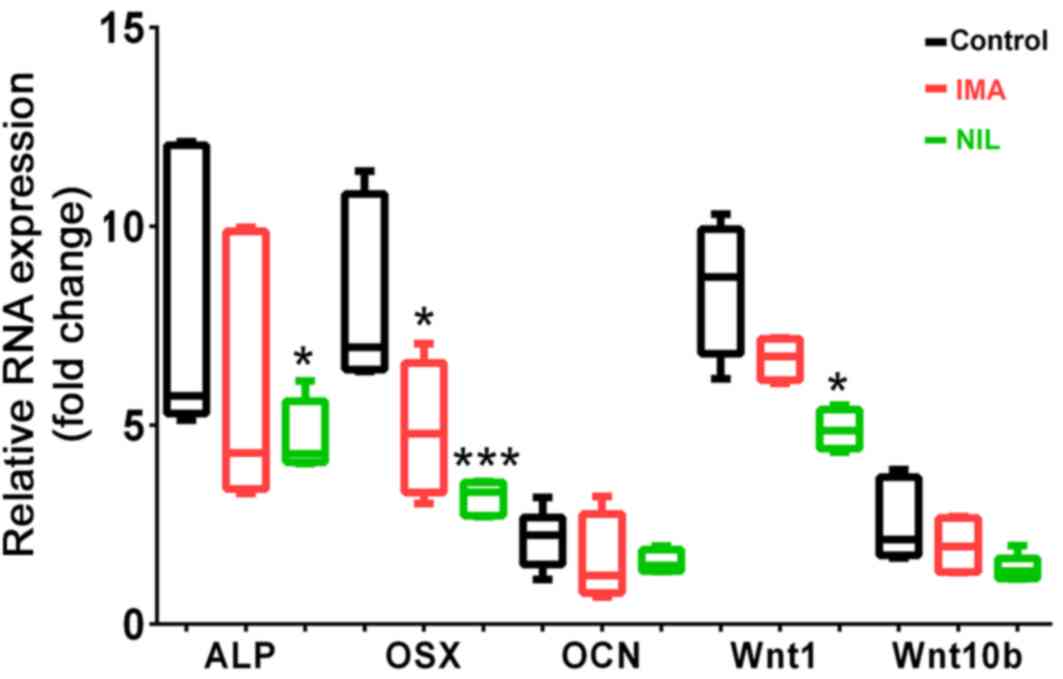
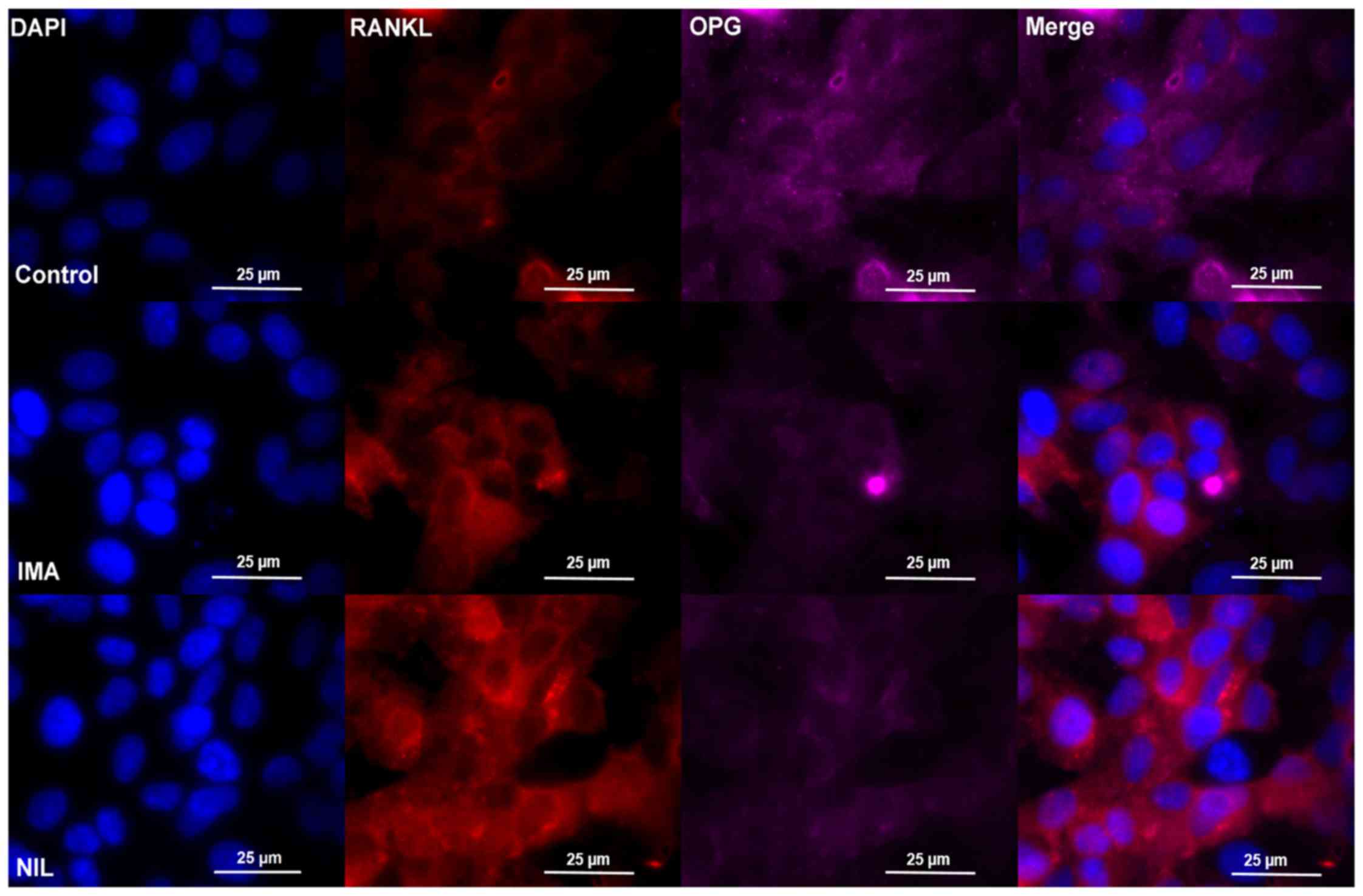
|
1
|
Champagne MA, Capdeville R, Krailo M, Qu
W, Peng B, Rosamilia M, Therrien M, Zoellner U, Blaney SM and
Bernstein M; Children's Oncology Group phase 1 study, : Imatinib
mesylate (STI571) for treatment of children with Philadelphia
chromosome-positive leukemia: Results from a children's oncology
group phase 1 study. Blood. 104:2655–2660. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Druker BJ, Tamura S, Buchdunger E, Ohno S,
Segal GM, Fanning S, Zimmermann J and Lydon NB: Effects of a
selective inhibitor of the Abl tyrosine kinase on the growth of
Bcr-Abl positive cells. Nat Med. 2:561–566. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Druker BJ, Talpaz M, Resta DJ, Peng B,
Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R,
Ohno-Jones S and Sawyers CL: Efficacy and safety of a specific
inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid
leukemia. N Engl J Med. 344:1031–1037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grigg A and Hughes T: Role of allogeneic
stem cell transplantation for adult chronic myeloid leukemia in the
imatinib era. Biol Blood Marrow Transplant. 12:795–807. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Millot F, Guilhot J, Nelken B, Leblanc T,
De Bont ES, Bekassy AN, Gadner H, Sufliarska S, Stary J,
Gschaidmeier H, et al: Imatinib mesylate is effective in children
with chronic myelogenous leukemia in late chronic and advanced
phase and in relapse after stem cell transplantation. Leukemia.
