Introduction
As a malignancy that develops in the bone,
osteosarcoma primarily affects children, adolescents and young
adults (1). Although the incidence
is low, osteosarcoma is one of the leading causes of
cancer-associated mortality among teenagers (13–19 years old) and
young adults (20–30 years old) worldwide (2). With the developmement and application
of systemic chemotherapy, the survival rate of patients with
osteosarcoma has improved significantly (3). However, this is challenged by the high
prevalence of tumor metastasis by the time of diagnosis, and
despite appropriate treatment, the 5-year surival rate of patients
with distant tumor metastasis remains poor (≥20%) (4). Therefore, early diagnosis and treatment
are prominent factors in the surival of patients with osteosarcoma.
Unclear pathogenesis is a principal cause of treatment failure in
osteosarcoma (3), and in-depth
investigation of the mechanisms of development and progression of
osteosarcoma may improve its diagnosis and treatment.
It has been demonstrated that the development and
progression of osteosarcoma is frequently associated with the
altered expression of specific long non-coding RNAs (lncRNAs)
(5), indicating the invovlement of
lncRNA in disease pathogenesis. Glucose metabolism serves a pivotal
role in cancer growth by providing energy for survival and cellular
proliferation (6). lncRNA heart and
neural crest derivatives expressed 2-antisense RNA 1 (HAND2-AS1) is
a recently identified lncRNA that serves as a tumor suppressor gene
in endometrioid endometrial carcinoma by inhibiting the invasion
and migration of cancer cells (7).
However, its involvement in other cancer types is unknown. It has
been reported that certain lncRNAs may interfere with glucose
metabolism in cancer cells, promoting or inhibiting growth,
development and progression (8). In
the present study, HAND2-AS1 was downregulated in osteosarcoma,
where its influence on glucose metabolism may regulate tumor
growth. This revealed a novel function for HAND2-AS1 and provides a
potential therapeutic target for osteosarcoma.
Materials and methods
Subjects
A total of 48 patients with osteosarcoma were
recruited. Patients were diagnosed by pathological examination and
treated in Jingzhou Central Hospital (Jingzhou, Hubei, China)
between January 2015 and January 2017. Inclusion criteria were as
follows: i) Osteosarcoma confirmed by pathological examinations;
ii) patients who were willing to participate; and iii) patients who
received surgical resection. Exclusion criteria were as follows: i)
Patients suffering from other types of malignancies; ii) patients
with other types of severe disease, such as metabolic diseases and
severe infections; and iii) patients who were treated in other
hospitals prior to the study. The cohort included 28 males and 20
females (age range, 12–67 years; mean age, 32±8.5 years), and 44
healthy volunteers were recruited as controls. The control group
included 26 males and 18 females (age range, 14–66 years; mean age,
34±7.9 years). No significant differences in age and sex were
present between the two groups. All patients provided written
informed consent.
Specimen collection and
processing
Tumor tissues and adjacent healthy tissues (within a
2-cm area around the tumor) were collected during surgical
resection, and confirmed by pathological examination. On the day of
admission, blood was extracted from the elbow vein of each of the
patients and healthy controls. The blood was stored at room
temperature for 2 h, followed by centrifugation at 1,250 × g at
room temperature for 20 min for serum. All specimens were stored in
liquid nitrogen for long-term use.
Cell lines and transfection
The human osteosarcoma MG-63 and SAOS-2 cell lines,
and the normal bone hFOB cell line were obtained from the American
Type Culture Collection (ATCC). The MG-63 and SAOS-2 cells were
cultured in Eagle's minimum essential medium (cat. no. 30-2003;
ATCC) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.). hFOB cells were cultured in McCoy's 5a modified
medium (cat. no. 30-2007; ATCC) containing 10% FBS. All cells were
cultured at 37°C with 5% CO2. HAND2-AS1 small
interfering RNA (siRNA; 5′-CCGAGGUGCUCCAAUAUUATT-3′) and negative
control siRNA (5′-UUCUCCGAACGUGUCACGUdTdT-3′); were purchased from
Shanghai GenePharma Co., Ltd. All three cell lines were cultured to
80–90% confluence, and 5×105 cells/sample were
transfected with 50 nM siRNA using Lipofectamine® 2000
reagent (cat. no. 11668-019; Invitrogen; Thermo Fisher Scientific,
Inc.). Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) was performed to measure the expression levels of
HAND2-AS1, and to confirm a <50% reduction prior to subsequent
experiments. Subsequent experiments were performed at 24 h after
transfections. The control group for transfection was
un-transfected cells, and the negative control group was negative
control siRNA-transfected cells.
