Introduction
Cisplatin is one of the most widely used
chemotherapeutic agents for human epithelial cancer types (1). This drug was the first platinum drug to
be approved by the USA Food and Drug Administration for cancer
treatment and has since been demonstrated to improve overall
survival, progression-free survival and recurrence-free survival
rates for patients with cancer (2–4).
Nevertheless, treatment failure is not uncommon in cases involving
tumors that are inherently resistant to cisplatin or that acquire a
resistant phenotype during treatment (5). Such resistance can lead to cancer
recurrence and poor survival (6). To
the best of our knowledge, there is currently no effective
pharmacological strategy available to avoid cisplatin
resistance.
Cisplatin activates specific signaling pathways in
cancer cells, leading to the development of a resistant phenotype
(7). Reactive oxygen species
(ROS)-mediated signaling regulates the responsiveness of cancer
cells to chemotherapeutic agents (8). Specifically, ROS function as secondary
messengers to activate and modify specific signaling pathways. In
mammalian cells, ROS are predominantly generated by enzymes in the
NADPH oxidase (NOX) family (9). This
family comprises seven members [NOX1-5, dual oxidase (DUOX)1 and
DUOX2] and their expression patterns vary depending on the cellular
context (10). Previous studies have
revealed that cisplatin treatment can promote the generation of ROS
and expression of NOX isoforms (11,12).
Curcumin is a natural polyphenol isolated from the
rhizome of Curcuma longa, which yields the common dietary
spice, turmeric (13). In Chinese
herbal medicine, purified curcumin has been used to alleviate
throbbing pain and pain caused by injury (14). Previously, curcumin has been reported
to exert chemosensitizing effects in the context of chemotherapy
based on paclitaxel or 5-fluorouracil. When administered in
combination with paclitaxel, curcumin exerted a synergistic growth
inhibitory effect on a human cervical cancer xenograft (15). Furthermore, the combination of
curcumin and 5-fluorouracil exerted synergistic cytotoxic activity
against breast cancer cells by suppressing nuclear factor-κB
(16). Curcumin has also been
demonstrated to enhance the cytotoxicity of cisplatin in a head and
neck cancer model (17). However, to
the best of our knowledge, the mechanism by which curcumin
sensitizes cancer types to cisplatin, particularly those with
acquired cisplatin resistance, remains unclear.
The current study explored the transcription
activating effects of cisplatin on different NOX isoforms in an
epithelial cancer model. It was identified that NOX5 was
upregulated differentially in response to cisplatin treatment.
Finally, the current study investigated whether curcumin treatment
could be an effective strategy for resensitizing cancer cells that
acquire cisplatin resistance in a xenograft model.
Materials and methods
Cell line
HONE1 cells were established in 1989 and have since
been used as a poorly differentiated nasopharyngeal carcinoma cell
line (18). The HONE1 cells used in
the current study were kindly provided by Professor S.W. Tsao (The
University of Hong Kong, Hong Kong, SAR, China). However, this cell
line has been contaminated with HeLa cells, likely at the time of
establishment (19). The HONE1 cells
were maintained at 37°C in a humidified atmosphere containing 5%
CO2 in RPMI-1640 medium supplemented with 10% fetal
bovine serum, 200 U/ml penicillin G sodium, 20 µg/ml streptomycin
sulfate and 0.5 µg/ml amphotericin B (all Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Development of cisplatin-resistant
HONE1
A cisplatin-resistant HONE1 cell line was developed
via long-term cisplatin treatment. HONE1 cells (3×105)
were exposed to cisplatin for 3 days at 37°C in a humidified
atmosphere containing 5% CO2, followed by a 3-day period
of growth recovery at 37°C in a humidified atmosphere containing 5%
CO2 in drug-free medium. This procedure was repeated for
28 cycles, with increasing concentrations of cisplatin, starting at
0.5 µM and increasing by 0.5 µM with each cycle to a maximum of
14.0 µM. The responses of parental and cisplatin-treated HONE1
cells to cisplatin were measured using an in vitro toxicity
test. The half-maximal inhibitory concentration (IC50)
values were determined from the dose-response curves and compared
between parental and cisplatin-treated HONE1 cells.
Plasmids and cell transfection
The pcDNA3.1-NOX5 plasmid and pcDNA3.1 empty vector
were purchased from Addgene, Inc. (Cambridge, MA, USA). HONE1 cells
were transfected with DNA plasmids for 48 h using Lipofectamine
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. At 48 h after
transfection, cells were subjected to in vitro toxicity
assay or western blotting.
