Introduction
Among the multimodal therapies, surgical resection
of primary tumors with the involved lymph nodes (LNs) offers the
best cure for patients with esophageal squamous cell carcinoma
(ESCC). Although the necessity of extensive LN dissection (LND)
remains debatable, the National Comprehensive Cancer Network (NCCN)
guidelines (1) and the Union for
International Cancer Control (UICC) staging manual (2) recommend that at least 12–15 nodes
should be removed. Furthermore, subsequent to weighing the benefits
and harm of radical lymphadenectomy, the 7th edition of the
American Joint Committee on Cancer (AJCC) suggests resecting as
many regional LNs as possible (3).
Additionally, numerous studies recommend an extensive removal of
6–30 LNs for survival improvement (4–9).
However, these studies and clinical guidelines focus on the extent
of LND or the total number of harvested LNs (HLNs). To the best of
our knowledge, no specifications have been made regarding the exact
stations of the HLNs, or the number of removed nodes from the
individual LN stations.
The total count of HLNs, alone, cannot provide the
full information of lymphadenectomy (10,11). The
association between nodal counts and survival can be modified
according to the type of lymphadenectomy performed. According to
previous study, the survival of patients with ESCCs undergoing en
bloc resection is significantly improved when compared with those
receiving transhiatal or transthoracic dissection, even with the
same threshold of 23 nodes (5).
Additionally, the association between higher negative LN counts and
improved prognosis was observed in patients undergoing 3-field LND
(3-FLND) but not 2-FLND (12).
Therefore, it is reasonable to extend the definition
of adequate LND (ALND) to optimize prognosis beyond total HLN
counts. In the present study, a novel individualized ALND strategy
was proposed for optimizing ESCC prognoses, which provided the
number of HLNs and considered the tumor location and the metastatic
status of LN zones.
Materials and methods
Patients
Between January 2009 and December 2013, patients
with ESCC who underwent curative esophagectomy at two independent
centers (Department of Thoracic Surgery, Affiliated Zhangzhou
Hospital of Fujian Medical University and Department of Thoracic
Surgery, An Xi Hospital) were enrolled in the present study
(Table I). All patients received
preoperative computed tomography (CT) and esophagoscopic biopsy
followed by pathological diagnosis. Positron emission tomography
(PET) was exclusively performed on suspicious stage-IV patients. If
patients met any of the exclusion criteria they were excluded from
the present study. The following exclusion criteria were used: i)
The patient had non-squamous cell carcinoma; ii) the patient had
undergone pre-operative chemotherapy or radiotherapy; iii) the
patient presented with distant metastasis; iv) the patient had a
postoperative survival time of <30 days; v) the patient had
non-primary esophageal carcinoma; and vi) the patient had <6
HLNs. According to the 6th UICC recommendation (13), a minimum number of 6 LNs need to be
resected in order to ensure accurate pN staging. Patients who
survive <30 days are likely to succumb to surgical
complications, which does not agree with the purpose of the present
study. Therefore, individuals whose survival time was <30 days
were excluded. A total of 350 consecutive patients with ESCC were
included in the cohort of the present study, 260 from Zhang Zhou
Hospital (14) and 90 from An Xi
Hospital.
 | Table I.Associations of demographic, clinical
and pathological characteristics with LND. |
Table I.
Associations of demographic, clinical
and pathological characteristics with LND.
|
| Total (n=350) | HLNs |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | n | % | M (P25,
P75) | P-value |
|---|
| Age (years) |
|
| 0.002a | 0.967b |
| Median
(P25, P75) | 60 (53, 67) |
|
|
|
| Sex |
|
|
| 0.109c |
|
Male | 259 | 74.0 | 30 (20, 43) |
|
|
Female | 91 | 26.0 | 29 (18, 38) |
|
| Tumor location |
|
|
| 0.223d |
|
CE/UTE | 48 | 13.7 | 24 (15, 39) |
|
|
MTE | 223 | 63.7 | 30 (20, 42) |
|
|
LTE | 79 | 22.6 | 30 (21, 41) |
|
| Tumor length
(cm) |
|
| 0.067a | 0.220b |
| Median
(P25, P75) | 4.0 (3.0, 4.5) |
|
|
|
| Primary tumor |
|
|
| 0.343d |
|
pT1 | 42 | 12.0 | 26 (15, 45) |
|
|
pT2 | 64 | 18.3 | 29 (18, 39) |
|
|
pT3 | 215 | 61.4 | 30 (21, 42) |
|
|
pT4 | 29 | 8.3 | 33 (24, 38) |
|
| Regional lymph
nodes |
|
|
| 0.003d |
|
pN0 | 174 | 49.7 | 27 (17, 39) |
|
|
pN1 | 84 | 24.0 | 32 (24, 42) |
|
|
pN2 | 68 | 19.4 | 31 (23, 41) |
|
|
pN3 | 24 | 6.9 | 39 (28, 47) |
|
| Histologic
grade* |
|
|
| 0.003d |
|
pG1 | 139 | 41.5 | 27 (17, 37) |
|
|
pG2 | 175 | 52.2 | 33 (23, 42) |
|
|
pG3 | 21 | 6.3 | 35 (26, 44) |
|
| Tumor stage |
|
|
| 0.006d |
| 0 | 3 | 0.9 | 15 (11, 56) |
|
| IA | 11 | 3.1 | 27 (10, 37) |
|
| IB | 41 | 11.7 | 24 (16, 39) |
|
|
IIA | 63 | 18.0 | 24 (17, 35) |
|
|
IIB | 70 | 20.0 | 33 (19, 44) |
|
|
IIIA | 71 | 20.3 | 32 (24, 42) |
|
|
IIIB | 50 | 14.3 | 29 (21, 46) |
|
|
IIIC | 41 | 11.7 | 35 (27, 44) |
|
| Skip
LNM# |
|
Yes | 90 | 51.1e | 32 (24, 44) | 0.499c |
| No | 86 | 48.9e | 33 (25, 44) |
|
| LVI |
|
|
|
<0.001c |
|
Yes | 72 | 20.6 | 36 (27, 50) |
|
| No | 278 | 79.4 | 28 (18, 39) |
|
| PNI |
|
|
|
<0.001c |
|
Yes | 62 | 17.7 | 40 (30, 52) |
|
| No | 288 | 82.3 | 27 (18, 38) |
|
| Fields of
lymphadenectomy |
|
|
|
<0.001c |
|
3-FLND | 185 | 52.9 | 35 (26, 47) |
|
|
2-FLND | 165 | 47.1 | 23 (17, 34) |
|
| Residual tumor |
|
|
| 0.998d |
| Rx | 9 | 2.6 | 30 (18, 36) |
|
| R0 | 337 | 96.3 | 29 (20, 41) |
|
| R1 | 4 | 1.1 | 25 (24, 41) |
|
| Positive lymph
nodes |
|
| 0.187a |
<0.001b |
| Median
(P25, P75) | 1 (0, 3) |
|
|
|
Baseline demographic information regarding the
patients with ESCC was collected on admission. The clinical and
pathological traits were recorded during hospitalization, and
postoperative radiotherapy and/or chemotherapy was also documented.
All pathological diagnoses made prior to 2010, including tumor
location, primary tumor (T stage), regional LNs (N stage),
histological grade (G stage) and TNM, were revised according to the
7th edition of the AJCC Cancer Staging System (15).
Follow-up
All patients were followed-up every 3 months in the
first 2 postoperative years and every 6 months thereafter. The last
follow-up was conducted in May 2016. Information regarding patient
mortality was confirmed by contacting the patient's family or
retrieving the information from the local mortality registration
department. The date of death or the last successful contact was
recorded as the last follow-up date. Patients who were still alive
at the last follow-up or with whom contact had been lost were coded
as censored. Overall survival (OS) of the patients was defined as
the time interval between the date of surgery and the date of the
last follow-up.
Local and distal LN zones
In order to alleviate the impacts from different
staging system, all lymph nodes documented with the Japan
Esophageal Society LN codes were transformed into the 7th AJCC LN
stations according to a report by Niwa et al (16) (Fig.