Glucose uptake assay
A total of 5×105 cells were cultured in
each well of a 6-well plate. Cells were incubated for 24 h and
washed twice with PBS. Glucose uptake was initiated by incubating
cells with Krebs-Ringer-HEPES (KRH) buffer [120 mM NaCl, 25 mM
HEPES (pH 7.4), 1.2 mM MgSO4, 1.3 mM CaCl2, 5
mM KCl and 1.3 mM KH2PO4] containing 1 µCi
[3H]-2-deoxyglucose (PerkinElmer, Inc.) for 25 min at 37°C. Cells
were washed twice with ice-cold KRH buffer to halt glucose uptake,
and lysed using lysis buffer [10 mM Tris-HCl (pH 8.0) and 0.2%
SDS]. Radioactivity was measured using liquid scintillation
spectrometry, and glucose uptake was presented as disintegrations
per min. The experiment was performed in triplicate.
RT-qPCR
TRIzol® reagent was used to extract the
total RNA from tumor tissues, adjacent healthy tissues and the 3
cell lines, MG-63, SAOS-2 and hFOB. The RNA concentration was
measured using a NanoDrop™ 2000 Spectrophotometer
(Thermo Fisher Scientific, Inc.), and samples with an A260/A280
ratio between 1.8 and 2.0 were reverse transcribed. The PrimeScript
RT reagent kit (Takara Bio, Inc.) was used to perform reverse
transcription reactions with poly(T) as the primer. Reaction
conditions were: 25°C for 6 min, 54°C for 20 min and 80°C for 5
min. The SYBR® Green Real-Time PCR Master mix (Thermo
Fisher Scientific, Inc.) was used to prepare the PCR. The sequences
of primers were as follows: HAND2-AS1 forward,
5′-GGGTGTTTACGTAGACCAGAACC-3′ and reverse,
5′-CTTCCAAAAGCCTTCTGCCTTAG-3′; and β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
PCR was conducted using the CFX96 Touch™ Real-Time PCR Detection
system (Bio-Rad Laboratories Inc.). PCR conditions were as follows:
95°C for 40 sec, followed by 40 cycles at 95°C for 15 sec and 55°C
for 45 sec. HAND2-AS1 expression was normalized to β-actin using
the 2−ΔΔCq method (9).
The experiment was performed in triplicate.
Cell proliferation assay
A 96-well plate was seeded at 4×103
cells/well and cultured at 37°C (5% CO2). At the 24-,
48-, 72- and 96-h time points, 10 µl Cell Counting Kit-8 (CCK-8)
solution was added to each well; cells were cultured for a further
4 h, and OD values were measured at 450 nm using the Fisherbrand™
accuSkan™ GO UV/Vis microplate spectrophotometer (Thermo Fisher
Scientific, Inc.). The experiment was performed in triplicate.
Western blot analysis
Radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.) was used to extract the total protein from cells,
and the bicinchoninic acid assay method was used to measure protein
concentration. Proteins were separated using SDS-PAGE with a 10%
gel (30 µg protein/lane), and transferred to PVDF membranes. The
membranes were blocked with 5% skimmed milk in PBS at room
temperature for 1 h, followed by incubation with the following
primary antibodies overnight at 4°C: Rabbit anti-glucose
transporter 1 (GLUT1; 1:2,000; cat. no. ab15309) and anti-GAPDH
(1:1,000; ab8245; all Abcam, Cambridge, UK). The following day, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibody (1:1,000; cat. no. MBS435036; MyBioSource, Inc.)
for 1 h at room temperature. Protein bands were visualized using an
Enhanced Chemiluminescence detection reagent (Sigma-Aldrich; Merck
KGaA), and Image J software, v1.8.0 (National Institutes of Health)
was used to normalize the expression levels of GLUT1 to the
endogenous β-actin control. The experiment was performed in
triplicate.