20:187–192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roy L, Guilhot J, Krahnke T,
Guerci-Bresler A, Druker BJ, Larson RA, O'Brien S, So C, Massimini
G and Guilhot F: Survival advantage from imatinib compared with the
combination interferon-alpha plus cytarabine in chronic-phase
chronic myelogenous leukemia: Historical comparison between two
phase 3 trials. Blood. 108:1478–1484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iijima R, Byrne RA, Dibra A, Ndrepepa G,
Spaulding C, Laarman GJ, Menichelli M, Valgimigli M, Di Lorenzo E,
Kaiser C, et al: Drug-eluting stents versus bare-metal stents in
diabetic patients with ST-segment elevation acute myocardial
infarction: A pooled analysis of individual patient data from seven
randomized trials. Rev Esp Cardiol. 62:354–364. 2009.(In English,
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hobernicht SL, Schweiger B, Zeitler P,
Wang M and Hunger SP: Acquired growth hormone deficiency in a girl
with chronic myelogenous leukemia treated with tyrosine kinase
inhibitor therapy. Pediatr Blood Cancer. 56:671–673. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deguchi Y, Kimura S, Ashihara E, Niwa T,
Hodohara K, Fujiyama Y and Maekawa T: Comparison of imatinib,
dasatinib, nilotinib and INNO-406 in imatinib-resistant cell lines.
Leuk Res. 32:980–983. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kantarjian H, Giles F, Wunderle L, Bhalla
K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W,
et al: Nilotinib in imatinib-resistant CML and Philadelphia
chromosome-positive ALL. N Engl J Med. 354:2542–2551. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jabbour E, El AS, Cortes J and Kantarjian
H: Nilotinib: A novel Bcr-Abl tyrosine kinase inhibitor for the
treatment of leukemias. Expert Opin Investig Drugs. 17:1127–1136.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shima H, Tokuyama M, Tanizawa A, Tono C,
Hamamoto K, Muramatsu H, Watanabe A, Hotta N, Ito M, Kurosawa H, et
al: Distinct impact of imatinib on growth at prepubertal and
pubertal ages of children with chronic myeloid leukemia. J Pediatr.
159:676–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berman E, Nicolaides M, Maki RG, Fleisher
M, Chanel S, Scheu K, Wilson BA, Heller G and Sauter NP: Altered
bone and mineral metabolism in patients receiving imatinib
mesylate. N Engl J Med. 354:2006–2113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fierro F, Illmer T, Jing D, Schleyer E,
Ehninger G, Boxberger S and Bornhäuser M: Inhibition of
platelet-derived growth factor receptorbeta by imatinib mesylate
suppresses proliferation and alters differentiation of human
mesenchymal stem cells in vitro. Cell Prolif. 40:355–366. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fitter S, Dewar AL, Kostakis P, To LB,
Hughes TP, Roberts MM, Lynch K, Vernon-Roberts B and Zannettino AC:
Long-term imatinib therapy promotes bone formation in CML patients.
Blood. 111:2538–2547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaeger BA, Tauer JT, Ulmer A, Kuhlisch E,
Roth HJ and Suttorp M: Changes in bone metabolic parameters in
children with chronic myeloid leukemia on imatinib treatment. Med
Sci Monit. 18:CR721–CR728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kroschwald L, Suttorp M, Tauer JT,
Zimmermann N, Gunther C and Bauer A: Offtarget effect of imatinib
and nilotinib on human vitamin D3 metabolism. Mol Med Rep.
17:1382–1388. 2018.PubMed/NCBI
|
|
18
|
Mehlig LM, Garve C, Tauer JT, Suttorp M
and Bauer A: Inhibitory effects of imatinib on vitamin D(3)
synthesis in human keratinocytes. Mol Med Rep. 11:3143–3147. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boyce BF and Xing L: Biology of RANK,
RANKL, and osteoprotegerin. Arthritis Res Ther. 9 (Suppl 1):S12007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mori K, Le GB, Berreur M, Riet A, Moreau
A, Blanchard F, Chevalier C, Guisle-Marsollier I, Léger J, Guicheux
J, et al: Human osteosarcoma cells express functional receptor
activator of nuclear factor-kappa B. J Pathol. 211:555–562. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong MY, Liu LQ, Liu SQ, Liu ZH and Gao
HF: Effects of osteoprotegerin, RANK and RANKL on bone destruction
and collapse in avascular necrosis femoral head. Am J Transl Res.
8:3133–3140. 2016.PubMed/NCBI
|
|
23
|
Stephens AS, Stephens SR and Morrison NA:
Internal control genes for quantitative RT-PCR expression analysis
in mouse osteoblasts, osteoclasts and macrophages. BMC Res Notes.