1A). Briefly, the supraclavicular and other deep cervical LNs
were grouped as the cervical LN zone; the left or right upper
paratracheal, anterior mediastinal, posterior mediastinal, left or
right lower paratracheal along with aorticopulmonary LNs were
categorized as the upper LN zone; the subcarinal, left or right
tracheobronchial and middle paraesophageal LNs were classified as
the middle LN zone; the lower paraesophageal, pulmonary ligament
and diaphragmatic LNs belonged to the lower LN zone; and LNs
located in celiac regions (paracardial, left gastric, common
hepatic, splenic and celiac LNs) were grouped as the celiac LN
zone. All LN zones anatomically situated nearer to or across the
center of the tumor location were grouped as local LN zones,
whereas distant LNs were referred to as the distal LN zones
(Fig. 1B). Skip LN metastases (SLNM)
were defined as the metastatic LN station situated in the distal LN
zones with the local LN zones free of tumor infiltration.
CT scanning
CT scans were performed using a LightSpeed scanner
(GE Healthcare). All patients were in the supine position and the
scan images were obtained from the level of the lower neck to upper
abdomen according to the following scanning protocols: 64×0.625
mm2 collimation, 0.984 pitch, 5 mm slice width, 1.25–2.5
mm reconstruction increment, 1.25–2.5 mm slice spacing, 60–100 ml
injection of intravenous contrast medium at a rate of 2.0–3.0 ml/s
at 12 kV and 50–600 mA.
Surgical and lymphadenectomy
procedure
The tri-incisional cervico-thoraco-abdominal
procedure (McKeown type) has been adopted as a standard surgical
approach (17). In the thoracic
stage, esophagectomy and mediastinal lymphadenectomy (including the
LNs located in the upper, middle and lower thoracic zones; Fig. 1A) were conducted via right-sided
posterolateral thoracotomy. In the abdominal stage, midline
laparotomy was conducted and followed by stomach mobilization,
gastric tube creation and celiac node resection (station 16–20;
Fig. 1A). In the cervical stage, the
gastric tube was pulled up to the neck through the retrosternal or
posterior mediastinal route. Subsequently, anastomosis of the
alimentary tract was performed via left-sided cervicotomy. Cervical
LND was not systematically undertaken for all patients. Cervical
LND was adopted for patients who met the following criteria: i) The
short radius of cervical LNs from the CT scan was >1 cm; or ii)
the ratio of the short to long radius was <0.8. Patients
receiving cervico-thoraco-abdominal LND were recorded as 3-FLND,
and 2-FLND referred to thoracoabdominal node resection. The LNs
located in the upper, middle, lower and celiac zones were dissected
systematically (Fig. 1A).
Statistical analysis
Sample size needed for the Cox proportional hazard
regression model was calculated according to the formula proposed
by Hsieh et al (18). The
estimated hazard ratio (HR) for ALND was 0.75, the overall event
rate in the present study was 0.449, and the statistical power was
set at 0.80 with a type I error rate of 0.05. The required total
sample size could be approximated at 330.
Due to the deviated distribution of the HLNs,
median, 25th and 75th percentiles were adopted in the present
study. Mann-Whitney U tests or Kruskal-Wallis H tests were used to
compare the median number of HLNs in the categorical groups. The
Benjamini-Hochberg corrections were applied for repeated
comparisons between two independent groups. The post hoc Bonferroni
corrections were used for examining pair-wise differences following
Kruskal-Wallis tests. Spearman correlation coefficients
(rs) were applied to evaluate the association between
HLNs and continuous variables, including age, tumor length and
number of positive LNs (PLNs).
The survival of patients with ESCC was calculated
using the Kaplan-Meier method. HLNs were divided into four
categories according to quartiles (<20, 20–29, 30–40 and
>40). The association between quartered HLNs and OS was
evaluated using the log-rank test. The percentage of total HLNs in
local zones was calculated by dividing the number of HLNs in the
local zones by the total number of HLNs. In order to determine the
optimal cut-points of local HLN percentages for maximum OS
difference, the X-tile algorithm was used (19). For N+ cases, LN ratios
(LNRs) were computed as the ratio of PLNs to HLNs. Locally weighted
smoothing scatter plot (LOESS) curves were plotted to identify the
thresholds of HLNs at the inflection points on the curves.
Prior to Cox regression analysis, the variables were
investigated for collinearity, and the variance inflation factor
threshold was set at 3. The proportional hazards assumption was
assessed using Schoenfeld residuals (20). Multivariate Cox regression analysis
was performed to verify the therapeutic values of ALND while the
other confounders were controlled, including sex, age, tumor
location, tumor length, regional LNs (N stage), depth of tumor
invasion (T stage), histological grade (G stage), perineural
lymphatic vascular invasion (PNLVI), chemoradiotherapy (CRT) and
medical centers. The HR and the corresponding 95% CI were used to
express the protective effect of ALND. Furthermore, stratified
analyses were performed for well-established prognostic factors,
including PNLVI, T stage, N stage, G stage, TNM, CRT, fields of LND
and SLNM, to verify the prognostic significance of ALND within each
stratum.
The statistical analyses were conducted using SPSS
version 19.0 (IBM Corp.). All statistical tests performed were
two-tailed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Lymphadenectomy of 350 patients with
ESCC
Details of demographic, clinical and pathological
characteristics are summarized in Table
I. The median value of HLNs was 29, with the lower and upper
quartile at 20 and 41. The total number of HLNs was correlated with
the count of PLNs (rs=0.187, P<0.001). Patients
undergoing 3-FLND had significantly more LNs resected when compared
with those receiving 2-FLND (P<0.001). Factors such as
lymphovascular invasion, perineural invasion, pG, pN and TNM
classification were associated with HLNs (P<0.05; Table I, Supplementary Table I).
There is no association between the
total number of HLNs and OS in patients with ESCC
The median follow-up duration was 1321 days. The
5-year OS rate of patients with ESCC was 54% (95% CI, 49–60%). A
higher count of HLNs was not identified to be associated with
improved OS (P=0.254; Fig. 2A).
Furthermore, stratified analyses based on T stage (Fig. 2B and C) and N stage (Fig. 2D and E) also yielded non-significant
results (P=0.743, P=0.534, P=0.396 and P=0.818 for T1-2, T3-4, N0
and N+ cases, respectively).
Selective lymphadenectomy based on
tumor locations is associated with improved survival of N0
patients
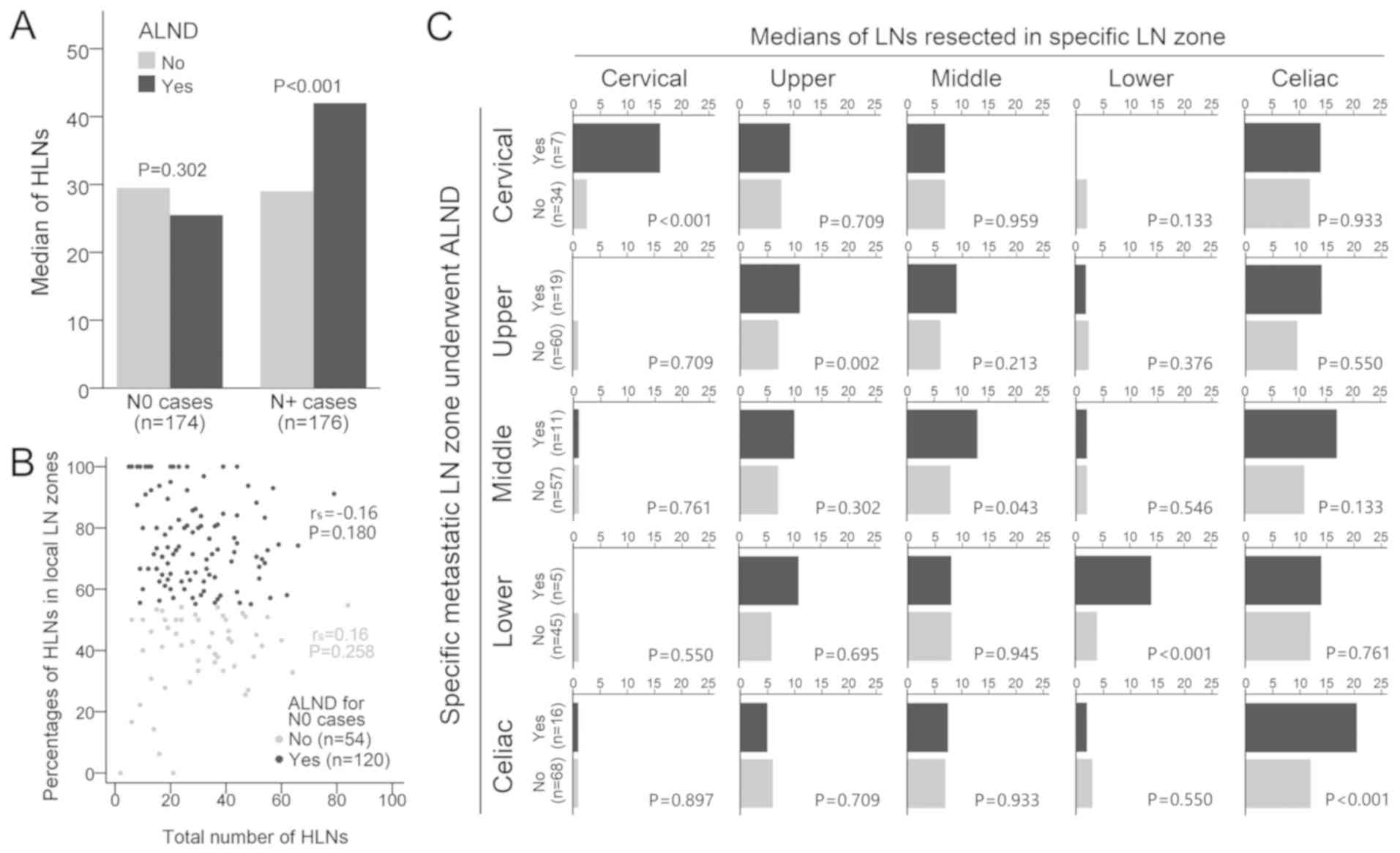
For all cases, more LNs were harvested in the local
zones compared with the distal zones, regardless of tumor location
and metastatic status (P<0.05; Fig.