Statistical analysis
SPSS software version 19.0 (IBM Corp.) was used for
all statistical analyses. Data are expressed as the mean ± standard
deviation. Comparisons between two groups and among multiple groups
were performed using the Student's t-test and one-way analysis of
variance, followed by the Tukey's test, respectively. Count data
were analyzed using the χ2 test. Receiver operating
characteristic (ROC) curve analysis was performed to evaluate the
diagnostic value of HAND2-AS1 serum levels in osteosarcoma, with
patients as true-positive samples and healthy controls as
true-negative samples. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of HAND2-AS1 in the tumor
tissues and adjacent healthy tissues of 48 patients with
osteosarcoma
Expression levels of HAND2-AS1 in the osteosarcoma
tissues and healthy tissues of 48 patients were measured using
RT-qPCR. As illustrated in Fig. 1,
expression levels of HAND2-AS1 were significantly lower in
osteosarcoma tissues compared with those in the adjacent tissues in
41 of the 48 patients (P<0.05). Only 3 patients displayed
significantly lower expression levels of HAND2-AS1 in adjacent
tissues compared with those in osteosarcoma tissues (P<0.05). No
significant differences were observed in the remaining 4
patients.
Comparison and diagnostic values of
HAND2-AS1 serum levels between patients with osteosarcoma and
healthy controls
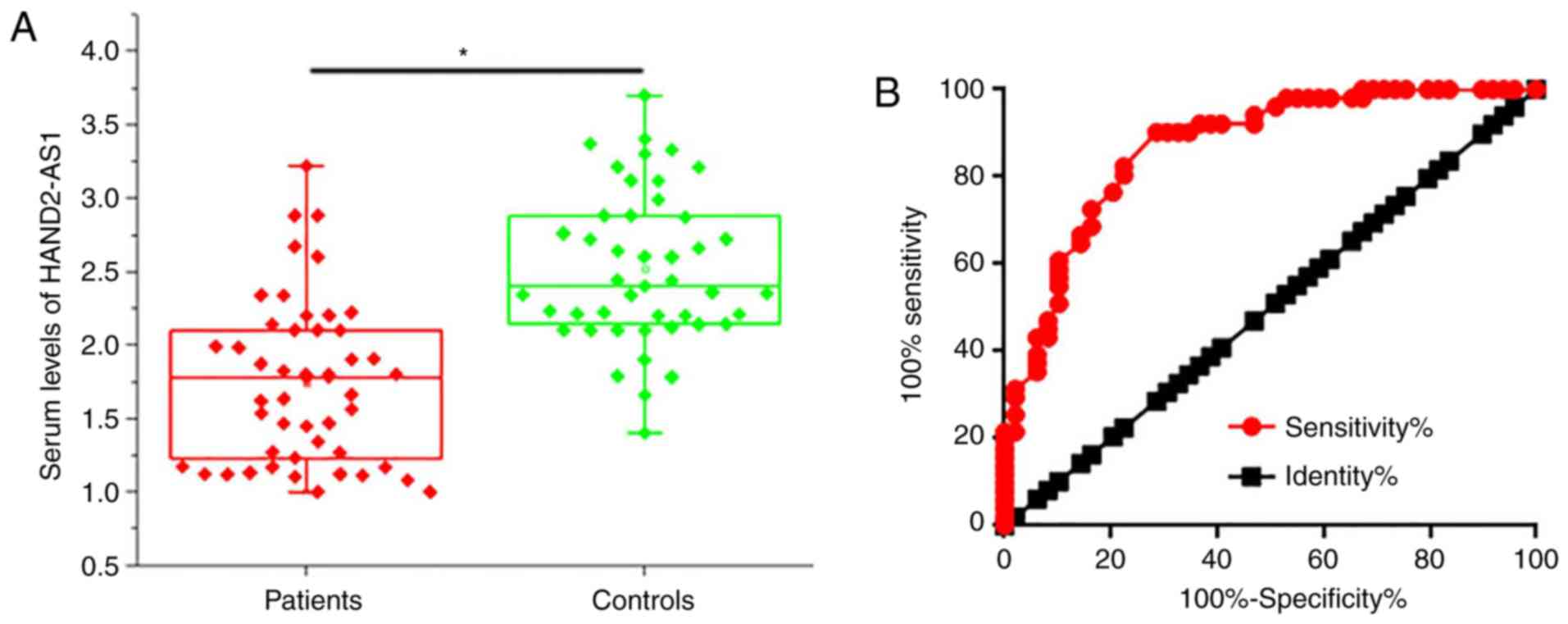
Serum levels of HAND2-AS1 were determined using
RT-qPCR. Serum levels of HAND2-AS1 were significantly higher in
control subjects compared with those in osteosarcoma patients
(P<0.05; Fig. 2A). ROC curve
analysis was performed with osteosarcoma patients as true-positive
and healthy controls as true-negative samples. The area under the
curve (AUC) was 0.8685, with a 95% confidence interval of
0.7989–0.9382 (P<0.0001; Fig.
