Introduction
Gastric cancer (GC) is the third leading cause of
cancer-associated death worldwide (1). In particular, the prevalence of GC is
high in Japan and other countries in East Asia (2). Although there has been a rise in the
overall survival rate due to curative resection (R0) with lymph
node dissection plus adjuvant chemotherapy, the survival rate of
patients with state II and stage III GC was 30% in 2011 (3). Peritoneal recurrence is the most
frequent type of recurrence in patients with GC and is associated
with poor prognosis (4). Peritoneal
dissemination is detected in 14% of patients with GC at the time of
initial diagnosis, and the median survival time for these patients
is ~4 months (5). Various treatment
regimens have been utilized to treat peritoneal recurrence,
including systemic chemotherapy, intraperitoneal chemotherapy,
hyperthermia and aggressive surgery; however, none of these
treatments have led to a satisfactory clinical outcome (6,7). Among
all cases diagnosed as curable without metastasis prior to surgery,
10–20% present peritoneal dissemination during surgery (8). Prophylactic treatment strategies,
including extensive intraoperative peritoneal lavage and
intraperitoneal chemotherapy are associated with improved survival
rates (9), and their effectiveness
is associated with the early detection of patients at high risk of
developing peritoneal metastasis (10).
Peritoneal fluid lavage cytology (CY) utilizes
peritoneal washing as a means to identify patients with a high
likelihood of peritoneal recurrence, and CY is a common clinical
practice in Japan. However, this procedure is not practiced in
Western countries, due to low sensitivity for the detection of
recurrence (11). In fact, evidence
suggests that patients with negative CY (CY0) occasionally develop
peritoneal recurrence post-surgery (12). A prospective randomized study
reported peritoneal recurrence in 14.6% of CY0 patients with stage
II–III GC that underwent R0 resection (3). This observation supports the need for
the discovery of novel biomarkers for the screening of patients
with a high risk of peritoneal recurrence.
Circulating microRNAs (miRNAs/miRs) in plasma or
serum have attracted attention as minimally invasive biomarkers for
the diagnosis and prognosis of various types of cancer, including
GC (13,14). miRNAs are small (23–35 nucleotides)
non-coding RNAs that negatively regulate the expression of target
genes at the post-transcriptional level via RNA interference
(13). miRNAs serve an important
role in several processes associated with carcinogenesis, including
cellular proliferation, apoptosis and differentiation, metastasis
(15). Grady and Tewai (16) reported abnormal expression of miRNAs
in cancer cells, and the role they serve in the commencement and
advancement of cancer in the form of oncogenes or tumor suppressor
genes (16). These miRNAs have been
identified in an extremely stable form within the exosomes in the
plasma and serum, and are protected from endogenous RNase activity
(17). Exosomes are vesicles with a
small diameter (50–150 nm) derived from the luminal membranes,
which are released following fusion with the cell membrane
(18). Proteins and selectively
packaged RNAs, including miRNAs, are encapsulated in exosomes in a
stable and intact form (19). These
exosomes may transfer the encapsulated components to other cells
(20–22). Exosome-encapsulated miRNAs
(ex-miRNAs) have recently attracted attention as promising
predictive and prognostic biomarkers in patients with cancer
(23–27). However, the potential role of plasma
ex-miRNAs in the prediction of peritoneal recurrence in patients
with GC, to the best of our knowledge, has not yet been
investigated.
The present study aimed to clarify the applicability
of circulating plasma ex-miRNAs for the prediction of peritoneal
recurrence in patients with stage II and III GC that underwent R0
resection.
Patients and methods
Study design
The inclusion criteria for the healthy controls
were: i) Did not have cancer; ii) No abnormalities in the blood
test results; iii) normal respiratory function; iv) normal
cardiovascular function; and v) normal gastrointestinal
examination. The inclusion criteria for the patients with GC were:
i) Histopathologically confirmed diagnosis of stage II or III GC;
ii) R0 resection (with no tumor cells at the margin); iii) no
evidence of hepatic, peritoneal or distant metastasis; iv) no tumor
cells in the peritoneal fluid on cytological analysis; v) aged
20–85 years; vi) no previous treatment for cancer except for the
initial gastric resection for the primary lesion; and vi) adequate
organ function. The present study first profiled peritoneal
recurrence-specific plasma ex-miRNAs using a miRNA array. Patients
with stage II GC with peritoneal recurrence after surgery (n=3),
stage II GC without peritoneal recurrence after surgery (n=3) and
healthy control subjects (n=3) were examined. The
clinicopathological characteristics of the patients are provided in
Table SI. The average age of the 6
patients with GC was 66 years (range, 63–71 years), and there were
4 men and 2 women. The average age of the 3 healthy controls was 65
years old (range, 62–71 years), and there were 2 men and 1 woman.
These patients and healthy controls were recruited between January
2006 and October 2006 at Teikyo University Hospital (Tokyo, Japan).
Peritoneal recurrence-specific ex-miRNAs were profiled with a miRNA
array using plasma exosomes as described below. Subsequently, the
potential of selected ex-miRNAs was investigated using other
samples collected from 129 patients with GC and 20 healthy
controls. The average age of the 129 patients with gastric cancer
was 68 years old (range, 36–82 years), and there were 90 men and 39
women. The average age of the 20 healthy controls was 60 years old
(range, 53–65 years), and there were 14 men and 6 women. These
patients were recruited between November 2006 and December 2015 at
Teikyo University Hospital and comprised 49 cases with stage II and
80 cases with stage III GC. The cancer stage was determined
according to the tumor-node-metastasis (TNM) classification by the
International Union Against Cancer (28). The inclusion criteria of patients
were as follows: Japanese patients with GC with TNM stage II or
III. Since peritoneal recurrence after surgery was not observed in
patients with stage I GC, these patients were excluded from the
present study. In addition, recurrent cases without peritoneal
dissemination were not included in the present study. The median
follow-up period was 3.4 years (range, 0.04–5.5 years). Blood
samples were collected prior to treatment, and the primary tumor
tissues and matched normal tissues adjacent to the tumor tissues
(≥4 cm away) of the same patient were collected during resection.