3A and B). These findings suggested a surgical preference to
dissect LNs in regions near the tumor location rather than far from
it. The optimal cut-off point for the percentages of HLNs in the
local LN zones to maximize survival differences in N0 patients was
set at 55% using the X-tile algorithm (P=0.011; Fig. 3C). However, no association was
observed in N+ patients (P=0.846; Fig. 3D).
Thresholds of HLNs from the metastatic
LN zones in N+ patients
For N+ patients, surgeons preferred to
dissect more LNs in the specific LN zone when metastasis was
evident (P<0.05; Table II) For
example, when the cervical LN zones were involved, surgeons would
resect more LNs in the cervical zone compared with in the
uninvolved area in the same zone (P<0.001; Table II). A similar dissection preference
was also observed in other LN zones (P<0.05; Table II), except for the nodes in celiac
zones.
 | Table II.Association of HLNs in specific LN
zones with metastases status for N+ patients
(n=176). |
Table II.
Association of HLNs in specific LN
zones with metastases status for N+ patients
(n=176).
|
| HLNs in specific LN
zones |
|---|
|
|
|
|---|
|
| Cervical | Upper | Middle | Lower | Celiac |
|---|
|
|
|
|
|
|
|
|---|
| LN zones metastases
status | M
(P25,P75) | M
(P25,P75) | M
(P25,P75) | M
(P25,P75) | M
(P25,P75) |
|---|
| Cervical |
| No
(n=135) | 0 (0, 2) | 7 (4, 11) | 8 (4, 11) | 2 (1, 4) | 12 (6, 18) |
| Yes
(n=41) | 3 (2, 7) | 6 (4, 13) | 7 (4, 12) | 2 (1, 5) | 12 (5, 17) |
|
P-valuea | <0.001 | 0.969 | 0.986 | 0.986 | 0.957 |
| Upper |
| No
(n=97) | 1 (0, 3) | 5 (2, 11) | 8 (4, 12) | 2 (1, 5) | 13 (8, 18) |
| Yes
(n=79) | 1 (0, 2) | 9 (5, 12) | 7 (4, 11) | 2 (1, 4) | 11 (5, 15) |
|
P-valuea | 0.478 | 0.009 | 0.784 | 0.851 | 0.131 |
| Middle |
| No
(n=108) | 1 (0, 3) | 6 (3, 11) | 7 (4, 11) | 2 (1, 5) | 12 (7, 18) |
| Yes
(n=68) | 1 (0, 2) | 8 (4, 11) | 9 (5, 13) | 2 (1, 4) | 12 (6, 17) |
|
P-valuea | 0.784 | 0.478 | 0.030 | 0.851 | 0.784 |
| Lower |
| No
(n=126) | 1 (0, 2) | 7 (4, 11) | 8 (4, 12) | 2 (0, 3) | 12 (6, 17) |
| Yes
(n=50) | 1 (0, 3) | 7 (2, 12) | 8 (4, 11) | 4 (3, 7) | 13 (6, 16) |
|
P-valuea | 0.986 | 0.969 | 0.862 | <0.001 | 0.969 |
| Celiac |
| No
(n=92) | 1 (0, 3) | 7 (4, 12) | 8 (4, 12) | 2 (1, 4) | 10 (4, 17) |
| Yes
(n=84) | 1 (0, 2) | 6 (3, 11) | 7 (4, 11) | 2 (1, 5) | 12 (8, 18) |
|
P-valuea | 0.280 | 0.243 | 0.604 | 0.933 | 0.243 |
Scatter plots were applied to depict the association
between the HLNs and LNRs in N+ patients. By identifying
the inflection points on the LOESS curves, the thresholds for ALND
were set at 8, 8, 8, 8 and 16 for cases with cervical, upper,
middle, lower and celiac metastases, respectively (Fig. 4A-E). Metastatic patients who received
ALND exhibited improved survival compared with those who did not
(P=0.009; Fig. 4F).