2B). Therefore, serum HAND2-AS1 level may hold potential
diagnostic value in osteosarcoma.
Correlation between serum levels of
HAND2-AS1 and clinicopathological features
Patients were divided into high and low expression
groups according to the median serum level of HAND2-AS1. The
χ2 test was performed to analyze correlations between
serum levels of HAND2-AS1 and clinicopathological patient data. As
displayed in Table I, there were no
significant correlations between the serum levels of HAND2-AS1 and
patients' age, sex and distant tumor metastasis. However, a
significant correlation was observed between serum levels of
HAND2-AS1 and tumor size.
 | Table I.Correlation between serum levels of
heart and neural crest derivatives expressed 2-antisense RNA 1 and
the clinicopathological data of the patients. |
Table I.
Correlation between serum levels of
heart and neural crest derivatives expressed 2-antisense RNA 1 and
the clinicopathological data of the patients.
| Variable | Group | Cases, n | High expression,
n | Low expression,
n | χ2
value | P-value |
|---|
| Sex | Male | 28 | 12 | 16 | 1.37 | 0.24 |
|
| Female | 20 | 12 | 8 |
|
|
| Age, years | >30 | 26 | 11 | 15 | 1.34 | 0.25 |
|
| <30 | 22 | 13 | 9 |
|
|
| Primary tumor
diameter, cm | >5 | 23 | 17 | 6 | 10.1 | 0.001 |
|
| ≥5 | 25 | 7 | 18 |
|
|
| Tumor distant
metastasis | Yes | 19 | 11 | 8 | 0.78 | 0.38 |
|
| No | 29 | 13 | 16 |
|
|
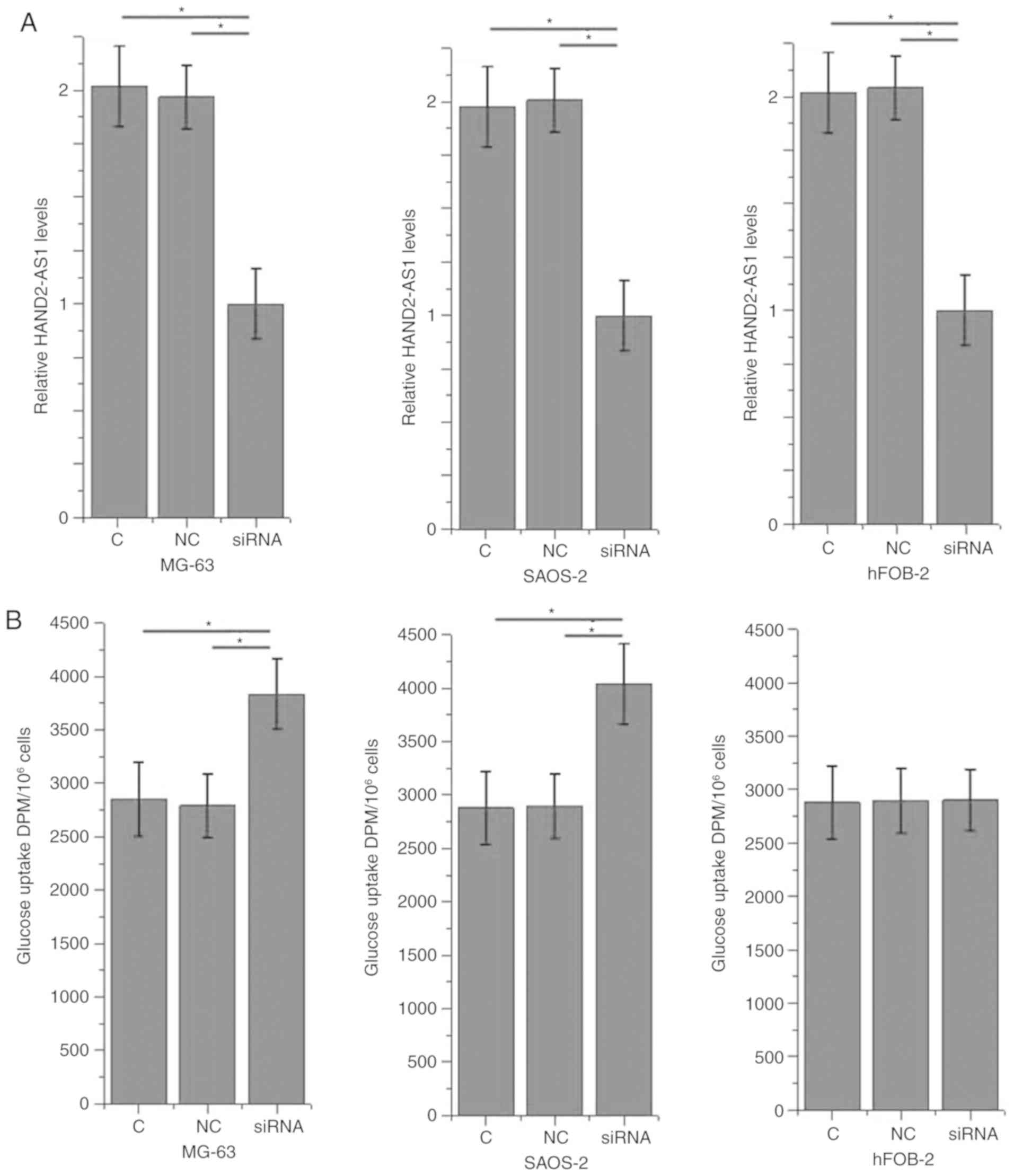
Effects of HAND2-AS1 siRNA silencing
on glucose uptake
The data in Table I
indicate that HAND2-AS1 is associated with tumor growth in
osteosarcoma. Glucose metabolism is critical for the survival and
proliferation of cancer cells (10).