Tissues were frozen in liquid nitrogen (−196°C) and stored at
−80°C. until further use. The patients received TS-1 for 1 year as
standard adjuvant chemotherapy. The study protocol conformed with
the guidelines of the Ethics and Indications Committee of Teikyo
University (Tokyo, Japan), and was approved by the Review Board of
Teikyo University (approval no. 09-081-3). Written informed consent
was obtained from all patients.
Purification of exosomes from plasma
samples
Peripheral blood was centrifuged at 1,200 × g for 10
min at 4°C to obtain plasma. Plasma (~1 ml) samples were used for
microarray analysis and reverse transcription-quantitative PCR
(RT-qPCR). The exosomes were purified from the plasma by
ultracentrifugation at 100,000 × g for 70 min at 4°C. The pellets
were stored at −80°C for microarray and RT-qPCR analyses.
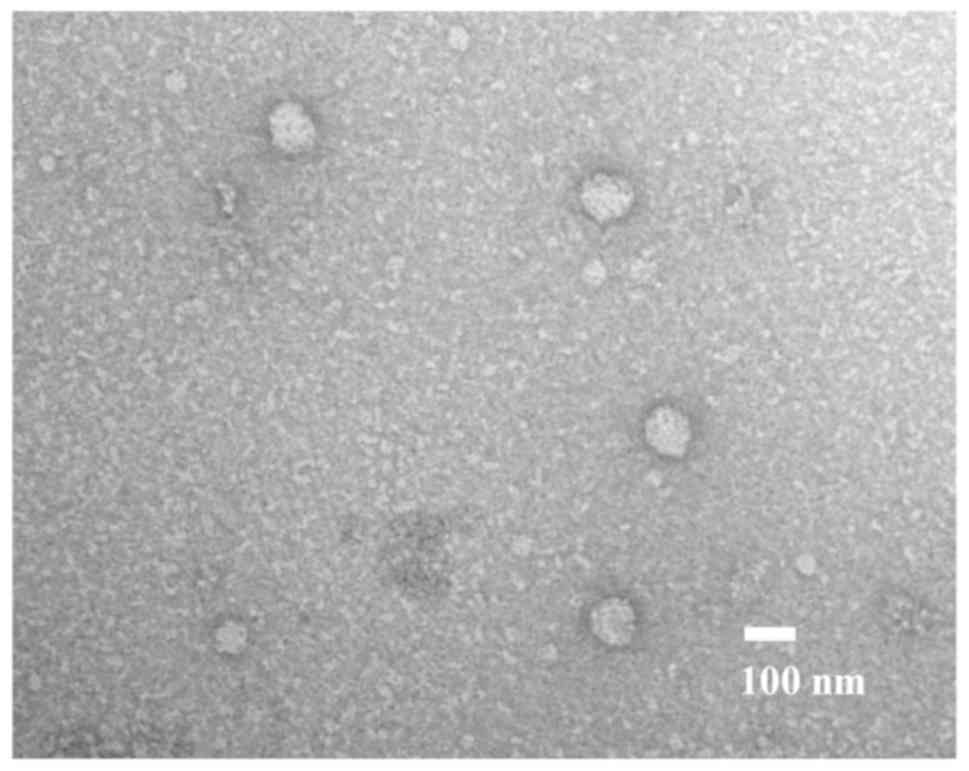
Transmission electron microscopy
The morphology of the isolated exosomes was
confirmed by transmission electron microscopy (Hitachi H-7600;
Hitachi, Ltd.), as previously described (23). Approximately 5 µl of isolated exosome
sample was placed on Parafilm. A carbon coated 400 mesh copper grid
was positioned on the top of the drop for 10 sec and washed with a
droplet of distilled water. The grid was contrasted by adding a
drop of 2% uranyl acetate on Parafilm and placing the grid on top
of drop for 10 sec and excess liquid was removed by gently using
absorbing paper. After drying, the sample was submitted to
transmission electron microscopy. A total of 10 fields of view per
sample were analyzed and samples were viewed at a magnification of
×200,000.
Total RNA extraction from exosomes and
tissues
Total RNAs (including miRNAs) from exosomes were
extracted using the miRNeasy serum/plasma kit (Qiagen, Inc.), and
total RNAs (including miRNAs) of the tissues were extracted using
the miRNeasy Mini kit (Qiagen, Inc.). The exosomes purified from 1
ml of plasma were diluted with 1 ml of QIAzol Lysis reagent
(Qiagen, Inc.). Subsequent extraction and analysis using cartridges
(Qiagen China Co., Ltd.) was performed according to the
manufacturer's protocol. The quality of the extracted RNA was
analyzed using an Agilent 2100 Bioanalyzer (Agilent Technologies,
Inc.).
miRNA microarray analysis
Exosomal miRNA expression profiles were investigated
with 3D-Gene Human miRNA Oligo chips ver. 20 (Toray Industries,
Inc.), according to the manufacturer's protocol. Fluorescence
signals were scanned and analyzed using a 3D-gene scanner (Toray
Industries, Inc.). A total of 2,578 genes were mounted on the chip.
The raw data from each spot were normalized by the subtraction of
the background signal mean intensity, as determined by the 95% CI
of the signal intensities of all blank spots. Any signal intensity
in both duplicate spots at >2 SD of the background signal
intensity was considered as a valid measurement.
RT-qPCR for miRNAs from exosomes and
tissues
The expression levels of miRNAs from plasma exosomes
and tissues were assayed using RT-qPCR. The reverse transcription
protocol was: 30 min at 16°C, 30 min at 42°C and 5 min at 85°C.