 | Figure 4.Thresholds for defining ALND in
N+ patients. Thresholds for ALND were identified as the
inflection points on LOESS curves to identify the corresponding
values on the horizontal axis, which indicated that the HLNs were
(A) 8, (B) 8, (C) 8, (D) 8 and (E) 16 for cases with cervical,
upper, middle, lower and celiac zone metastases, respectively. (F)
Improved overall survival was observed in N+ patients
who received ALND (log-rank, P=0.009). ALND, adequate lymph node
dissection; HLN, harvested lymph node; LNR, lymph node ratio;
LOESS, locally weighted smoothing scatter. |
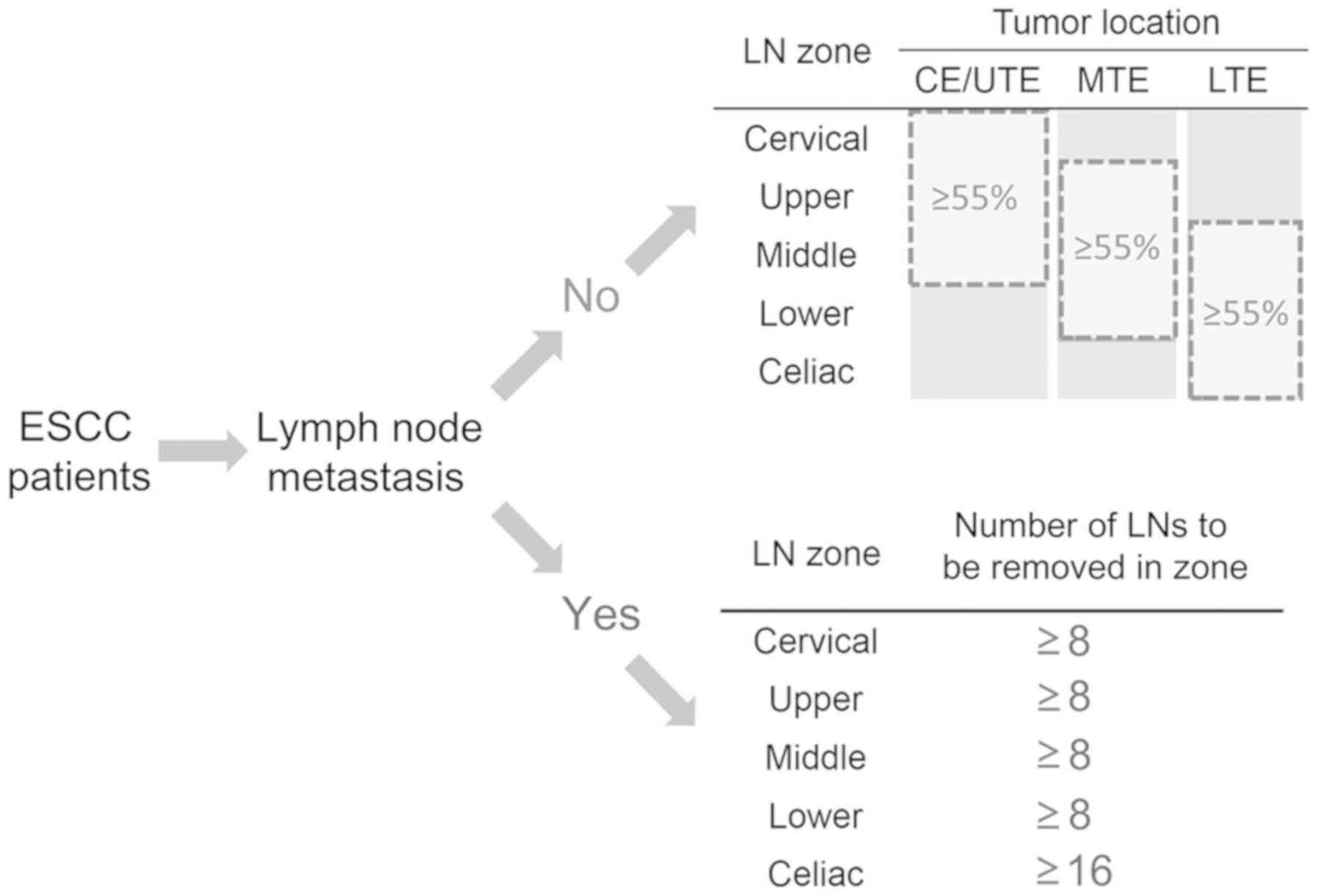
Definition of ALND beyond HLNs
According to the aforementioned analyses, ALND was
designated as an LND strategy that considered tumor location and
metastatic nodal zones (Fig. 5). For
N0 patients, ALND was defined as a resection of >55% of the LNs
distributed in the LN zones adjacent to the tumor location (local
LN zones). For N+ patients, ALND was defined as a
sufficient LN resection from the involved LN zones. For instance,
for N+ patients with metastases in the cervical and
celiac zones, ALND could be achieved by resecting at least 8 and 16
nodes in the two zones, respectively. Other uninvolved nodal zones
were subjected to standard lymphadenectomy.
For N0 patients, those who received ALND did not
yield more LNs compared with those who did not receive ALND
(P=0.302; Fig. 6A). Furthermore, the
percentages of HLNs in the local LN zones were not correlated with
the total number of HLNs, regardless of whether they received ALND
(rs=−0.16, P=0.180; Fig.
6B) or not (rs=0.16, P=0.258; Fig. 6B). However, N+ patients
that underwent ALND yielded more LNs compared with those without
ALND (P<0.001; Fig. 6A). A higher
count of HLNs in the ALND group was primarily due to more
aggressive resection in the metastatic nodal zones, but not in the
uninvolved zones (P<0.05; Fig.
6C). For example, when lymph node metastasis (LNM) was detected
in the celiac zone, in order to achieve ALND, surgeons would resect
more LNs in the abdomen only (P<0.001; Fig. 6C).
ALND is associated with improved
survival in patients with ESCC
Since several factors were associated with ALND
(tumor location, pT stage, pN stage, fields of LND and CRT, all
P<0.05; Table III), stratified
Cox regressions were performed with these factors and other
well-established prognostic factors, such as PNLVI, pG and the
presence of SLNMs. The therapeutic values of ALND were confirmed
for all cases with the exception of pT1-2 cases (HR=0.42, 95%
CI=0.15–1.18, P=0.100, Model 3; Table
IV). When using the whole cohort (Model 15; Table IV), ALND was associated with
improved survival (HR=0.45, 95% CI=0.30–0.66, P<0.001). For
cases with ≥15 HLNs (adequately staged ESCCs), ALND was associated
with improved survival in N0 (HR=0.45, 95% CI=0.20–0.97, P=0.043)
and N+ patients (HR=0.41, 95% CI=0.22–0.75, P=0.004;
Table V).
 | Table III.Associations of demographic, clinical
and pathological characteristics with ALND. |
Table III.
Associations of demographic, clinical
and pathological characteristics with ALND.
|
| ALND |
|
|---|
|
|
|
|
|---|
|
| No (n=183) | Yes (n=167) |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | n |
| % | n |
| % | P-value |
|---|
| Age (years) |
|
|
|
|
|
| 0.549a |
| Median
(P25, P75) |
| 60 (53, 67) |
|
| 59 (53, 66) |
|
|
| Sex |
|
|
|
|
|
| 0.729b |
|
Male | 134 |
| 73.2 | 125 |
| 74.9 |
|
|
Female | 49 |
| 26.8 | 42 |
| 25.1 |
|
| Tumor location |
|
|
|
|
|
|
<0.001b |
|
CE/UTE | 24 |
| 13.1 | 24 |
| 14.4 |
|
|
MTE | 134 |
| 73.2 | 89 |
| 53.3 |
|
|
LTE | 25 |
| 13.7 | 54 |
| 32.3 |
|
| Tumor length
(cm) |
|
|
|
|
|
| 0.295a |
| Median
(P25, P75) |
| 4.0 (3.0, 5.0) |
|
| 4.0 (3.0, 5.0) |
|
|
| pT |
|
|
|
|
|
| 0.015b |
|
pT1 | 22 |
| 12.0 | 20 |
| 12.0 |
|
|
pT2 | 22 |
| 12.0 | 42 |
| 25.1 |
|
|
pT3 | 122 |
| 66.7 | 93 |
| 55.7 |
|
|
pT4 | 17 |
| 9.3 | 12 |
| 7.2 |
|
| pN |
|
|
|
|
|
|
<0.001b |
|
pN0 | 54 |
| 29.5 | 120 |
| 71.8 |
|
|
pN1 | 55 |
| 30.1 | 29 |
| 17.4 |
|
|
pN2 | 52 |
| 28.4 | 16 |
| 9.6 |
|
|
pN3 | 22 |
| 12.0 | 2 |
| 1.2 |
|
| pG* |
|
|
|
|
|
| 0.216b |
|
pG1 | 68 |
| 37.8 | 71 |
| 45.8 |
|
|
pG2 | 98 |
| 54.4 | 77 |
| 49.7 |
|
|
pG3 | 14 |
| 7.8 | 7 |
| 4.5 |
|
| Skip
LNM# |
| No | 67 |
| 51.9 | 19 |
| 40.4 | 0.176b,c |
|
Yes | 62 |
| 48.1 | 28 |
| 59.6 |
|
| PNLVI |
|
|
|
|
|
| 0.133b |
| No | 126 |
| 68.9 | 127 |
| 76.0 |
|
|
Yes | 57 |
| 31.1 | 40 |
| 24.0 |
|
| Fields of LND |
|
|
|
|
|
| 0.047b |
|
3-FLND | 77 |
| 42.1 | 88 |
| 52.7 |
|
|
2-FLND | 106 |
| 57.9 | 79 |
| 47.3 |
|
| CRT |
|
|
|
|
|
| 0.012b |
| No | 84 |
| 45.9 | 99 |
| 59.3 |
|
|
Yes | 99 |
| 54.1 | 68 |
| 40.7 |
|
| HLNs |
|
|
|
|
|
| 0.835a |
| Median
(P25, P75) |
| 29 (21, 40) |
|
| 29 (19, 44) |
|
|
 | Table IV.Stratified analysis of the
therapeutic benefits of ALND. |
Table IV.
