardiovasc
Intervent Radiol. 40:1401–1407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Tollenaere C, Lievens Y, Vandecasteele
K, Vermaelen K and Surmont V: Unresectable stage III non-small-cell
lung cancer: Have we made any progress? World J Respirol.
5:140–151. 2015. View Article : Google Scholar
|
|
30
|
Stinchcombe TE and Bogart JA: Novel
approaches of chemoradiotherapy in unresectable stage IIIA and
stage IIIB non-small cell lung cancer. Oncologist. 17:682–693.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caglar HB, Baldini EH, Othus M, Rabin MS,
Bueno R, Sugarbaker DJ, Mentzer SJ, Jänne PA, Johnson BE and Allen
AM: Outcomes of patients with stage III nonsmall cell lung cancer
treated with chemotherapy and radiation with and without surgery.
Cancer. 115:4156–4166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Zhang J, Shi F, Zhang C, Jiao Q
and Zhu H: Better cancer specific survival in young small cell lung
cancer patients especially with AJCC stage III. Oncotarget.
8:34923–34934. 2017.PubMed/NCBI
|
|
33
|
Lu Q, Cao W, Huang L, Wan Y, Liu T, Cheng
Q, Han Y and Li X: CT-guided percutaneous microwave ablation of
pulmonary malignancies: Results in 69 cases. World J Surg Oncol.
10:802012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
NCCN guidelines for Non-Small Cell Lung
Cancer (Ver. 4.2018-April 2018). https://www.nccn.org/store/login/login.aspxReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
|
|
35
|
Postmus PE, Kerr KM, Oudkerk M, Senan S,
Waller DA, Vansteenkiste J, Escriu C and Peters S; ESMO Guidelines
Committee, : Early and locally advanced non-small- cell lung cancer
(NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 28 (Suppl 4):iv1–iv21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Howington JA, Blum MG, Chang AC, Balekian
AA and Murthy SC: Treatment of stage I and II non-small cell lung
cancer: Diagnosis and management of lung cancer, 3rd ed: American
College of Chest Physicians evidence-based clinical practice
guidelines. Chest 143 (5 Suppl). e278S–e313S. 2013. View Article : Google Scholar
|
|
37
|
Wei Z, Ye X, Yang X, Zheng A, Huang G, Li
W, Wang J, Han X, Meng M and Ni Y: Microwave ablation combined with
EGFR-TKIs versus only EGFR-TKIs in advanced NSCLC patients with
EGFR-sensitive mutations. Oncotarget. 8:56714–56725. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ni Y, Bi J, Ye X, Fan W, Yu G, Yang X,
Huang G, Li W, Wang J, Han X, et al: Local microwave ablation with
continued EGFR tyrosine kinase inhibitor as a treatment strategy in
advanced non-small cell lung cancers that developed extra-central
nervous system oligoprogressive disease during EGFR tyrosine kinase
inhibitor treatment: A pilot study. Medicine (Baltimore).
95:e39982016. View Article : Google Scholar : PubMed/NCBI
|