Complementary DNA (cDNA) was synthesized from total RNA using
TaqMan MicroRNA primers specific for miRNA-21 (miR-21), miRNA-92a
(miR-92a), Caenorhabditis elegans miR-39 (Cel-miR-39) and
miRNA-16 (miR-16) (Thermo Fisher Scientific, Inc.) and the TaqMan
Micro-RNA Reverse Transcription kit (Thermo Fisher Scientific,
Inc.). Cel-miR-39 was selected as the external control, while
miR-16 served as an internal control, as previously described
(17). In the tissues, cDNA was
synthesized from total RNA using TaqMan miRNA primers specific for
miR-21 (assay ID 000397), miR-92a (assay ID 000431) and RNA, U6
small nuclear 6, pseudogene (assay ID 001093; Thermo Fisher
Scientific, Inc.) and the TaqMan Micro-RNA Reverse Transcription
kit (Thermo Fisher Scientific, Inc.). U6 small nuclear 6 was used
as an internal control. qPCR was performed using TaqMan Universal
PCR Master mix (Thermo Fisher Scientific, Inc.) and the StepOne™
system (Thermo Fisher Scientific, Inc.). PCR reaction mixtures were
incubated at 95°C for 10 min for denaturation, followed by 45
amplification cycles of 95°C for 15 sec and 60°C for 1 min,
followed by an extension at 40°C for 30 sec. The experiments were
repeated three times. Relative quantification of miRNA expression
was performed using the 2−ΔΔCq method, as previously
described (24,29).
RT-qPCR for prostaglandin E receptor 4
(EP4) and programmed cell death protein 4 (PDCD4) mRNA in
tissues
Total RNA was extracted from primary cancer tissues
collected from patients with GC using the miRNeasy Mini kit
(Qiagen, Inc.). Random hexamer primers and SuperScript II reverse
transcriptase (Thermo Fisher Scientific, Inc.) were used to obtain
cDNA according to the manufacturer's protocol. RT-qPCR for EP4,
PDCD4 and GAPDH (internal control) was performed using
the LightCycler (Roche Applied Science). Primers for PDCD4
(cat. no. Hs00377253) and EP4 (cat. no. Hs00168761) were
purchased (Thermo Fisher Scientific, Inc.). The sequences for these
primers have not been disclosed by the supplier. The amplification
of these mRNAs was performed using the TaqMan Universal Master mix
II (Thermo Fisher Scientific, Inc.). The thermocycling conditions
were as follows: 95°C For 10 min; followed by 45 cycles of 95°C for
15 sec, 60°C for 1 min, and 40°C for 30 sec. The mRNA expression
levels of EP4 and PDCD4 were normalized to
GAPDH mRNA expression.
Postoperative surveillance
The follow-up program comprised interim history,
physical examination, hematology and blood chemistry and was
performed every 3 months for the first postoperative year and every
6 months thereafter. Computed tomography or abdominal
ultrasonography were examined every 6 months. Evidence of
peritoneal recurrence was comprehensively diagnosed using CT, tumor
marker (CA125), paracentesis and autopsy Primary tumor tissues from
48 patients with stage II GC (n=24) and stage III GC (n=24) were
examined. A significant negative correlation was identified between
miR-21 and PDCD4 mRNA expression (P<0.01), as well as
between miR-92a and EP4 mRNA expression (P<0.01). These
results indicated that PDCD4 expression may be negatively
regulated by miR-21, whereas EP4 mRNA expression may be
negatively regulated by miR-92a.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Experiments were repeated three times. In the
clinicopathological study and the survival study, patients were
split into two groups, with one group exhibiting high expression
levels of ex-miR-21 and ex-miR-92a, and the other group exhibiting
low expression levels of these markers. The association between
miRNA expression and clinicopathological characteristics was
analyzed using Student's t-test, a χ2 test or a one-way
ANOVA with a post-hoc Tukey's test. Overall survival (OS) and
peritoneal recurrence-free survival (PRFS) were analyzed using the
Kaplan-Meier survival curve method, and the resulting data were
examined using log-rank and Wilcoxon tests. Cox proportional hazard
regression analysis was used to estimate the univariate and
multivariate hazard ratios for OS and PRFS. Multivariate analysis
was performed for the factors that exhibited significance in the
univariate analysis. Correlations were determined using Pearson's
rank correlation analysis. Target genes of miR-21 and miR-92a were
determineded using miRBase (http://www.mirbase.org/index.shtml) and miRWalk 2.0
(http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/index.html)
databases. All P-values are two-sided, and P<0.05 was considered
to indicate a statistically significant difference. Statistical
analyses were performed using JMP v9.0 software (SAS Institute,
Inc.).
Results
Identification of exosomes in
plasma
As presented in Fig.
1, exosomes were identified following the ultracentrifugation
of samples from patients with GC. In these samples, round
microvesicles with diameters of 50–150 nm were observed.
Ex-miRNA profile of patients with
GC
The clinical characteristics and background
information (sex, age, nationality and medical history) of 6
patients with GC and 3 healthy controls whose samples were used in
miRNA microarray analyses are described in Table SI. As presented in Table I, the top five upregulated and
downregulated ex-miRNAs in the samples collected from all the
patients are shown in Table SI were
reported. Among the upregulated miRNAs, miR-21-5p (miR-21;
MIMAT0000076) expression was markedly altered in the recurrence
group compared with the healthy control and non-recurrence groups.
Among the downregulated miRNAs, miR-92a-3p (miR-92a; MIMAT0000092)
expression exhibited the greatest alterations in the samples from
the recurrence group compared with those from the healthy control
and non-recurrence groups. Therefore, miR-21 and miR-92a were
selected as biomarkers with potential application for the
prediction of peritoneal recurrence in patients with GC.
 | Table I.Five most up- or downregulated miRNAs
in plasma exosomes of patients with stage II GC with peritoneal
recurrence according to miRNA array analysis. |
Table I.