Stratified analysis of the
therapeutic benefits of ALND.
|
|
| ALND |
|---|
|
|
|
|
|---|
| Modela | Stratified
factors | Hazard
ratiob | (95% CI) | P-value |
|---|
| PNLVI |
| 1 | No (n=253) | 0.52 | (0.31, 0.88) | 0.014 |
| 2 | Yes (n=97) | 0.26 | (0.13, 0.52) | <0.001 |
| Primary tumor |
| 3 | pT1-2 (n=106) | 0.42 | (0.15, 1.18) | 0.100 |
| 4 | pT3-4 (n=244) | 0.44 | (0.28, 0.68) | <0.001 |
| Regional lymph
nodes |
| 5 | pN0 (n=174) | 0.45 | (0.22, 0.92) | 0.029 |
| 6 | pN+
(n=176) | 0.47 | (0.26, 0.82) | 0.008 |
| Histological
grade |
| 7 | pG1 (n=139) | 0.45 | (0.25, 0.78) | 0.005 |
| 8 | pG2-3 (n=196) | 0.38 | (0.21, 0.68) | 0.001 |
| CRT |
| 9 | No (n=183) | 0.37 | (0.21, 0.65) | 0.001 |
| 10 | Yes (n=167) | 0.56 | (0.32, 0.99) | 0.047 |
| Fields of LND |
| 11 | 2-FLND (n=165) | 0.41 | (0.22, 0.74) | 0.004 |
| 12 | 3-FLND (n=185) | 0.47 | (0.27, 0.82) | 0.007 |
| Skip LNM (for N+
cases) |
| 13 | No (n=86) | 0.41 | (0.17, 1.00) | 0.049 |
| 14 | Yes (n=90) | 0.44 | (0.20, 0.99) | 0.046 |
| All cases |
| 15 | Combined
(n=350) | 0.45 | (0.30, 0.66) | <0.001 |
 | Table V.Association between ALND and
prognoses in adequately staged patients with ESCC stratified by
nodal statusa. |
Table V.
Association between ALND and
prognoses in adequately staged patients with ESCC stratified by
nodal statusa.
|
| ALND |
|
|
|
|---|
|
|
|
|
|
|
|---|
| pN | no (n, %) | yes (n, %) | Hazard
ratiob | (95% CI) | P-value |
|---|
| N0 | 45,
30.8 | 101, 69.2 | 0.45 | (0.20, 0.97) | 0.043 |
| N+ | 118, 72.4 | 45,
27.6 | 0.41 | (0.22, 0.75) | 0.004 |
Furthermore, the protective role of ALND was
examined in several relatively homogeneous subgroups. No
significant associations between ALND and survival rate were found
for subgroups of pN1 (HR=0.44, 95% CI=0.19–1.01, P=0.170; Fig. 7A), pN2 (HR=0.45, 95% CI=0.18–1.10,
P=0.157; Fig. 7B) and and pN2-3
(HR=0.52, 95% CI=0.23–1.18, P=0.069; Fig. 7D). However, trends toward improved
survival with ALND were observed pN1-2 (HR=0.49, 95% CI=0.28–0.88,
P=0.033; Fig. 7C). Additionally,
ALND was associated with improved survival in local diseases
(T1-3/N0-1; HR=0.50, 95% CI=0.30–0.84, P<0.001; Fig. 7E) and locally advanced diseases
(T4/Nany or T1-3/N2-3; HR=0.32, 95% CI=0.15–0.68, P=0.022; Fig. 7F).
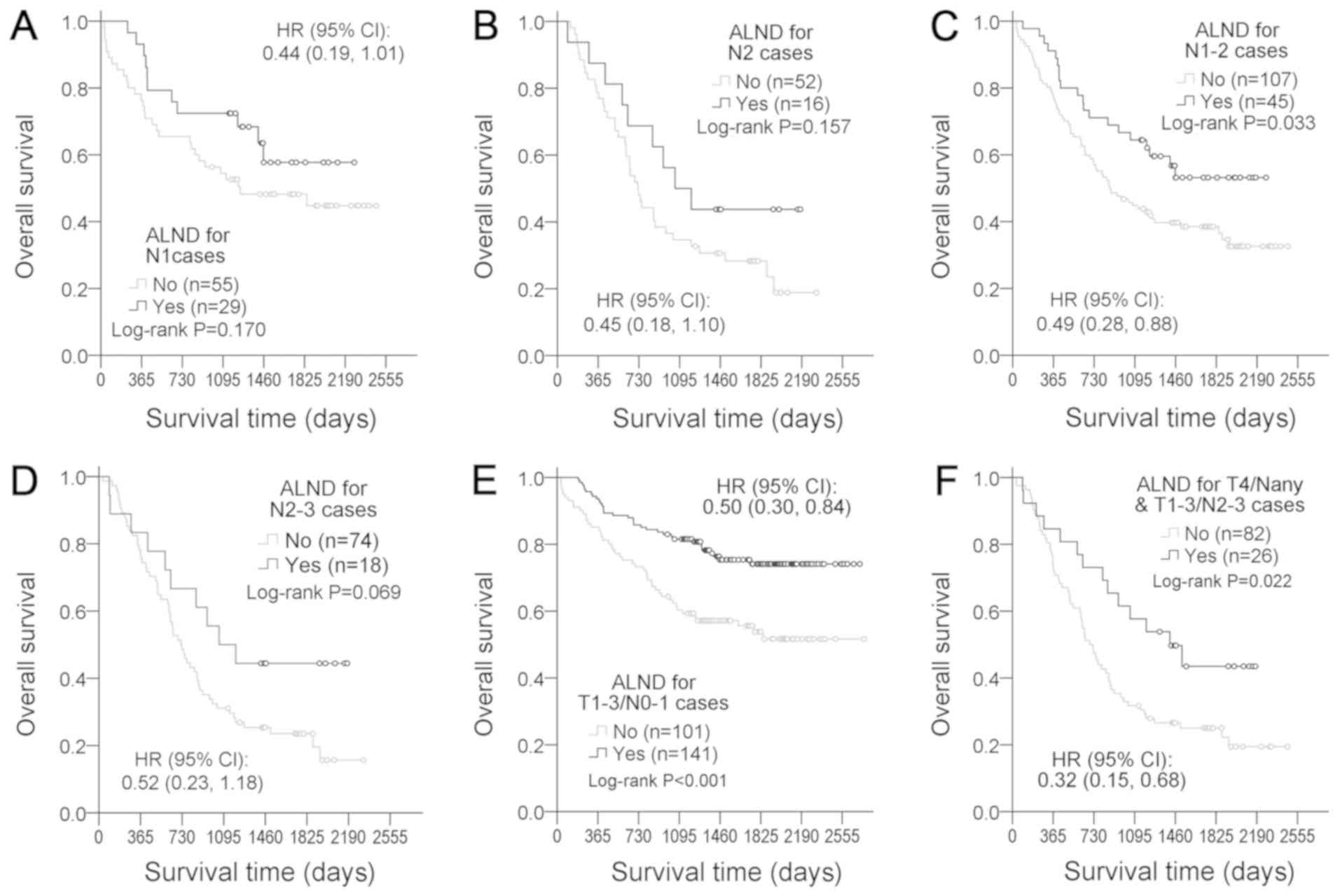 | Figure 7.Therapeutic effect of ALND in the
relative homogeneous subgroups of patients with esophageal squamous
cell carcinoma. ALND efficacy was further evaluated in (A) pN1
cases, (B) pN2 cases, (C) pN1-2, (D) pN2-3, (E) local disease
patients (T1-3/N0-1) and (F) locally advanced disease (T4/Nany and
T1-3/N2-3). HRs of ALND were adjusted for sex, age, tumor length,
PNLVI, number of positive LNs, pT, pG, chemoradiotherapy, tumor
location and medical center. ALND, appropriate lymph node
dissection; HR, hazard ratio; PNLVI, perineural lymphatic vascular
invasion. |
Finally, in order to illustrate the efficacy of the
proposed ALND, the current cohort was analyzed with five other LND
recommendations proposed by the NCCN (1) (Fig. 8A),
UICC (2) (Fig. 8B), Rizk et al (6) (Fig. 8C),
Peyre et al (5) (Fig. 8D) and Schwarz et al (9) (Fig. 8E).
The results indicated that none of the recommended LNDs
outperformed the proposed ALND (Fig.
8F).