In vitro toxicity assay
Parental HONE1 cells or cisplatin-resistant HONE1
cells were treated with 0–100 µM (0.195, 0.39, 0.78, 1.56, 3.13,
6.25, 12.5, 25, 50 and 100 µM) cisplatin for 72 h at 37°C. HONE1
cells transfected with a NOX5-expressing vector or empty vector
were treated with 0–32 µM (2, 4, 8, 16 and 32 µM) cisplatin for 72
h at 37°C. The relative cell viability was determined using an
in vitro toxicology assay kit, the Sulforhodamine B (SRB)
assay (cat. no. TOX6; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) according to the manufacturer's protocol. The percentage
of viable cells was calculated as follows: Number of
cisplatin-treated viable cells/number of viable untreated control
cells ×100%. IC50 values were determined from
dose-response curves.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HONE1 cells and
cisplatin-resistant HONE1 cells using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
cDNA synthesis was performed using the High Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The qPCR analysis
was performed using a FastStart Universal Probe Master mix (Roche
Applied Science, Mannheim, Germany) on a LightCycler®
480 device (Roche Applied Science). GAPDH was used as a reference
gene. Reactions were performed at 95°C for 10 min followed by 45
cycles of 95°C for 15 sec and 60°C for 1 min. The following primers
were used for qPCR: NOX1 forward, 5′-AAGGATCCTCCGGTTTTACC-3′ and
reverse, 5′-TTTGGATGGGTGCATAACAA-3′; NOX2 forward,
5′-GAAGAAAGGCAAACACAACACA-3′ and reverse,
5′-CTCATTCACAGCCCAGTTCC-3′; NOX3 forward,
5′-CACACCATGTTTTCATCGTCTT-3′ and reverse,
5′-GTTTGGCCTCGAACAATCC-3′; NOX4 forward, 5′-GCTGACGTTGCATGTTTCAG-3′
and reverse, 5′-CGGGAGGGTGGGTATCTAA-3′; NOX5 forward,
5′-CGAGGAGGCTCAATACGG-3′ and reverse, 5′-TCTTGCCCAGTGCAGATGT-3′;
DUOX1 forward, 5′-TCCCCAAGGAGTATGACCTG-3′ and reverse,
5′-TCCCCGGAGATTTTCCAC-3′; DUOX2 forward, 5′-AGGCTGTGACAAAGCAGCA-3′
and reverse, 5′-CCTGGTTGATGTCCAGCAC-3′; and GAPDH forward,
5′-AGCCACATCGCTCAGACAC-3′ and reverse, 5′-GCCCAATACGACCAAATCC-3′.
The gene expression levels were evaluated using the comparative
threshold cycle method (2−ΔΔCq) (20). All experiments were repeated three
times.
Liposomal curcumin preparation
The phospholipids, dipalmitoylphosphatidylcholine
and dimyristoylphosphatidylgylcyerol, were mixed in a 1:1 ratio.
Subsequently, 0.013 g of curcumin and 0.1 g of the 1:1 mixture of
the two phospholipids were dissolved in 10 ml of a chloroform and
methanol mixture (2:1 ratio). This curcumin-liposome mixture was
then subjected to thin-film evaporation (21) and the solvent was evaporated using a
rotary evaporator until a dry lipid film was formed. This lipid
film was hydrated for approximately 1 h with 5 ml of PBS at 50°C in
a rotating flask. Empty liposomes were prepared using the same
protocol without curcumin and were used as a control to study the
effects of phospholipids on cells and xenografts. The final
concentration of liposomal curcumin was 10 mM.
Treatment with cisplatin, liposomal
curcumin or empty liposomes
Cisplatin-resistant HONE1 cells were plated in
96-well plates and treated with cisplatin alone (8 µM), liposomal
curcumin alone (2 µM) or in combination for 72 h at 37°C. Empty
liposomes were used as controls for the liposomal curcumin
treatments. Drug cytotoxicity was determined using an SRB assay
(cat. no. TOX6; Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol.
Western blotting
Cell lysates were prepared in a cell lysis buffer
containing 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.5%
sodium deoxycholate, 0.01% phenylmethylsulfonyl fluoride and 0.02%
protease inhibitor (Roche Applied Science) and incubated for 30 min
on ice. Protein concentrations were measured using a BCA protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 20
µg of protein was loaded per lane. NOX5, Akt/phosphorylated (p)-Akt
and the reference protein, β-actin, were separated by SDS-PAGE on
an 8% gel using a Mini-protein III system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The separated proteins were then
transferred onto polyvinylidine difluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA) in a semi-dry transfer cell (Bio-Rad
Laboratories, Inc.). The PVDF membrane was blocked at room
temperature with 5% non-fat milk in TBS with Tween-20 for 1 h. The
membrane was then incubated overnight with an anti-NOX5 monoclonal
antibody (1:1,000; cat. no. ab191010; Abcam, Cambridge, UK),
anti-Akt (pan) antibody (1:1,000; cat. no. 4691; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-phospho (p)-Akt (Ser473)
antibody (1:1,000; cat. no. 4060; Cell Signaling Technology, Inc.)
or anti-β-actin antibody (1:5,000; cat. no. A2228; Sigma-Aldrich;
Merck KGaA) at 4°C. Next, the membranes were incubated with a
horseradish peroxidase-labeled anti-rabbit secondary antibody
(1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.) for 1 h
at room temperature. Protein bands were visualized using an
enhanced chemiluminescence system (ECL Plus Western Blotting
Detection system; GE Healthcare, Chicago, IL, USA) and exposure of
membranes to X-ray film according to the manufacturer's protocol.
ImageJ software (National Institutes of Health, Bethesda, MD, USA)
was used for densitometry analysis.