Five most up- or downregulated miRNAs
in plasma exosomes of patients with stage II GC with peritoneal
recurrence according to miRNA array analysis.
| A, Upregulated |
|---|
|
|---|
|
|
|
| Fold change |
|---|
|
|
|
|
|
|---|
| Ranks | microRNA | MirBase no. | Peritoneal
recurrent GC vs. healthy controls | Peritoneal
recurrent GC vs. non-recurrent GC |
|---|
| 1 | miR-21-5p | MIMAT 0000076 | 3.28 | 2.71 |
| 2 | miR-204-3p | MIMAT 0022693 | 3.14 | 2.21 |
| 3 | miR-6879-5p | MIMAT 0027658 | 3.12 | 2.17 |
| 4 | miR-3928-3p | MIMAT 0018205 | 3.07 | 2.07 |
| 5 | miR-4476 | MIMAT 0019003 | 3.03 | 2.14 |
|
| B,
Downregulated |
|
|
|
|
| Fold
change |
|
|
|
|
|
| Ranks |
microRNA | MirBase
no. | Peritoneal
recurrent GC vs. healthy controls | Peritoneal
recurrent GC vs. non-recurrent GC |
|
| 1 | miR-92a-3p | MIMAT 0000092 | 0.31 | 0.36 |
| 2 | miR-6850-3p | MIMAT 0027601 | 0.34 | 0.37 |
| 3 | miR-3944-3p | MIMAT 0018360 | 0.37 | 0.42 |
| 4 | miR-23b-3p | MIMAT 0000418 | 0.38 | 0.45 |
| 5 | miR-4686 | MIMAT 0019773 | 0.41 | 0.50 |
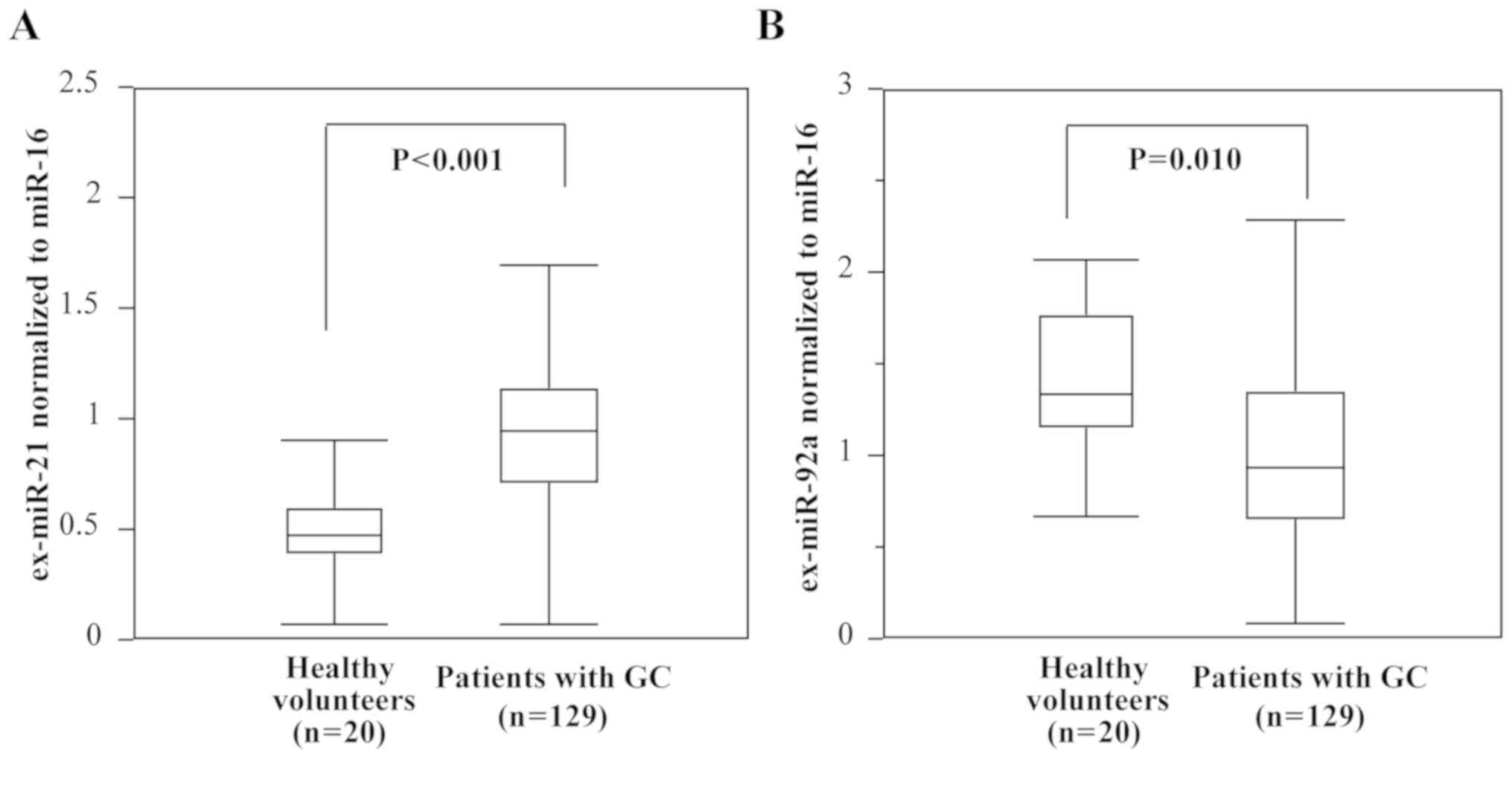
Comparison of ex-miR-21 and ex-miR-92a
levels in patients with GC and healthy controls
The expression levels of ex-miR-21 were determined
to be significantly higher in patients with GC than in healthy
controls, whereas those of ex-miR-92a were significantly lower in
patients with GC than in healthy controls (Fig. 2).
Clinicopathological characteristics
and ex-miR-21 and ex-miR-92a expression
In Table II, the
associations between the expression levels of ex-miR-21 and
ex-miR-92a, and clinicopathological characteristics were presented.
The patients were split into two groups as follows: One exhibiting
high expression levels of ex-miR-21 and ex-miR-92a, and the other
with low expression levels of these markers. The cut-off levels
were determined as 0.93 for ex-miR-21 and 1.04 for ex-miR-92a,
which were the mean levels. A statistically significant association
was observed between ex-miR-21 expression and TNM stage. Sex, tumor
size, differentiation, lymphatic invasion, venous invasion and
lymph node metastasis were not identified to exhibit a significant
association with the levels of ex-miR-21. The analysis of
ex-miR-92a levels revealed no significant association with any of
the clinicopathological characteristics.
 | Table II.Association between
clinicopathological characteristics and plasma levels of ex-miR-92a
and ex-miR-21. |
Table II.