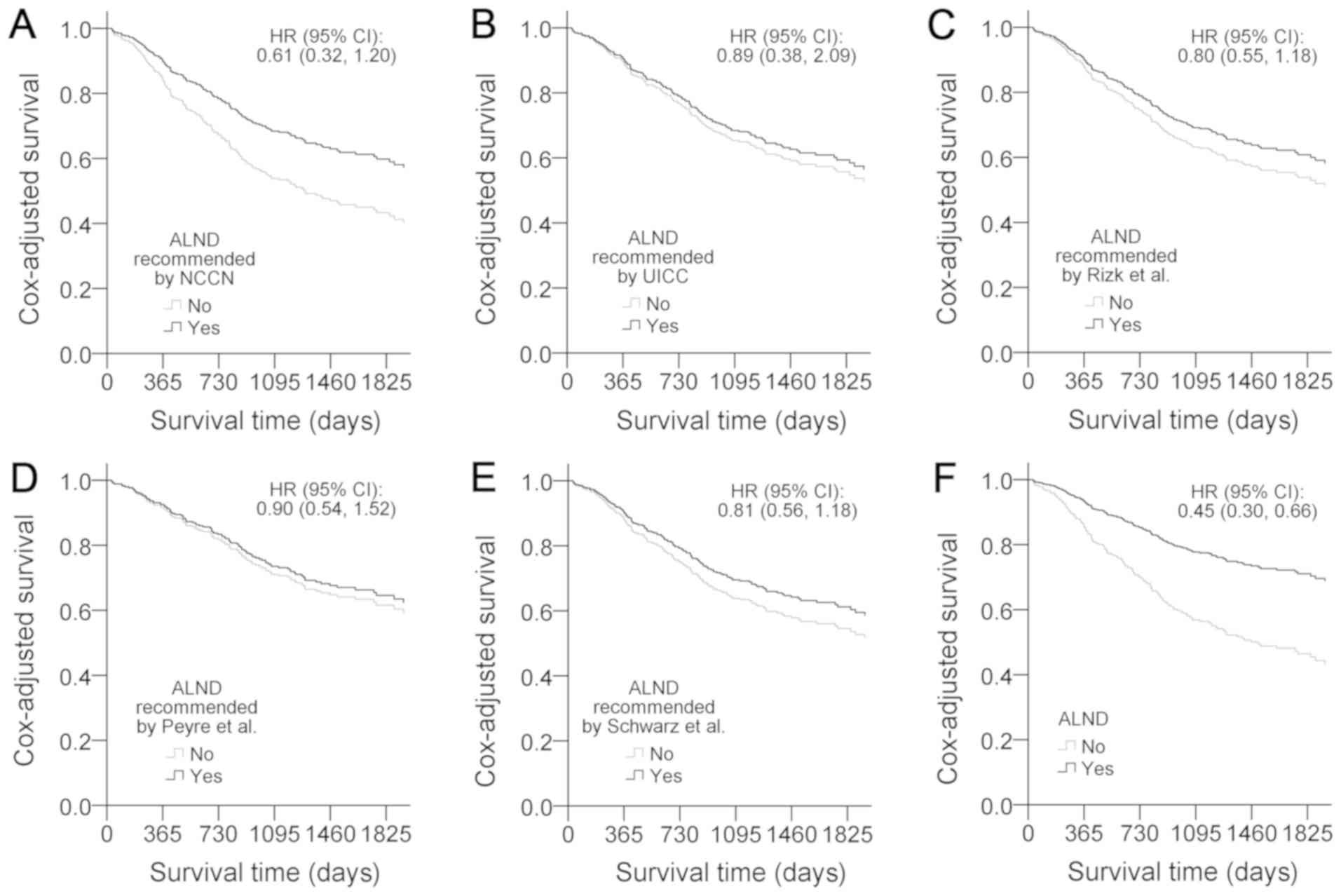 | Figure 8.Comparisons of Cox-adjusted survival
curves of ALND. Cox-adjusted survival curves were generated using
multiple Cox regression, which included sex, age, tumor location,
tumor length, pG, pN, PNLVI, chemoradiotherapy, medical centers and
ALND. (A) ALND recommendation from the NCCN guidelines (1) is ≥15 HLNs. (B) At least 12 total HLNs
are required for T1b-3/N0-1 cases according to the UICC (2). (C) Rizk et al (6) recommended optimal T stage-dependent
lymphadenectomy, and set the thresholds at 10, 20 and 30 HLNs for
pT1, pT2 and pT3/4 cases, respectively. (D) Peyre et al
(5) defined ALND by the removal of
≥23 nodes. (E) Schwarz et al (9) recommended a resection of ≥30 LNs to
achieve ALND. (F) Although the survival curves for cases receiving
ALND demonstrated improved prognosis, none of the five
recommendations were verified as a significant factor by Cox
regression models; the ALND proposed in the present study was
significant. ALND, adequate lymph node dissection; HLNs, harvested
lymph nodes; LN, lymph node; NCCN, National Comprehensive Cancer
Network; pG, histological grade; pN, LN metastases; PNLVI,
perineural lymphatic vascular invasion; UICC, Union for
International Cancer Control. |
Discussion
In the present study, no significant association
between the total number of HLNs and OS was identified. This lack
of association between more extensive LND and improved survival has
also been documented by other studies, including an international
multicenter study (21), a long
follow-up case cohort in a high-volume center (22), nation-wide cohorts (23,24),
randomized clinical trials (25–27),
retrospectively analyzed cases receiving right-sided transthoracic
or left-sided thoracoabdominal approaches (28), patients with early-stage ESCC
(29) and patients with ESCC
undergoing neoadjuvant chemotherapy (30). Additionally, evidence indicates that
the survival benefits from higher HLNs or radical LND can be
attributable, at least, partly to stage migration (improved staging
rather than improved therapeutic benefit of the dissection itself)
(31–33). Since most cases in the present study
had more than 15 HLNs, which indicated that the metastatic nodes
could have already been removed (2),
further nodal resection may not bring additional benefits to
survival.
It is well known that the depth of tumor invasion is
associated with nodal metastases, which causes a nodal metastatic
pattern that is predisposed to tumor location (34). Additionally, a recent study claimed
that the extent of LND should be estimated by the dissected zones
and modified according to the tumor location (35). In the present study, surgeons tended
to harvest more nodes in the region adjacent to the tumor location
(the local LN zones) in order to remove potential metastatic nodes.
However, as indicated in the present study, this selective LND had
a protective effect for N0 patients only.
Since the presence of abundant longitudinal
lymphatic drainage in the submucosa facilitates the spread of
cancer cells to distant LNs (36),
SLNMs were frequently observed in the present study (51% of
N+ patients). Additionally, the direction of metastatic
lymphatic flow from the tumor may be altered according to the depth
of invasion (37), which can reduce
the accuracy of predicting metastatic LN sites. Therefore,
selective lymphadenectomy based on the site of primary tumors may
fail to capture these skipped or unexpected metastatic nodes, which
may partly explain the lack of association between the percentages
of local HLNs and survival rates of N+ patients.
In order to successfully remove nodes with cancerous
infiltration, lymphadenectomy for the N+ patients should
focus on the metastatic LN zones. It has been reported that
micrometastases are highly prevalent in pathologically negative
nodes (38,39), and sufficient dissection may block
the spreading of tumor cells. However, to the best of our
knowledge, no previous study has specified the number of LNs that
need to be resected in the exact site. By using LOESS curves,
cut-offs of LN counts for adequately removing potentially
metastatic nodes in specific zones were set. In the cohort of the
present study, N+ patients with sufficient LNs resected
from the metastatic zones exhibited improved survival compared with
those who did not receive ALND, even in the cases of patients with
SLNMs.
By integrating the requirements for removing the
potentially involved LNs in the N0 and N+ patients, a
novel definition of ALND was proposed. The total numbers of HLNs in
the aforementioned strategy were not addressed out of the following
considerations: i) In the present study, most cases received a
radical resection, which yielded a high LN count (median HLNs
value=29); and ii) no statistical association was evident between a
higher LN total and improved survival in the present study.
Although non-significant results were observed in
pN1 and pN2 patients, trends toward improved survival were observed
for ALND in these subgroups. Additionally, following the merging of
pN1 and pN2 subgroups, significantly improved survival was
indicated, which suggested the protective role of ALND. However,
the present study failed to verify the protective outcome in pN2-3
cases. Therefore, ALND may have limited effects on cases with high
pN stages. The results were consistent with the current opinions
that radical surgery has limited value for cases with systemic
nodal spread diseases (40,41).