Xenograft model
All animal experiments were performed according to
the institutional guidelines and were approved by the Institutional
Committee on the Use of Live Animals in Teaching and Research
(protocol no. 3474-14) at the Animal Laboratory, Department of
Surgery, University of Hong Kong (Hong Kong, SAR, China). A total
of 24 five-week-old male athymic nu/nu mice (weight, 18 to 22 g)
were used. Mice were obtained from the Laboratory Animal Unit of
the University of Hong Kong. The mice were maintained under
pathogen-free conditions, in a temperature (21°C) and humidity
(50%) controlled environment with a 14-h light/10-h dark cycle.
Mice were given ad libitum access to food and water.
Cisplatin-resistant HONE1 cells (2×106) in RPMI-1640
medium were injected subcutaneously in the right flanks of the
mice. The tumor size was measured daily in two dimensions using
calipers and the tumor volume was calculated using the following
formula: Volume (mm3) = (L × W2)/2, where L
is the length (mm) and W is the width (mm). When the tumor volume
reached 150 mm3, the mice were randomly assigned into
four groups to receive empty liposomes, liposomal curcumin alone,
cisplatin alone or liposomal curcumin combined with cisplatin.
Liposomal curcumin (25 mg/kg) or an equal volume of empty liposomes
was administered via intraperitoneal injection thrice weekly.
Cisplatin (2.5 mg/kg) was administrated via intraperitoneal
injection twice weekly. The tumor volume was measured every day
with calipers. Following 32 days of treatment, all mice were
sacrificed with an excessive dosage of pentobarbital (100–150
mg/kg; Alfasan International BV, Woerden, The Netherlands) and the
tumors were harvested.
Statistical analysis
All statistical tests were performed using SPSS
software version 20.0 (IBM Corp., Armonk, NY, USA). Three
independent repeats of all experiments were performed. The data are
expressed as the mean ± standard deviation. Differences in measured
variables between the experimental and control groups were assessed
using Student's t-test or one-way analysis of variance followed by
a Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cisplatin-resistant HONE1 cells can be
generated by chronic exposure to cisplatin
To confirm the ability to induce cisplatin
resistance in HONE1 cells, the cells were treated with 0–100 µM
cisplatin and the changes in the IC50 values between
parental and cisplatin-resistant HONE1 cells were determined.
Fig. 1A demonstrates that the
IC50 value of cisplatin-resistant HONE1 cells (21.9 µM)
was markedly higher compared with that of parental HONE1 cells (5.5
µM). These data indicated that long-term cisplatin treatment
reduced the responsiveness of HONE1 cells to cisplatin.
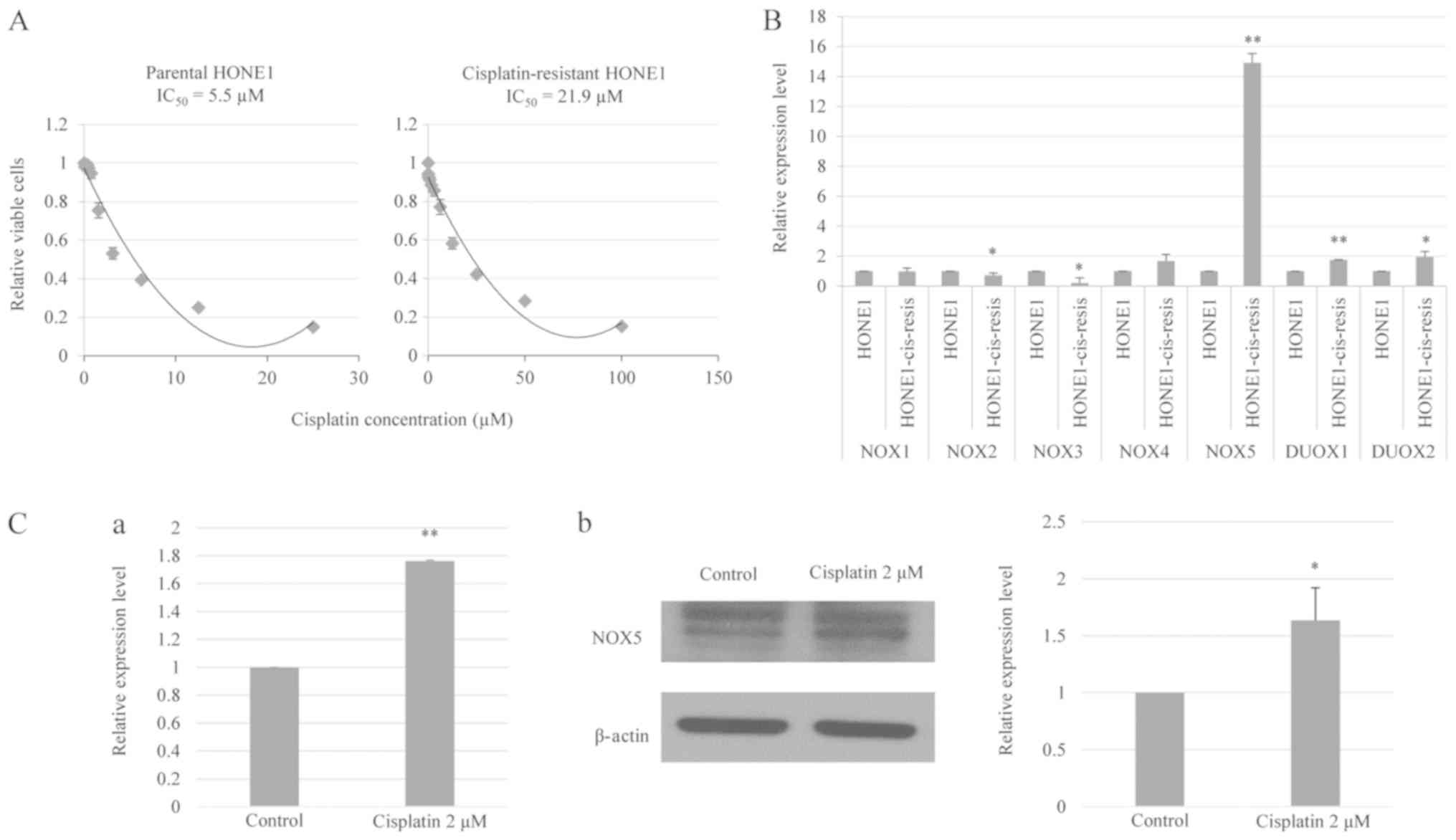 | Figure 1.NOX5 is overexpressed in
cisplatin-resistant HONE1 cells and is induced by cisplatin in