Association between
clinicopathological characteristics and plasma levels of ex-miR-92a
and ex-miR-21.
|
| ex-miR-92a | ex-miR-21 |
|---|
|
|
|
|
|---|
| Variables | High (n=61), n
(%) | Low (n=68), n
(%) | P-value | High (n=58), n
(%) | Low (n=71), n
(%) | P-value |
|---|
| Sex |
|
| 0.33 |
|
| 0.57 |
|
Male | 40 (65.6) | 50 (73.5) |
| 42 (72.4) | 48 (67.6) |
|
|
Female | 21 (34.4) | 18 (26.5) |
| 16 (27.6) | 23 (32.4) |
|
| Tumor size, cm |
|
| 0.90 |
|
| 0.26 |
|
<5 | 20 (32.8) | 23 (33.8) |
| 16 (27.6) | 27 (38.0) |
|
| ≥5 | 41 (67.2) | 45 (66.2) |
| 42 (72.4) | 44 (62.0) |
|
|
Differentiation |
|
| 0.80 |
|
| 0.80 |
|
Well/moderate | 9 (14.8) | 9
(13.2) |
| 9 (15.5) | 9 (12.7) |
|
|
Poorly/other | 52 (85.2) | 59 (86.8) |
| 49 (84.5) | 62 (87.3) |
|
| Lymphatic
invasion |
|
| 0.10 |
|
| 0.26 |
|
Ly(−) | 9 (14.8) | 15 (22.1) |
| 8 (13.8) | 16 (22.5) |
|
|
Ly(+) | 52 (85.2) | 53 (77.9) |
| 50 (86.2) | 55 (77.5) |
|
| Venous
invasion |
|
| 0.75 |
|
| 0.26 |
|
V(−) | 12 (19.7) | 12 (17.6) |
| 8 (13.8) | 16 (22.5) |
|
|
V(+) | 49 (80.3) | 56 (82.4) |
| 50 (86.2) | 55 (77.5) |
|
| Lymph node
metastasis |
|
| 0.11 |
|
| 0.07 |
|
pN(−) | 8 (13.1) | 17 (25.0) |
| 7 (12.1) | 18 (25.4) |
|
|
pN(+) | 53 (86.9) | 51 (75.0) |
| 51 (87.9) | 53 (74.6) |
|
| TNM Stage |
|
| 0.27 |
|
| 0.03 |
| II | 22 (36.1) | 27 (39.7) |
| 16 (27.6) | 33 (46.5) |
|
|
III | 39 (63.9) | 41 (60.3) |
| 42 (72.4) | 38 (53.5) |
|
Sensitivity, specificity, and accuracy
of ex-miR-21 and ex-miR-92a in the detection of peritoneal
recurrence
As presented in Table
III, the sensitivity, specificity and accuracy of ex-miR-21 and
ex-miR-92a was investigated for the detection of peritoneal
recurrence. In this analysis, >60% sensitivity, specificity and
accuracy were identified. The specificity and accuracy of ex-miR-21
were markedly higher than those of ex-miR-92a. The levels of
ex-miR21 and ex-miR92a between peritoneal recurrence cases and
peritoneal recurrence-free cases were determined (Table IV). The results revealed that
ex-miR-21 expression was significantly higher in peritoneal
recurrence cases than in peritoneal recurrence-free cases. By
contrast, ex-miR-92a expression was significantly lower in
peritoneal recurrence cases than in peritoneal recurrence-free
cases.
 | Table III.Sensitivity, specificity and accuracy
of ex-miR-21 and ex-miR-92a for predicting peritoneal
recurrence. |
Table III.
Sensitivity, specificity and accuracy
of ex-miR-21 and ex-miR-92a for predicting peritoneal
recurrence.
| Analysis item | ex-miR-21 | ex-miR-92a |
|---|
| Sensitivity
(%) | 45/73 (61.6) | 46/73 (63.0) |
| Specificity
(%) | 43/56 (76.8) | 34/56 (60.7) |
| Accuracy (%) | 88/129 (68.2) | 80/129 (62.0) |
 | Table IV.Comparison of peritoneal recurrence
and ex-miR-21and ex-miR-92a levels. |
Table IV.
Comparison of peritoneal recurrence
and ex-miR-21and ex-miR-92a levels.
| miR | Peritoneal
recurrence cases | Peritoneal
recurrence-free cases | P-value |
|---|
| Ex-miR-21 | 1.04±0.37 | 0.79±0.19 | 0.042a |
| Ex-miR-92a | 0.86±0.41 | 1.28±0.65 | 0.031a |
Association of ex-miR-21 and ex-miR92a
levels
The present study examined the combination of
ex-miR-21 and ex-miR-92a to clarify the association of these miRNAs
(Table V). The numbers of patients
in the ex-miR-21high/ex-miR-92alow,
ex-miR-21low/ex-miR-92alow,
ex-miR-21high/ex-miR92ahigh and
ex-miR-21low/ex-miR-92ahigh groups were 28
(21.7%), 40 (31.0%), 30 (23.3%) and 31 (24.0%), respectively.
 | Table V.Combination of ex-miR-21and
ex-miR-92a levels. |
Table V.
Combination of ex-miR-21and
ex-miR-92a levels.