4:4102011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tauer JT, Hofbauer LC, Jung R, Gerdes S,
Glauche I, Erben RG and Suttorp M: Impact of long-term exposure to
the tyrosine kinase inhibitor imatinib on the skeleton of growing
rats. PLoS One. 10:e01311922015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gandia P, Arellano C, Lafont T, Huguet F,
Malard L and Chatelut E: Should therapeutic drug monitoring of the
unbound fraction of imatinib and its main active metabolite
N-desmethyl-imatinib be developed? Cancer Chemother Pharmacol.
71:531–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suttorp M, Schulze P, Glauche I, Gohring
G, von Neuhoff N, Metzler M, Sedlacek P, de Bont ESJM, Balduzzi A,
Lausen B, et al: Front-line imatinib treatment in children and
adolescents with chronic myeloid leukemia: Results from a phase III
trial. Leukemia. 32:1657–1669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tibullo D, Giallongo C, La CP, Berretta S,
Stagno F, Chiarenza A, Conticello C, Palumbo GA and Di Raimondo F:
Effects of imatinib mesylate in osteoblastogenesis. Exp Hematol.
37:461–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tibullo D, Barbagallo I, Giallongo C, La
CP, Branca A, Conticello C, Stagno F, Chiarenza A, Palumbo GA and
Di Raimondo F: Effects of second-generation tyrosine kinase
inhibitors towards osteogenic differentiation of human mesenchymal
cells of healthy donors. Hematol Oncol. 30:27–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Sullivan S, Naot D, Callon KE, Watson M,
Gamble GD, Ladefoged M, Karsdal MA, Browett P, Cornish J and Grey
A: Imatinib mesylate does not increase bone volume in vivo. Calcif
Tissue Int. 88:16–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Sullivan S, Tay ML, Lin JM, Bava U,
Callon K, Cornish J, Naot D and Grey A: Tyrosine kinase inhibitors
regulate OPG through inhibition of PDGFRβ. PLoS One.
11:e01647272016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Sullivan S, Naot D, Callon K, Porteous
F, Horne A, Wattie D, Watson M, Cornish J, Browett P and Grey A:
Imatinib promotes osteoblast differentiation by inhibiting PDGFR
signaling and inhibits osteoclastogenesis by both direct and
stromal cell-dependent mechanisms. J Bone Miner Res. 22:1679–1689.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
O'Sullivan S, Horne A, Wattie D, Porteous
F, Callon K, Gamble G, Ebeling P, Browett P and Grey A: Decreased
bone turnover despite persistent secondary hyperparathyroidism
during prolonged treatment with imatinib. J Clin Endocrinol Metab.
94:1131–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vandyke K, Fitter S, Dewar AL, Hughes TP
and Zannettino AC: Dysregulation of bone remodeling by imatinib
mesylate. Blood. 115:766–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Narayanan KR, Bansal D, Walia R, Sachdeva
N, Bhansali A, Varma N and Marwaha RK: Growth failure in children
with chronic myeloid leukemia receiving imatinib is due to
disruption of GH/IGF-1 axis. Pediatr Blood Cancer. 60:1148–1153.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Millot F, Guilhot J, Baruchel A, Petit A,
Leblanc T, Bertrand Y, Mazingue F, Lutz P, Vérité C, Berthou C, et
al: Growth deceleration in children treated with imatinib for
chronic myeloid leukaemia. Eur J Cancer. 50:3206–3211. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou L, An N, Haydon RC, Zhou Q, Cheng H,
Peng Y, Jiang W, Luu HH, Vanichakarn P, Szatkowski JP, et al:
Tyrosine kinase inhibitor STI-571/Gleevec down-regulates the
beta-catenin signaling activity. Cancer Lett. 193:161–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suknuntha K, Thita T, Togarrati PP,
Ratanachamnong P, Wongtrakoongate P, Srihirun S, Slukvin I and
Hongeng S: Wnt signaling inhibitor FH535 selectively inhibits cell
proliferation and potentiates imatinib-induced apoptosis in myeloid
leukemia cell lines. Int J Hematol. 105:196–205. 2017. View Article : Google Scholar : PubMed/NCBI
|