parental HONE1 cells. (A) In vitro toxicity assay of
cisplatin treatment in parental and cisplatin-resistant HONE1
cells. Cisplatin-resistant HONE1 cells demonstrated a higher
IC50 value compared with parental HONE1 cells. (B)
RT-qPCR revealed the mRNA expression levels of NOX family enzymes,
NOX1-5 and DUOX1-2, in parental and cisplatin-resistant HONE1
cells. Cisplatin-resistant HONE1 cells demonstrated a significantly
higher expression of NOX5, DUOX1 and DUOX2 compared with parental
HONE1 cells. Notably, NOX5 was the most highly upregulated gene in
cisplatin-resistant HONE1 cells. *P<0.05, **P<0.01 vs.
parental HONE1 cells. (C) Cisplatin treatment induced the
expression of NOX5 in HONE1 cells. (C-a) RT-qPCR analysis of mRNA
expression level of NOX5 in HONE1 cells treated with cisplatin.
(C-b) Western blot analysis of the protein expression level of NOX5
in HONE1 cells treated with cisplatin. *P<0.05, **P<0.01 vs.
control. Data are presented as the mean ± standard deviation, n=3.
HONE1-cis-resis, cisplatin-resistant HONE1 cells; NOX, NADPH
oxidase; DUOX, dual oxidase; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction;
IC50, half-maximal inhibitory concentration. |
NOX5 demonstrates differential
upregulation in cisplatin-resistant HONE1 cells
The expression levels of NOX family members were
evaluated in both parental HONE1 and cisplatin-resistant HONE1
cells using qPCR. Previous studies have indicated that cisplatin
treatment suppresses gene transcription, as indicated by the
reduced transcriptional activity of certain gene promoters
following genotoxic stress (22).
The current study observed a significant decrease in the expression
of NOX2 and NOX3 in cisplatin-resistant HONE1 cells compared with
parental HONE1 cells. By contrast, a significant upregulation of
NOX5, DUOX1 and DUOX2 was identified in cisplatin-resistant HONE1
cells compared with parental HONE1 cells (Fig. 1B). Specifically, the
cisplatin-resistant HONE1 cells demonstrated a 15-fold increase in
the expression level of NOX5 relative to the parental line. This
suggested that NOX5 upregulation may confer a particular adaptive
advantage to HONE1 cells under genotoxic stress and may serve an
important role in the development of a cisplatin-resistant
phenotype (23).
NOX5 expression in HONE1 cells is
induced by exposure to cisplatin
To confirm the transcription activating effect of
cisplatin on NOX5 expression, parental HONE1 cells were treated
with cisplatin (2 µM) and the change in NOX5 expression was
measured using qPCR and western blotting. Fig. 1C demonstrates that cisplatin
treatment significantly increased the NOX5 mRNA and protein levels
in the HONE1 cells. In summary, these data indicate that
cisplatin-induced NOX5 expression may be associated with the
development of a cisplatin-resistant phenotype in cancer.