Two or three-field lymphadenectomy could produce
different postoperative lymph node distributions, which can
influence the chances of ALND and survival. Therefore, in the
present study, the protective role of ALND within each stratum was
evaluated. The results revealed significant associations between
ALND and improved survival in the two strata. Therefore, it was
likely that the association between ALND and prognosis was not
modified by the fields of lymphadenectomy.
In order to determine the efficacy of the proposed
ALND, the current cohort was examined using five other recommended
guidelines. The findings indicated that none of the recommended
guidelines outperformed the proposed ALND. The difference in
efficacy may be due to two reasons. Firstly, the multicenter
populations included in studies by Rizk et al (6), Peyer et al (5) and Schwarz et al (9) were primarily composed of patients with
adenocarcinoma (57–60%), which has been reported to have a
different lymphatic spread pattern from that of squamous cell
carcinoma (42). Secondly, all three
studies reported few HLNs during lymphadenectomy, with the median
LN counts ranging between 8 and 17, which indicates that the
observed survival benefits from an extensive LND were likely to be
confounded by inadequate staging (31,32).
Therefore, the ALND proposed in the present study was more
applicable to patients with ESCC receiving radical
lymphadenectomy.
Although ALND in neoadjuvant chemotherapy (nCT)
patients could not be evaluated in the present study, the impact of
nCT on lymphadenectomy has been reported elsewhere. The nCT may
affect the preoperative LND strategy and the preferences/habits of
nodal dissection during surgery, but not the therapeutic value of
LND (30,43). In our clinical centers, a small
number of ESCC cases (<13%) received nCT and were not included
in the present study. Investigations into the effect of nCT on ALND
will be conducted in the future when a sufficient sample pool is
available.
There are several limitations of the present study.
Although the proportion of pN3 patients in the present study (6.9%)
was similar to that of a previous large-scale study (6.1%)
(44), which consisted of 1195
patients with ESCC treated with surgery alone, the sample size of
pN3 in the present study was small (n=28). Furthermore, 52.9% of
patients were treated with 3-FLND and the rest of the patients were
treated with extended 2-FLND, which indicates a different
dissection preference from what is predominantly practiced in
Europe and North America, where the standard is 2-FLND (45). This dissection preference limits the
application of ALND when the cervical LN zone is involved. In
addition, as it is difficult to predict specific nodal metastases
even with PET-CT and endoscopic ultrasound, only pathological
examination results were used as indicators for LNM, which may
weaken the protective effect of ALND when LNM status cannot be
clearly demonstrated preoperatively. Although the existing
techniques can hardly accurately predict metastatic nodal sites,
novel diagnosis methods will enhance the preoperative diagnostic
accuracy in the future. Additionally, more studies are needed to
validate the efficacy of this novel ALND.
In conclusion, a novel LND strategy was proposed for
the optimization of the survival of patients with ESCC undergoing
radical 2- or 3-FLND. The ALND proposed in the present study was a
metastatic status-dependent LND, which considered the tumor
location and metastatic nodal zones. With the exception of patients
with high pN stages, patients receiving ALND exhibited improved OS
compared with those who did not receive ALND. The competitive
advantage of ALND is that when compared with the traditional 2- or
3-FLND, this LND strategy can achieve optimal overall survival
without harvesting much more LNs or extending the LND range.
Therefore, the present study suggested that the proposed ALND may
complement the existing surgical guidelines to improve
individualized therapeutic efficacy.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Daoshu Luo
(Department of Anatomy, Fujian Medical University) and Professor
Bin Wang (Department of Pathology, Fujian Medical University) for
their consultation on lymph node coding and pathological
staging.
Funding
The present study was supported by grants from
National Key R&D Program of China (grant no. 2017YFC0907100),
Natural Science Foundation of Fujian Province (grant no.
2015J01305), Professor Academic Development Foundation of Fujian
Medical University (grant no. JS14005), Industry-University
Research Project of Educational Department of Fujian Province
(grant no. JA13137) and Science and Technology Project of Anxi
Tieguanyin Group Co., Ltd. Fujian (grant no. 2013B013).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH, ZL and XP conceived and designed the study. WC,
YC and SY participated in the acquisition of clinical data, were
involved in manuscript writing regarding surgical methods and CT
scanning, and interpreted the results from a clinical perspective.
ZL, FH, RF and YJ performed the data analysis and interpretation.
ZL wrote the manuscript. ZH reviewed and edited the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work is appropriately
investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Fujian Medical University. All patients enrolled in this study
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALND
|
adequate lymph node dissection
|
|
CRT
|
chemoradiotherapy
|
|
ESCCs
|
esophageal squamous cell
carcinomas
|
|
HLNs
|
the number of harvested lymph
nodes
|
|
LNs
|
lymph nodes
|
|
LND
|
lymph node dissection
|
|
LNM
|
lymph node metastasis
|
|
LOESS
|
locally weighted smoothing scatter
|
|
LNRs
|
lymph node ratios
|
|
OS
|
overall survival
|
|
PNLVI
|
perineural lymphatic vascular
invasion
|
|
SLNM
|
skip lymph node metastasis
|
|
3-FLND
|
3-field lymph node dissection
|
References
|
1
|
Vashist YK, Loos J, Dedow J, Tachezy M,
Uzunoglu G, Kutup A, Yekebas EF and Izbicki JR: Glasgow prognostic
score is a predictor of perioperative and long-term outcome in
patients with only surgically treated esophageal cancer. Ann Surg
Oncol. 18:1130–1138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Sullivan B, Brierley J and International
Union against Cancer: UICC manual of clinical oncology. John Wiley
and Sons Ltd.; Chichester, West Sussex, UK; Hoboken, NJ: 2015,
View Article : Google Scholar
|
|
3
|
Edge SB and American Joint Committee on
Cancer, . AJCC cancer staging manual. Springer; New York: 2010
|
|
4
|
Hu Y, Hu C, Zhang H, Ping Y and Chen LQ:
How does the number of resected lymph nodes influence TNM staging
and prognosis for esophageal carcinoma? Ann Surg Oncol. 17:784–790.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peyre CG, Hagen JA, DeMeester SR, Altorki
NK, Ancona E, Griffin SM, Hölscher A, Lerut T, Law S, Rice TW, et
al: The number of lymph nodes removed predicts survival in
esophageal cancer: An international study on the impact of extent
of surgical resection. Ann Surg. 248:549–556. 2008.PubMed/NCBI
|
|
6
|
Rizk NP, Ishwaran H, Rice TW, Chen LQ,
Schipper PH, Kesler KA, Law S, Lerut TE, Reed CE, Salo JA, et al:
Optimum lymphadenectomy for esophageal cancer. Ann Surg. 251:46–50.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groth SS, Virnig BA, Whitson BA, DeFor TE,
Li ZZ, Tuttle TM and Maddaus MA: Determination of the minimum
number of lymph nodes to examine to maximize survival in patients
with esophageal carcinoma: Data from the surveillance epidemiology
and end results database. J Thorac Cardiovasc Surg. 139:612–620.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen YJ, Schultheiss TE, Wong JY and
Kernstine KH: Impact of the number of resected and involved lymph
nodes on esophageal cancer survival. J Surg Oncol. 100:127–132.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwarz RE and Smith DD: Clinical impact
of lymphadenectomy extent in resectable esophageal cancer. J
Gastrointest Surg. 11:1384–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rizk N: Surgery for esophageal cancer:
Goals of resection and optimizing outcomes. Thorac Surg Clin.
23:491–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Phillips AW, Lagarde SM, Navidi M, Disep B
and Griffin SM: Impact of extent of lymphadenectomy on survival,
post neoadjuvant chemotherapy and transthoracic esophagectomy. Ann
Surg. 265:750–756. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baba Y, Watanabe M, Shigaki H, Iwagami S,
Ishimoto T, Iwatsuki M and Baba H: Negative lymph-node count is
associated with survival in patients with resected esophageal
squamous cell carcinoma. Surgery. 153:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bogoevski D, Onken F, Koenig A, Kaifi JT,
Schurr P, Sauter G, Izbicki JR and Yekebas EF: Is it time for a new
TNM classification in esophageal carcinoma? Ann Surg. 247:633–641.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin Z, Chen W, Chen Y, Peng X, Zhu K, Lin
Y, Lin Q and Hu Z: A new classification of lymph node metastases
according to the lymph node stations for predicting prognosis in
surgical patients with esophageal squamous cell carcinoma.