In the present study, the effects of HAND2-AS1 siRNA silencing on
glucose uptake were investigated in osteosarcoma cells. HAND2-AS1
siRNA silencing significantly promoted glucose uptake in the human
osteosarcoma MG-63 and SAOS-2 cell lines (P<0.05; Fig. 3), but not in the normal bone hFOB
cell line.
Effects of HAND2-AS1 siRNA silencing
on GLUT1 expression
Compared with that in normal bone cells (hFOB),
HAND2-AS1 expression was significantly downregulated (Fig. 4A), while GLUT1 protein was
significantly upregulated (Fig. 4B),
in the osteosarcoma MG-63 and SAOS-2 cell lines. HAND2-AS1 siRNA
silencing led to significantly upregulated expression of GLUT1 in
MG-63 and SAOS-2 cells (P<0.05), but not in hFOB cells (Fig. 4C).
 | Figure 4.Effects of HAND2-AS1 siRNA silencing
on GLUT1 expression. The effects of HAND2-AS1 siRNA silencing on
GLUT1 expression were investigated by western blotting. Compared
with normal bone hFOB cells, in MG-63 and SAOS-2 cells, (A) The
non-coding RNA, HAND2-AS1 was significantly downregulated, while
(B) GLUT1 expression was significantly upregulated. (C) HAND2-AS1
siRNA silencing led to significantly upregulated expression of
GLUT1 in MG-63 and SAOS-2 cells, but not in hFOB cells. *P>0.05.
n=3. HAND2-AS1, heart and neural crest derivatives expressed
2-antisense RNA 1; GLUT1, glucose transporter 1; siRNA, small
interfering RNA; C, control; NC, negative control. |
Effects of HAND2-AS1 siRNA silencing
on cell proliferation
The proliferation rates of the 3 cell lines were
detected using the CCK-8 assay. HAND2-AS1 siRNA silencing
significantly promoted the proliferation of the human osteosarcoma
MG-63 and SAOS-2 cell lines (P<0.05; Fig. 5), but not the cells of the normal
bone cell line, hFOB.