High NOX5 expression confers
resistance to cisplatin
Based on the aforementioned results, it was
suggested that NOX5 expression may affect the responsiveness of
cancer cells to cisplatin. To address the functional implication of
this possibility, the current study transfected HONE1 cells with a
NOX5-expressing vector or empty vector and compared the changes in
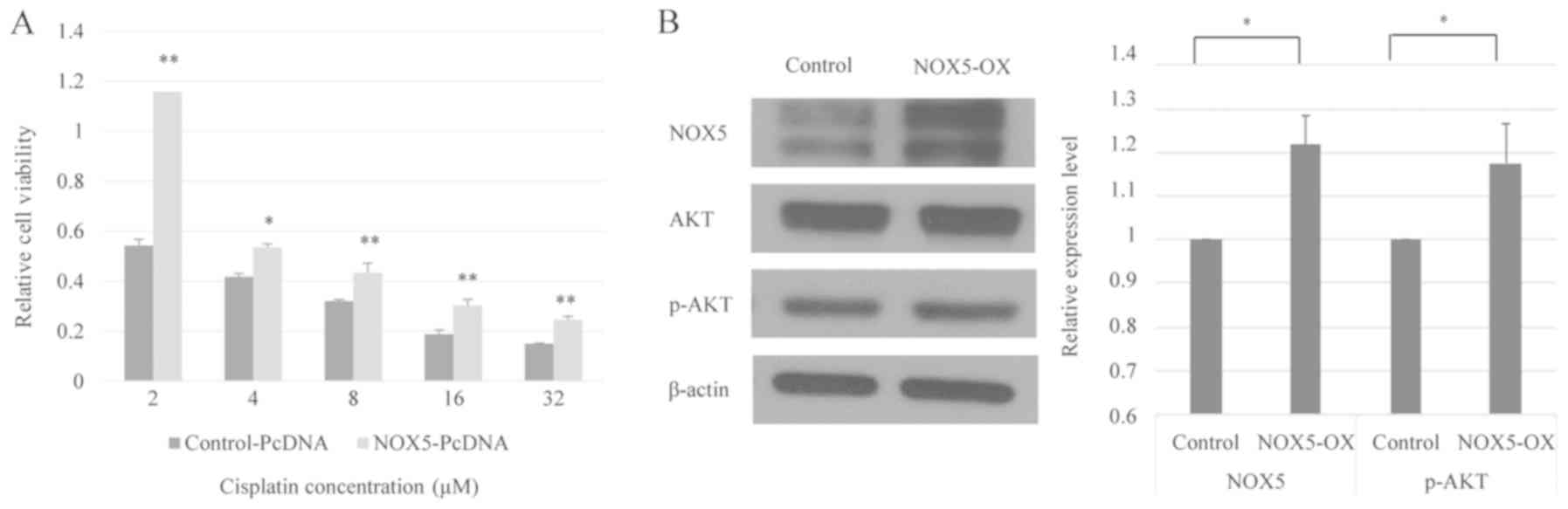
cisplatin sensitivity between the cells. As demonstrated in
Fig. 2A, NOX5-overexpressing HONE1
cells exhibited significantly higher cell viability when exposed to
cisplatin compared with control HONE1 cells. Therefore, it can be
hypothesized that NOX5-mediated signaling is involved in the
modulation of cisplatin sensitivity in HONE1 cells.
NOX5 activates the Akt signaling
cascade in cancer
Activation of the phosphoinositide 3-kinase
(PI3K)/Akt pathway contributes to the development of cisplatin
resistance in human cancer types (24). Western blot analysis revealed that
the overexpression of NOX5 in HONE1 cells did not affect the total
Akt protein level. The level of phosphorylated Akt (p-Akt) was
normalized to total Akt using the following formula:
(p-Akt/β-actin)/(Akt/β-actin) (25).
However, NOX5 overexpression significantly increased the level of
p-Akt, indicating that NOX5 could enhance PI3K/Akt signaling in
HONE1 cells (Fig. 2B).
Liposomal curcumin can suppress NOX5
expression and sensitize cisplatin-resistant HONE1 cells to
cisplatin
As demonstrated in Fig.
3A, cisplatin and curcumin treatment significantly decreased
the levels of NOX5 mRNA and protein in cisplatin-resistant HONE1
cells compared with cisplatin treatment alone. The chemosensitizing
effect of curcumin was then examined by treating
cisplatin-resistant HONE1 cells with a combination of low
concentrations of cisplatin (8 µM) and/or liposomal curcumin (2
µM). Treatment with liposomal curcumin (2 µM) or cisplatin alone (8
µM) exhibited a weak cytotoxic effect on cisplatin-resistant HONE1
cells (94.1 and 85.6% viability, respectively). However, a
combination of liposomal curcumin (2 µM) and cisplatin (8 µM)
significantly decreased the percentage of viable cells to 60.1%
(Fig. 3B). These data indicated that
the combined use of curcumin and cisplatin could effectively
increase the sensitivity of cisplatin-resistant cancer cells to
cisplatin.
Liposomal curcumin increases the
growth inhibitory effects of cisplatin in vivo
Finally, the in vivo chemosensitizing effect
of liposomal curcumin was analyzed in the cisplatin-resistant
HONE1-inoculated nude mice (Fig. 4).
In mice treated with liposomes alone, the tumor volumes were
associated with the time course of treatment. Compared with mice
treated with liposome alone, the tumor volumes were not
significantly smaller in the mice treated with either cisplatin or
liposomal curcumin, indicating that the selected drug
concentrations had little effect on the cisplatin-resistant HONE1
×enografts. In comparison, treatment with a combination of
liposomal curcumin and cisplatin significantly inhibited the growth
of the HONE1 ×enograft, as indicated by a significant reduction in
the tumor volume on days 24 and 28, relative to the cisplatin alone
group (Fig. 4A). Fig. 4B and C demonstrate the tumor weights
and sizes on day 32 in the four comparative groups. No significant
difference was identified in the weight of cisplatin-resistant
HONE1 tumors treated with liposomal curcumin alone or cisplatin
alone compared with those treated with liposome. By contrast, a
significant decrease in the tumor weight was identified when the
cisplatin-resistant HONE1 tumors were treated with a combined
treatment of liposomal curcumin and cisplatin compared with
liposome treatment alone or cisplatin treatment alone.