| Combination of
ex-miRs | Patients, n
(%) |
|---|
|
ex-miR-21high/ex-miR-92alow | 28 (21.7) |
|
ex-miR-21low/ex-miR-92alow | 40 (31.0) |
|
ex-miR-21high/ex-miR-92ahigh | 30 (23.3) |
|
ex-miR-21low/ex-miR-92ahigh | 31 (24.0) |
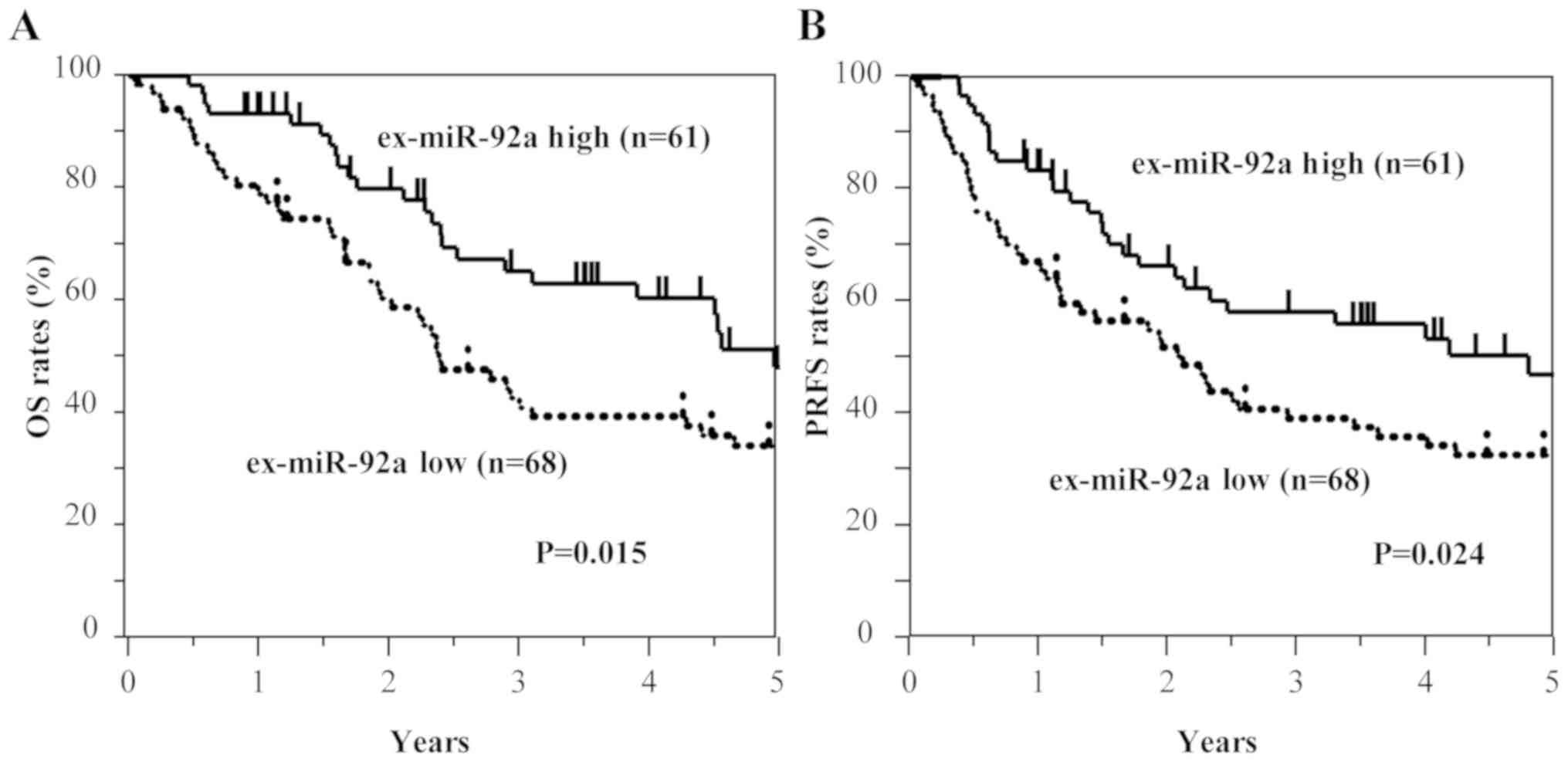
Kaplan-Meier survival curves of OS and
PRFS based on ex-miRNA levels
Among all patients with GC, including those with
stage II and III GC (n=129), those with high ex-miR-21 expression
exhibited significantly worse OS and PRFS compared with those with
low ex-miR-21 levels (Fig. 3).
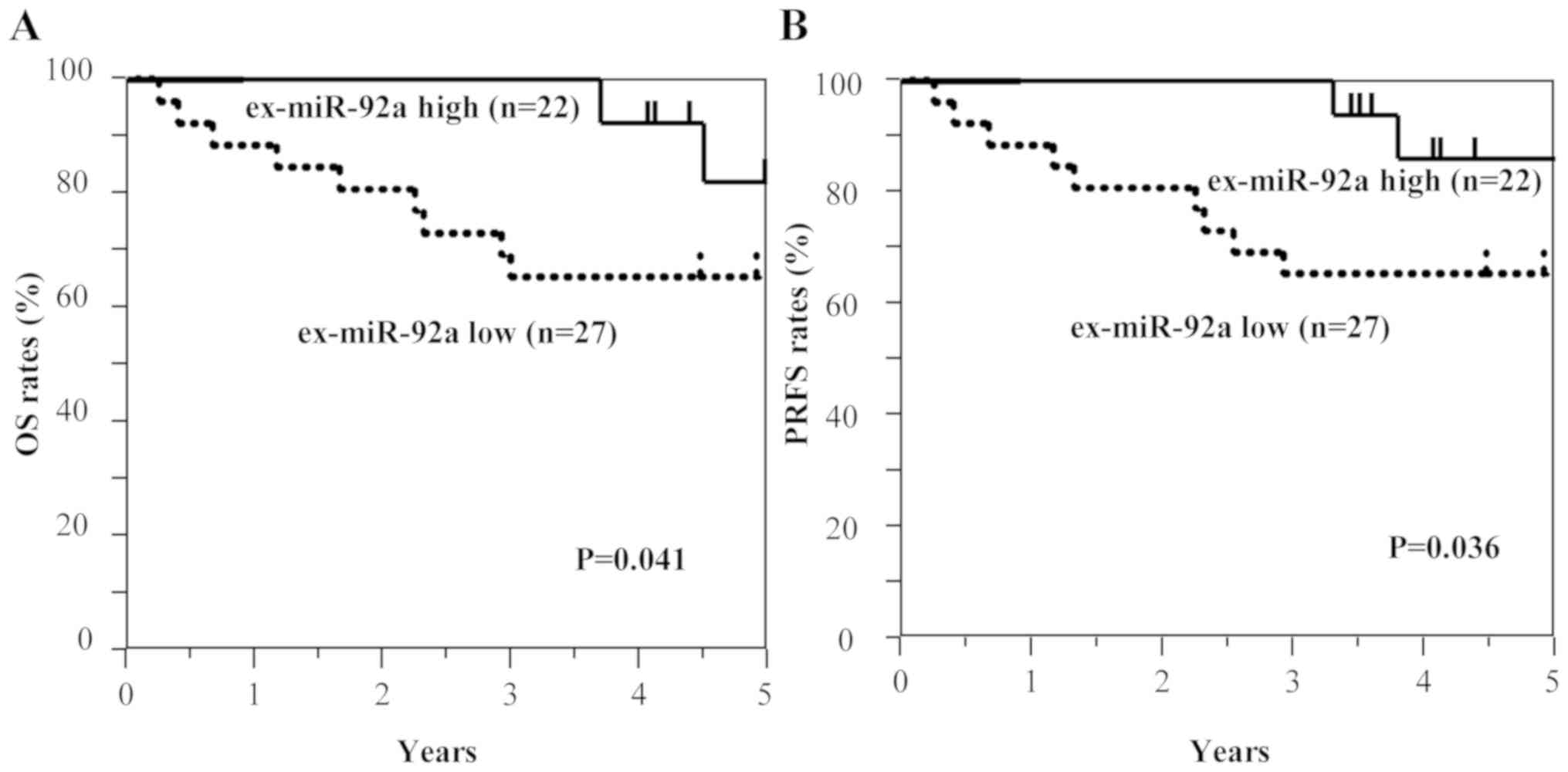
Additionally, the present study analyzed the data at each tumor
stage. In the analysis of patients with stage II GC (n=49), OS and
PRFS rates were significantly lower for patients with high
ex-miR-21 levels compared with patients with low ex-miR-21
expression (Fig. 4). In the analysis
of patients with stage III GC (n=80), OS and PRFS rates were
significantly lower for patients with high ex-miR-21 expression
compared with patients with low ex-miR-21 expression (Fig. 5).
By contrast, the low ex-miR-92a expression group
exhibited significantly worse OS and PRFS compared with the high
ex-miR-92a expression group among all patients with GC (Fig. 6). In the analysis of each tumor
stage, the patients with stage II GC and low ex-miR-92a levels
exhibited significantly worse OS and PRFS compared with patients
with high ex-miR-92a expression (n=49; Fig. 7). Among patients with stage III GC
(n=80), the low ex-miR-92a expression group exhibited significantly
worse OS and PRFS compared with the high ex-miR-92a expression
group (Fig. 8).
These results suggest that high ex-miR-21 expression
and low ex-miR-92a expression were associated with peritoneal
recurrence and poor prognosis in patients with stage II and III
GC.
Univariate and multivariate Cox
analyses for OS and PRFS
For univariate analysis, sex, tumor size, lymph node
metastasis, lymphatic invasion, venous invasion, differentiation,
stage, ex-miR-21 expression and ex-miR-92a expression were
examined. Multivariate analysis was performed for the variables
that exhibited significance in univariate analysis. The results of
the univariate and multivariate Cox analyses for OS and PRFS in all
patients (n=129) were presented in Table VI. In the univariate analysis for OS
and PRFS, tumor size, lymph node metastasis, stage, and ex-miR-21
and ex-miR-92a levels were significant, whereas stage, and
ex-miR-21 and ex-miR-92a levels were determined to be significant
for OS and PRFS in the multivariate analysis.