Discussion
In the present study, a novel lncRNA with
characterized functionality in endometrioid endometrial carcinoma,
and the growth of osteosarcoma tumors, was reported. It was also
observed that the action of this lncRNA in osteosarcoma is likely
achieved by inhibiting osteosarcoma cell proliferation through
disruption of glucose metabolism. This highlighted a potential
therapeutic target for osteosarcoma.
The pathogenesis of osteosarcoma is influenced by
numerous lncRNAs and their varied roles in the onset, development
and progression of cancer. Upregulation of lncRNA-highly
upregulated in liver cancer (HULC) was previously observed in
osteosarcoma tissues compared with that in adjacent healthy
tissues, and reducing the expression level of lncRNA HULC was
suggested to be a potential treatment for osteosarcoma (11). Overexpression of nuclear paraspeckle
assembly transcript 1 is involved in the development of drug
resistance in osteosarcoma cells. Therefore, knockdown of this
oncogenic lncRNA may improve the outcome of drug treatment in
osteosarcoma (12). By contrast,
growth arrest specific 5 is considered to be a tumor suppressor
lncRNA and displays a downregulated expression pattern in
osteosarcoma (13). lncRNA HAND2-AS1
is a recently identified lncRNA with decreased expression levels in
endometrioid endometrial carcinoma tissues (7), indicating its potential role as a tumor
suppressor in this disease. In the present study, significantly
lower expression levels of lncRNA HAND2-AS1 in tumor tissues
(compared with adjacent healthy tissues) was observed in the
majority of patients with osteosarcoma, indicating its potential
role as a tumor suppressor.
The survival rate of osteosarcoma patients with
distant tumor metastasis is low, and an increase in the early
diagnostic rate is required to improve treatment outcomes. The
blood not only transports nutrients around the body, but also
delivers signaling molecules, and the development of human diseases
is usually accompanied by alterations in certain blood constituents
(14,15). Detecting these alterations in the
blood may provide guidance for the treatment of human diseases
(16,17). In the present study it was observed
that serum levels of lncRNA HAND2-AS1 were significantly lower in
osteosarcoma patients compared with those in healthy controls. ROC
curve analysis also revealed that serum HAND2-AS1 may be used to
effectively distinguish osteosarcoma patients from healthy
individuals. These data suggest that serum HAND2-AS1 may serve as a
potential diagnostic marker for osteosarcoma. It is worth noting
that HAND2-AS1 is a recently identified lncRNA with an unknown
expression pattern in the majority of human diseases. Therefore,
multiple markers may be used to improve diagnostic accuracy.
The present study revealed an association between
HAND2-AS1 and tumor growth, but not metastasis. Glucose metabolism
provides energy for all cell types, although abnormal glucose
metabolism is a unique marker of cancerous cells (6). HAND2-AS1 siRNA silencing significantly
promoted glucose uptake in 2 osteosarcoma cell lines, indicating
that HAND2-AS1 may be an inhibitor of glucose uptake in
osteosarcoma. As a key component of glucose metabolism, GLUT1 is
ordinarily upregulated in cancer cells (18). HAND2-AS1 siRNA silencing
significantly promoted the expression of GLUT1, and also promoted
proliferation in 2 osteosarcoma cell lines. The data suggest that
HAND2-AS1 may inhibit the proliferation of osteosarcoma cells by
targeting glucose uptake through the downregulation of GLUT1.
Additionally, HAND2-AS1 siRNA silencing had no
significant effects on cells of the normal bone hFOB cell line.
Therefore, HAND2-AS1 may serve as a potential therapeutic target
for osteosarcoma specifically.