Discussion
Cisplatin is one of the most powerful chemotherapy
drugs; it has been widely used to treat a number of types of human
epithelial cancers, including ovarian carcinoma, lung carcinoma,
breast carcinoma and head and neck carcinoma (26). The administration of cisplatin in
combination with other chemotherapy drugs has been demonstrated to
be effective for the treatment of epithelial cancers (1). Nevertheless, cisplatin resistance is a
major challenge in the context of cisplatin-based chemotherapy
(27). The balance between the
influx and efflux rates of cisplatin determines the level of
cisplatin accumulation inside a cell. Cisplatin-resistant cells
exhibit decreased intracellular cisplatin accumulation due to
enhanced efflux and reduced influx (28). Furthermore, cisplatin may be
inactivated by sulfur-containing macromolecules, including
glutathione (GSH) and metallothionein (29), and upregulated levels of these
molecules have been observed in certain cisplatin-resistant cells
(30). Cancer may minimize the
genotoxic effects of cisplatin by increasing the ability of cells
to remove cisplatin-induced DNA adducts, which prevents
cisplatin-induced apoptosis (27).
Previously, it has been revealed that cisplatin-resistant cancer
cells exhibit reduced expression of the pro-apoptotic protein, Bax,
which leads to the inhibition of cisplatin-triggered apoptosis
(29). The current study
demonstrated that the exposure of cancer cells to sub-lethal doses
of cisplatin could promote the development of cisplatin resistance.
Furthermore, it was demonstrated that the signaling pathway
mediated by the ROS-generating enzyme NOX5 may serve an important
role in this process.
ROS-activated signaling pathways mediate cisplatin
resistance in numerous human cancer types. Mitochondrial
dysfunction increases the levels of ROS. Subsequently, this
activates the eukaryotic initiation factor 2α (eIF2α)-activating
transcription factor 4 pathway and upregulates the intracellular
level of GSH, resulting in cisplatin resistance (31). In addition, ROS promote activation of
the ataxia telangiectasia and Rad3-related protein-checkpoint
kinase 1 pathway, resulting in an enhanced DNA damage response and
cisplatin resistance (32). ROS are
mainly generated in cells by the mitochondrial electron transport
chain and NOX enzymes (33).
Although ROS produced in the mitochondria may contribute to
cisplatin resistance (31), the
associations of NOX enzymes with cisplatin resistance are less well
understood. The expression of the seven NOX family members varies
depending on the cellular context and may change in response to
different external stimuli (34). To
identify NOX enzymes associated with cisplatin resistance, the
current study profiled changes in the expression of NOX enzymes in
both cisplatin-resistant and parental cells. NOX5 was identified to
be the most significantly upregulated enzyme in cisplatin-resistant
cells, suggesting that NOX5 may mediate cisplatin resistance. The
observation that NOX5 expression was increased following cisplatin
treatment further supports the hypothesis that epithelial cells may
upregulate the expression of NOX5 in response to cisplatin-induced
genotoxic stress. Additionally, it was revealed that
NOX5-overexpressing cells were more resistant to cisplatin
treatment, which further supports the aforementioned hypothesis. In
summary, the data reveal a significant association between NOX5 and
cisplatin resistance in epithelial cancers.
ROS may promote activation of the PI3K/Akt signaling
pathway via suppression of phosphatase and tensin homolog activity
(35). Accordingly, the current
study suggested that the upregulation of NOX5 may modulate the
activation of the PI3K/Akt pathway in epithelial cancer cells.
PI3K/Akt pathway activation may increase NF-κB transcription
activity and promote the upregulation of numerous anti-apoptotic
proteins, including B-cell lymphoma-extra large, survivin, cellular
inhibitor of apoptosis protein (c-IAP)1 and c-IAP2 (36). Accordingly, increased levels of NOX5
may enable epithelial cancer cells to resist cisplatin-induced
apoptosis and provide a selective survival advantage. Therefore,
NOX5 may be a useful target for sensitizing epithelial cancer cells
to cisplatin. However, to the best of our knowledge, no specific
NOX5 inhibitor is currently available for clinical use (37).
NOX5 expression is regulated by the transcription
factor, signal transducer and activator of transcription 5 (STAT5)
(38). The clinically approved agent
curcumin has been identified as an effective suppressor of STAT5
(39), therefore the current study
suggests that curcumin may inhibit NOX5-mediated cisplatin
resistance. Given the low bioavailability and poor solubility of
curcumin, the current study developed a liposomal form of curcumin
to increase bioavailability in the xenograft model used. The
results demonstrated that treatment with liposomal curcumin
inhibits cisplatin-induced NOX5 expression and enhances the
sensitivity of resistant cancer cells to cisplatin. The anti-cancer
activity of curcumin has been investigated in a number of clinical
trials and its efficacy and safety in cancer treatment have been
well-documented (40). Previously,
curcumin has been reported to enhance the sensitivity of cancer
cells to cisplatin treatment by suppressing the NF-κB signaling
pathway activity, flap endonuclease 1 expression and cyclin D1
expression (17,41,42). The
current data reveal that targeting NOX5 may be a novel mechanism by
which curcumin sensitizes epithelial cancer cells to cisplatin.