 | Table VI.Univariate and multivariate Cox
analyses for OS in patients with stage II and III gastric
cancer. |
Table VI.
Univariate and multivariate Cox
analyses for OS in patients with stage II and III gastric
cancer.
| A, OS |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Regression
coefficient | Hazard ratio (95%
CI) | P-value | Regression
coefficient | Hazard ratio (95%
CI) | P-value |
|---|
| Sex | 0.10 | 1.10
(0.66–1.94) | 0.720 |
|
|
|
| Tumor size | 0.65 | 1.92
(1.13–3.44) | 0.015 | 0.19 | 1.21
(0.68–2.26) | 0.518 |
| Lymph node
metastasis | 0.80 | 2.23
(1.09–5.38) | 0.026 | −0.39 | 0.68
(0.23–2.01) | 0.474 |
| Lymphatic
invasion | 0.40 | 1.50
(0.80–3.13) | 0.219 |
|
|
|
| Venous
invasion | 0.57 | 1.78
(0.92–3.85) | 0.087 |
|
|
|
|
Differentiation | 0.13 | 1.14
(0.61–2.38) | 0.691 |
|
|
|
| Stage | 1.47 | 4.34
(2.43–8.35) | 0.001 | 1.67 | 5.30
(2.45–13.41) | 0.001 |
| ex-miR-21 | 1.16 | 3.20
(1.95–5.34) | 0.011 | 1.02 | 2.77
(1.66–4.71) | 0.027 |
| ex-miR-92a | −0.30 | 0.41
(0.21–0.68) | 0.014 | −0.78 | 0.46
(0.27–0.75) | 0.022 |
|
| B, PRFS |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Variables | Regression
coefficient | Hazard ratio
(95% CI) | P-value | Regression
coefficient | Hazard ratio
(95% CI) | P-value |
|
| Sex | 0.68 | 1.97
(1.18–3.46) | 0.009 | 0.15 | 1.16
(0.66–2.11) | 0.616 |
| Tumor size | 0.94 | 2.56
(1.26–6.15) | 0.008 | −0.31 | 0.73
(0.26–2.12) | 0.552 |
| Lymph node
metastasis | 0.49 | 1.63
(0.87–3.39) | 0.130 |
|
|
|
| Lymphatic
invasion | 0.52 | 1.69
(0.90–3.50) | 0.104 |
|
|
|
| Venous
invasion | 0.19 | 1.21
(0.65–2.51) | 0.567 |
|
|
|
|
Differentiation | 1.65 | 5.19
(2.92–9.95) | 0.001 | 1.80 | 6.07
(2.87–14.73) | 0.001 |
| Stage | 1.11 | 3.03
(1.88–4.78) | 0.012 | 1.02 | 2.76
(1.36–5.27) | 0.025 |
| ex-miR-21 | −0.54 | 0.58
(0.31–0.83) | 0.023 | −0.69 | 0.67
(0.36–0.91) | 0.032 |
| ex-miR-92a |
|
|
|
|
|
|
Subsequently, the present study conducted analysis
at each tumor stage. In the univariate analysis of patients with
stage II GC, tumor size, ex-miR-21 levels and ex-miR-92a levels
exhibited significance for OS and PRFS (Table VII). In the multivariate analysis
of patients with stage II GC, ex-miR-21 and ex-miR-92a levels were
significantly associated with OS and PRFS. In the univariate
analysis of patients with stage III GC, lymph node metastasis, and
ex-miR-21 and ex-miR-92a levels were significantly associated with
OS and PRFS (Table VIII), whereas
ex-miR-21 and ex-miR-92a levels exhibited significance for OS and
PRFS in the multivariate analysis.