Oncotarget. 7:76261–76273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rice TW, Blackstone EH and Rusch VW: 7th
edition of the AJCC cancer staging manual: Esophagus and
esophagogastric junction. Ann Surg Oncol. 17:1721–1724. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niwa Y, Koike M, Hattori M, Iwata N,
Takami H, Hayashi M, Tanaka C, Kobayashi D, Kanda M, Yamada S, et
al: The prognostic relevance of subcarinal lymph node dissection in
esophageal squamous cell carcinoma. Ann Surg Oncol. 23:611–618.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato H and Nakajima M: Treatments for
esophageal cancer: A review. Gen Thorac Cardiovasc Surg.
61:330–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsieh FY and Lavori PW: Sample-size
calculations for the Cox proportional hazards regression model with
nonbinary covariates. Control Clin Trials. 21:552–560. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schoenfeld D: Partial residuals for the
proportional hazards regression model. Biometrika. 69:239–241.
1982. View Article : Google Scholar
|
|
21
|
Nafteux P, Lerut A, Moons J, Hölscher AH,
Bollschweiler E, van Berge Henegouwen MI, Lagarde SM, van Lanschot
JJ, Messager M, Mariette C, et al: International multicenter study
on the impact of extracapsular lymph node involvement in primary
surgery adenocarcinoma of the esophagus on overall survival and
staging systems. Ann Surg. 262:809–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lagergren J, Mattsson F, Zylstra J, Chang
F, Gossage J, Mason R, Lagergren P and Davies A: Extent of
lymphadenectomy and prognosis after esophageal cancer surgery. JAMA
Surg. 151:32–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Schaaf M, Johar A, Wijnhoven B,
Lagergren P and Lagergren J: Extent of lymph node removal during
esophageal cancer surgery and survival. J Natl Cancer Inst.
107(pii): djv0432015.PubMed/NCBI
|
|
24
|
Talsma AK, Damhuis RA, Steyerberg EW,
Rosman C, van Lanschot JJ and Wijnhoven BP: Determinants of
improved survival after oesophagectomy for cancer. Br J Surg.
102:668–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hulscher JB, van Sandick JW, de Boer AG,
Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van
Dekken H, Obertop H, et al: Extended transthoracic resection
compared with limited transhiatal resection for adenocarcinoma of
the esophagus. N Engl J Med. 347:1662–1669. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chu KM, Law SY, Fok M and Wong J: A
prospective randomized comparison of transhiatal and transthoracic
resection for lower-third esophageal carcinoma. Am J Surg.
174:320–324. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishihira T, Hirayama K and Mori S: A
prospective randomized trial of extended cervical and superior
mediastinal lymphadenectomy for carcinoma of the thoracic
esophagus. Am J Surg. 175:47–51. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu PK, Wang BY, Chou TY, Huang CS, Wu YC
and Hsu WH: The total number of resected lymph node is not a
prognostic factor for recurrence in esophageal squamous cell
carcinoma patients undergone transthoracic esophagectomy. J Surg
Oncol. 103:416–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chao YK, Liu HP, Hsieh MJ, Wu YC, Liu YH,
Yeh CH, Chang HK and Tseng CK: Impact of the number of lymph nodes
sampled on outcome in ypT0N0 esophageal squamous cell carcinoma
patients. J Surg Oncol. 106:436–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyata H, Yamasaki M, Makino T, Miyazaki
Y, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M and
Doki Y: Therapeutic value of lymph node dissection for esophageal
squamous cell carcinoma after neoadjuvant chemotherapy. J Surg
Oncol. 112:60–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rizk N, Venkatraman E, Park B, Flores R,
Bains MS and Rusch V; American Joint Committee on Cancer staging
system, : The prognostic importance of the number of involved lymph
nodes in esophageal cancer: Implications for revisions of the
American Joint Committee on Cancer staging system. J Thorac
Cardiovasc Surg. 132:1374–1381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barbour AP, Rizk NP, Gonen M, Tang L,
Bains MS, Rusch VW, Coit DG and Brennan MF: Lymphadenectomy for
adenocarcinoma of the gastroesophageal junction (GEJ): Impact of
adequate staging on outcome. Ann Surg Oncol. 14:306–316. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lerut T, Nafteux P, Moons J, Coosemans W,
Decker G, De Leyn P, Van Raemdonck D and Ectors N: Three-field
lymphadenectomy for carcinoma of the esophagus and gastroesophageal
junction in 174 R0 resections: Impact on staging, disease-free
survival, and outcome: A plea for adaptation of TNM classification
in upper-half esophageal carcinoma. Ann Surg. 240:962–974. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen J, Liu S, Pan J, Zheng X, Zhu K, Zhu
J, Xiao J and Ying M: The pattern and prevalence of lymphatic
spread in thoracic oesophageal squamous cell carcinoma. Eur J
Cardiothorac Surg. 36:480–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tachimori Y, Ozawa S, Numasaki H,
Matsubara H, Shinoda M, Toh Y, Udagawa H, Fujishiro M, Oyama T, Uno
T, et al: Efficacy of lymph node dissection by node zones according
to tumor location for esophageal squamous cell carcinoma.
Esophagus. 13:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brotons ML, Bolca C, Fréchette E and
Deslauriers J: Anatomy and physiology of the thoracic lymphatic
system. Thorac Surg Clin. 22:139–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Motoyama S, Maruyama K, Sato Y, Usami S,
Nakatsu T, Saito H, Minamiya Y and Ogawa J: Status of involved
lymph nodes and direction of metastatic lymphatic flow between
submucosal and t2-4 thoracic squamous cell esophageal cancers.
World J Surg. 33:512–517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Izbicki JR, Hosch SB, Pichlmeier U,
Rehders A, Busch C, Niendorf A, Passlick B, Broelsch CE and Pantel
K: Prognostic value of immunohistochemically identifiable tumor
cells in lymph nodes of patients with completely resected
esophageal cancer. N Engl J Med. 337:1188–1194. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Imamura Y, Hayashi N, Sato N, Kinoshita K,
Kurashige J, Saito S, Hirashima K, Karashima R, Hiyoshi Y, Nagai Y,
et al: Extensive lymphatic spread of cancer cells in patients with
thoracic esophageal squamous cell carcinoma: Detection of CEA-mRNA
in the three-field lymph nodes. J Surg Oncol. 102:509–515. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang LS, Chow KC, Chi KH, Liu CC, Li WY,
Chiu JH and Huang MH: Prognosis of esophageal squamous cell
carcinoma: Analysis of clinicopathological and biological factors.
Am J Gastroenterol. 94:1933–1940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng YZ, Zhao W, Hu Y, Ding-Lin XX, Wen
J, Yang H, Liu QW, Luo KJ, Huang QY, Chen JY and Fu JH: Aggressive
surgical resection does not improve survival in operable esophageal
squamous cell carcinoma with N2-3 status. World J Gastroenterol.
21:8644–8652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stein HJ, Feith M, Bruecher BL, Naehrig J,
Sarbia M and Siewert JR: Early esophageal cancer: Pattern of
lymphatic spread and prognostic factors for long-term survival
after surgical resection. Ann Surg. 242:566–575. 2005.PubMed/NCBI
|
|
43
|
Smyth EC, Fassan M, Cunningham D, Allum
WH, Okines AF, Lampis A, Hahne JC, Rugge M, Peckitt C, Nankivell M,
et al: Effect of pathologic tumor response and nodal status on
survival in the medical research council adjuvant gastric
infusional chemotherapy trial. J Clin Oncol. 34:2721–2727. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su D, Zhou X, Chen Q, Jiang Y, Yang X,
Zheng W, Tao K, Wu J, Yan Z, Liu L, et al: Prognostic nomogram for
thoracic esophageal squamous cell carcinoma after radical
esophagectomy. PLoS One. 10:e01244372015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Haverkamp L, Seesing MF, Ruurda JP, Boone
J and V Hillegersberg R: Worldwide trends in surgical techniques in
the treatment of esophageal and gastroesophageal junction cancer.
Dis Esophagus. 30:1–7. 2017.
|