In conclusion, the current study revealed that the
NOX5/ROS/Akt axis is associated with acquired cisplatin resistance
in human epithelial cancer cells. The use of curcumin to target
this axis can sensitize cisplatin-resistant cancer cells to
cisplatin treatment both in an in vitro cell line model and
in an in vivo xenograft model. Further investigation is
required to examine the efficacy of curcumin as a treatment to
overcome cisplatin resistance in a clinical setting.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Health and
Medical Research Fund (grant nos. 12133541 and 03143326) and the S.
K. Yee Medical Foundation Grant and Seed Funding for Basic Research
(The University of Hong Kong; grant nos. 201511159256, 201611159282
and 201611159279).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TSW and WG conceived the study and designed the
experiments. SC and MJZ performed the experiments and acquired the
data. TSW, WG and JYWC analyzed and interpreted the data. TSW, WG
and SC drafted the manuscript. JYWC critically revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed according to
the institutional guidelines and were approved by the Institutional
Committee on the Use of Live Animals in Teaching and Research
(protocol no. 3474-14) at the Animal Laboratory, Department of
Surgery, University of Hong Kong.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS
and Wang WY: Phase III study of concurrent chemoradiotherapy versus
radiotherapy alone for advanced nasopharyngeal carcinoma: Positive
effect on overall and progression-free survival. J Clin Oncol.
21:631–637. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trimbos JB, Vergote I, Bolis G, Vermorken
JB, Mangioni C, Madronal C, Franchi M, Tateo S, Zanetta G, Scarfone
G, et al: Impact of adjuvant chemotherapy and surgical staging in
early-stage ovarian carcinoma: European Organisation for Research
and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm
trial. J Natl Cancer Inst. 95:113–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK,
Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE and
Ensley JF: Chemoradiotherapy versus radiotherapy in patients with
advanced nasopharyngeal cancer: Phase III randomized Intergroup
study 0099. J Clin Oncol. 16:1310–1317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu RY, Dong Z, Liu J, Yin JY, Zhou L, Wu
X, Yang Y, Mo W, Huang W, Khoo SK, et al: Role of eIF3a in
regulating cisplatin sensitivity and in translational control of
nucleotide excision repair of nasopharyngeal carcinoma. Oncogene.
30:4814–4823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Liu J, Li L, Nie D, Tao Q, Wu J,
Fan J, Lin C, Zhao S and Ju D: Inhibition of autophagy potentiated
the antitumor effect of nedaplatin in cisplatin-resistant
nasopharyngeal carcinoma cells. PLoS One. 10:e01352362015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Sá Junior PL, Câmara DAD, Porcacchia
AS, Fonseca PMM, Jorge SD, Araldi RP and Ferreira AK: The roles of
ROS in cancer heterogeneity and therapy. Oxid Med Cell Longev.
2017:24679402017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takac I, Schröder K and Brandes RP: The
Nox family of NADPH oxidases: Friend or foe of the vascular system?
Curr Hypertens Rep. 14:70–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD,
Kwon KB, Park C, Park BH, Lee HK, Chung SY, et al: Roles of NADPH
oxidases in cisplatin-induced reactive oxygen species generation
and ototoxicity. J Neurosci. 30:3933–3946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rybak LP, Mukherjea D, Jajoo S and
Ramkumar V: Cisplatin ototoxicity and protection: Clinical and
experimental studies. Tohoku J Exp Med. 219:177–186. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ammon HP and Wahl MA: Pharmacology of
Curcuma longa. Planta Med. 57:1–7. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun J, Chen F, Braun C, Zhou YQ, Rittner
H, Tian YK, Cai XY and Ye DW: Role of curcumin in the management of
pathological pain. Phytomedicine. 48:129–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sreekanth CN, Bava SV, Sreekumar E and
Anto RJ: Molecular evidences for the chemosensitizing efficacy of
liposomal curcumin in paclitaxel chemotherapy in mouse models of
cervical cancer. Oncogene. 30:3139–3152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vinod BS, Antony J, Nair HH,
Puliyappadamba VT, Saikia M, Narayanan SS, Bevin A and Anto RJ:
Mechanistic evaluation of the signaling events regulating
curcumin-mediated chemosensitization of breast cancer cells to
5-fluorouracil. Cell Death Dis. 4:e5052013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duarte VM, Han E, Veena MS, Salvado A, Suh
JD, Liang LJ, Faull KF, Srivatsan ES and Wang MB: Curcumin enhances
the effect of cisplatin in suppression of head and neck squamous
cell carcinoma via inhibition of IKKβ protein of the NFκB pathway.
Mol Cancer Ther. 9:2665–2675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glaser R, Zhang HY, Yao KT, Zhu HC, Wang
FX, Li GY, Wen DS and Li YP: Two epithelial tumor cell lines (HNE-1
and HONE-1) latently infected with Epstein-Barr virus that were
derived from nasopharyngeal carcinomas. Proc Natl Acad Sci USA.