In conclusion, HAND2-AS1 is downregulated in
osteosarcoma and may inhibit the growth of osteosarcoma tumors
through its interaction with glucose metabolism.
Acknowledgements
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
SC, XX, SL and BH were responsible for the
conception and design of the study. SC and XX performed the
experiments. SC, XX, SL and BH analyzed and interpreted the data.
SC and XX drafted the article. SC, XX, SL and BH were responsible
for the revision of the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The protocols of the present study were approved by
the Ethics Review Committee of Jingzhou Central Hospital (Jingzhou,
China). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Botter SM, Neri D and Fuchs B: Recent
advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu C and Jiang DL: Expression of POSTN
mRNA is associated with osteosarcoma prognosis. Int J Clin Exp
Pathol. 9:10664–10669. 2016.
|
|
5
|
Li J, Liu L, Li J, Chen Y, Jiang XW,
Ouyang YR, Liu YQ, Zhong H, Li H and Xiao T: Microarray expression
profile of long noncoding RNAs in human osteosarcoma. Biochem
Biophys Res Commun. 433:200–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hay N: Reprogramming glucose metabolism in
cancer: Can it be exploited for cancer therapy? Nat Rev Cancer.
16:635–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Wang CC, Lee WYW, Trovik J, Chung
TKH and Kwong J: Long non-coding RNA HAND2-AS1 inhibits invasion
and metastasis in endometrioid endometrial carcinoma through
inactivating neuromedin U. Cancer Lett. 413:23–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng X, Han H, Liu GP, Ma YX, Pan RL,
Sang LJ, Li RH, Yang LJ, Marks JR, Wang W and Lin A: LncRNA wires
up Hippo and Hedgehog signaling to reprogramme glucose metabolism.
EMBO J. 36:3325–3335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boroughs LK and DeBerardinis RJ: Metabolic
pathways promoting cancer cell survival and growth. Nat Cell Biol.
17:351–359. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong D and Wang Y: Knockdown of lncRNA
HULC inhibits proliferation, migration, invasion and promotes
apoptosis by sponging miR-122 in osteosarcoma. J Cell Biochem.
119:1050–1061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Yang Q, Wang L, Wang S, Sun F, Xu D
and Jiang J: Knockdown of the oncogene LncRNA NEAT1 restores the
availability of miR-34c and improves the sensitivity to
cisplatin in osteosarcoma. Biosci Rep. 38(pii): BSR201803752018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y and Kong D: LncRNA GAS5 represses
osteosarcoma cells growth and metastasis via sponging miR-203a.
Cell Physiol Biochem. 45:844–855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawashima M, Yamamura M, Taniai M,
Yamauchi H, Tanimoto T, Kurimoto M, Miyawaki S, Amano T, Takeuchi T
and Makino H: Levels of interleukin-18 and its binding inhibitors
in the blood circulation of patients with adult-onset Still's
disease. Arthritis Rheum. 44:550–560. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yaman Agaoglu F, Kovancilar M, Dizdar Y,
Darendeliler E, Holdenrieder S, Dalay N and Gezer U: Investigation
of miR-21, miR-141 and miR-221 in blood circulation of patients
with prostate cancer. Tumour Biol. 32:583–588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sabbisetti VS, Waikar SS, Antoine DJ,
Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee
M, et al: Blood kidney injury molecule-1 is a biomarker of acute
and chronic kidney injury and predicts progression to ESRD in type
I diabetes. J Am Soc Nephrol. 25:2177–2186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao M, Zhou Y, Zhu B, Wan M, Jiang T, Tan
Q, Liu Y, Jiang J, Luo S, Tan Y, et al: IFI44L promoter methylation
as a blood biomarker for systemic lupus erythematosus. Ann Rheum
Dis. 75:1998–2006. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kunkel M, Reichert TE, Benz P, Lehr HA,
Jeong JH, Wieand S, Bartenstein P, Wagner W and Whiteside TL:
Overexpression of Glut-1 and increased glucose metabolism in tumors
are associated with a poor prognosis in patients with oral squamous
cell carcinoma. Cancer. 97:1015–1024. 2003. View Article : Google Scholar : PubMed/NCBI
|