86:9524–9528. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strong MJ, Baddoo M, Nanbo A, Xu M,
Puetter A and Lin Z: Comprehensive high-throughput RNA sequencing
analysis reveals contamination of multiple nasopharyngeal carcinoma
cell lines with HeLa cell genomes. J Virol. 88:10696–10704. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang D, Veena MS, Stevenson K, Tang C, Ho
B, Suh JD, Duarte VM, Faull KF, Mehta K, Srivatsan ES and Wang MB:
Liposome-encapsulated curcumin suppresses growth of head and neck
squamous cell carcinoma in vitro and in xenografts through the
inhibition of nuclear factor kappaB by an Akt-independent pathway.
Clin Cancer Res. 14:6228–6236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spitkovsky D, Schulze A, Boye B and
Jansen-Dürr P: Down-regulation of cyclin A gene expression upon
genotoxic stress correlates with reduced binding of free E2F to the
promoter. Cell Growth Differ. 8:699–710. 1997.PubMed/NCBI
|
|
23
|
Matsumoto Y, Takano H and Fojo T: Cellular
adaptation to drug exposure: Evolution of the drug-resistant
phenotype. Cancer Res. 57:5086–5092. 1997.PubMed/NCBI
|
|
24
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chua BT, Gallego-Ortega D, Ramirez de
Molina A, Ullrich A, Lacal JC and Downward J: Regulation of
Akt(ser473) phosphorylation by choline kinase in breast carcinoma
cells. Mol Cancer. 8:1312009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basu A and Krishnamurthy S: Cellular
responses to Cisplatin-induced DNA damage. J Nucleic Acids.
2010:2013672010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3:1351–1371.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hall MD, Okabe M, Shen DW, Liang XJ and
Gottesman MM: The role of cellular accumulation in determining
sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol
Toxicol. 48:495–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kartalou M and Essigmann JM: Mechanisms of
resistance to cisplatin. Mutat Res. 478:23–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Godwin AK, Meister A, O'Dwyer PJ, Huang
CS, Hamilton TC and Anderson ME: High resistance to cisplatin in
human ovarian cancer cell lines is associated with marked increase
of glutathione synthesis. Proc Natl Acad Sci USA. 89:3070–3074.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang SF, Chen MS, Chou YC, Ueng YF, Yin
PH, Yeh TS and Lee HC: Mitochondrial dysfunction enhances cisplatin
resistance in human gastric cancer cells via the ROS-activated
GCN2-eIF2α-ATF4-xCT pathway. Oncotarget. 7:74132–74151.
2016.PubMed/NCBI
|
|
32
|
Meng Y, Chen CW, Yung MMH, Sun W, Sun J,
Li Z, Li J, Li Z, Zhou W, Liu SS, et al: DUOXA1-mediated ROS
production promotes cisplatin resistance by activating ATR-Chk1
pathway in ovarian cancer. Cancer Lett. 428:104–116. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tong L, Chuang CC, Wu S and Zuo L:
Reactive oxygen species in redox cancer therapy. Cancer Lett.
367:18–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skonieczna M, Hejmo T, Poterala-Hejmo A,
Cieslar-Pobuda A and Buldak RJ: NADPH oxidases: Insights into
selected functions and mechanisms of action in cancer and stem
cells. Oxid Med Cell Longev. 2017:94205392017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nogueira V and Hay N: Molecular pathways:
Reactive oxygen species homeostasis in cancer cells and
implications for cancer therapy. Clin Cancer Res. 19:4309–4314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu M, Qi B, Xiaoxiang W, Xu J and Liu X:
Baicalein increases cisplatin sensitivity of A549 lung
adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed
Pharmacother. 90:677–685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Altenhöfer S, Radermacher KA, Kleikers PW,
Wingler K and Schmidt HH: Evolution of NADPH oxidase inhibitors:
Selectivity and mechanisms for target engagement. Antioxid Redox
Signal. 23:406–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fulton DJR: Nox5 and the regulation of
cellular function. Antioxid Redox Signal. 11:2443–2452. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Blasius R, Reuter S, Henry E, Dicato M and
Diederich M: Curcumin regulates signal transducer and activator of
transcription (STAT) expression in K562 cells. Biochem Pharmacol.
72:1547–1554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Prasad S, Tyagi AK and Aggarwal BB: Recent
developments in delivery, bioavailability, absorption and
metabolism of curcumin: The golden pigment from golden spice.
Cancer Res Treat. 46:2–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baharuddin P, Satar N, Fakiruddin KS,
Zakaria N, Lim MN, Yusoff NM, Zakaria Z and Yahaya BH: Curcumin
improves the efficacy of cisplatin by targeting cancer stem-like
cells through p21 and cyclin D1-mediated tumour cell inhibition in
non-small cell lung cancer cell lines. Oncol Rep. 35:13–25. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zou J, Zhu L, Jiang X, Wang Y, Wang Y,
Wang X and Chen B: Curcumin increases breast cancer cell
sensitivity to cisplatin by decreasing FEN1 expression. Oncotarget.
9:11268–11278. 2018. View Article : Google Scholar : PubMed/NCBI
|