 | Table VII.Univariate and multivariate Cox
analyses for OS and PRFS in patients with stage II gastric
cancer. |
Table VII.
Univariate and multivariate Cox
analyses for OS and PRFS in patients with stage II gastric
cancer.
| A, OS |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Regression
coefficient | Hazard ratio (95%
CI) | P-value | Regression
coefficient | Hazard ratio (95%
CI) | P-value |
|---|
| Sex | 1.63 | 3.03
(0.98–11.27) | 0.065 |
|
|
|
| Tumor size | 1.11 | 5.09
(1.00–12.72) | 0.041 | 1.20 | 3.03
(0.98–11.27) | 0.055 |
| Lymph node
metastasis | −0.19 | 0.83
(0.28–2.59) | 0.740 |
|
|
|
| Lymphatic
invasion | −0.24 | 0.78
(0.25–2.90) | 0.691 |
|
|
|
| Venous
invasion | 1.25 | 3.48
(0.69–63.39) | 0.153 |
|
|
|
|
Differentiation | −0.66 | 0.52
(0.17–1.72) | 0.265 |
|
|
|
| ex-miR-21 | 1.15 | 3.17
(1.28–10.93) | 0.024 | 1.66 | 5.25
(1.49–18.65) | 0.035 |
| ex-miR-92a | −1.44 | 0.24
(0.04–0.88) | 0.031 | −1.40 | 0.25
(0.04–0.93) | 0.037 |
|
| B, PRFS |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Variables | Regression
coefficient | Hazard ratio
(95% CI) | P-value | Regression
coefficient | Hazard ratio
(95% CI) | P-value |
|
| Sex | 1.23 | 3.43
(1.08–13.08) | 0.036 | 1.28 | 2.94
(0.95–10.93) | 0.061 |
| Tumor size | −0.19 | 0.83
(0.27–2.58) | 0.737 |
|
|
|
| Lymph node
metastasis | 0.14 | 1.53
(0.33–7.07) | 0.580 |
|
|
|
| Lymphatic
invasion | 1.28 | 3.59
(0.71–65.45) | 0.141 |
|
|
|
| Venous
invasion | −0.67 | 0.51
(0.17–1.70) | 0.257 |
|
|
|
|
Differentiation | 1.06 | 2.88
(1.80–9.00) | 0.022 | 1.54 | 3.36
(1.49–10.65) | 0.031 |
| ex-miR-21 | −1.48 | 0.23
(0.04–0.85) | 0.026 | −1.43 | 0.24
(0.04–0.91) | 0.035 |
| ex-miR-92a |
|
|
|
|
|
|
 | Table VIII.Univariate and multivariate Cox
analyses for OS and PRFS in patients with stage III gastric
cancer. |
Table VIII.
Univariate and multivariate Cox
analyses for OS and PRFS in patients with stage III gastric
cancer.
| A, OS |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Regression
coefficient | Hazard ratio (95%
CI) | P-value | Regression
coefficient | Hazard ratio (95%
CI) | P-value |
|---|
| Sex | −0.28 | 0.76
(0.43–1.38) | 0.352 |
|
|
|
| Tumor size | −0.05 | 0.95
(0.52–1.84) | 0.864 |
|
|
|
| Lymph node
metastasis | 1.54 | 4.69
(1.03–82.91) | 0.045 | 1.26 | 3.54
(0.74–63.31) | 0.131 |
| Lymphatic
invasion | 0.48 | 1.61
(0.74–4.23) | 0.243 |
|
|
|
| Venous
invasion | 0.44 | 1.72
(0.71–3.85) | 0.234 |
|
|
|
|
Differentiation | −0.10 | 0.90
(0.40–2.60) | 0.833 |
|
|
|
| ex-miR-21 | 0.89 | 2.42
(1.40–4.31) | 0.014 | 1.26 | 3.54
(1.74–3.31) | 0.025 |
| ex-miR-92a | −0.65 | 0.52
(0.30–0.89) | 0.017 | −0.55 | 0.58
(0.33–0.99) | 0.047 |
|
| B, PRFS |
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Variables | Regression
coefficient | Hazard ratio
(95% CI) | P-value | Regression
coefficient | Hazard ratio
(95% CI) | P-value |
|
| Sex | −0.01 | 0.99
(0.56–1.86) | 0.962 |
|
|
|
| Tumor size | 1.79 | 5.99
(1.32–5.94) | 0.041 | 1.41 | 4.11
(0.87–7.36) | 0.080 |
| Lymph node
metastasis | 0.58 | 1.78
(0.83–4.65) | 0.150 |
|
|
|
| Lymphatic
invasion | 0.38 | 1.47
(0.75–3.21) | 0.273 |
|
|
|
| Venous
invasion | −0.24 | 0.79
(0.35–2.27) | 0.625 |
|
|
|
|
Differentiation | 0.81 | 2.26
(1.33–3.94) | 0.024 | 0.87 | 2.07
(1.41–3.98) | 0.028 |
| ex-miR-21 | −0.56 | 0.57
(0.34–0.96) | 0.033 | −0.43 | 0.51
(0.28–0.91) | 0.044 |
| ex-miR-92a |
|
|
|
|
|
|
These observations indicated that ex-miR-21 and
ex-miR-92a levels were independent predictive biomarkers for
peritoneal recurrence and prognosis in patients with stage II and
III GC.
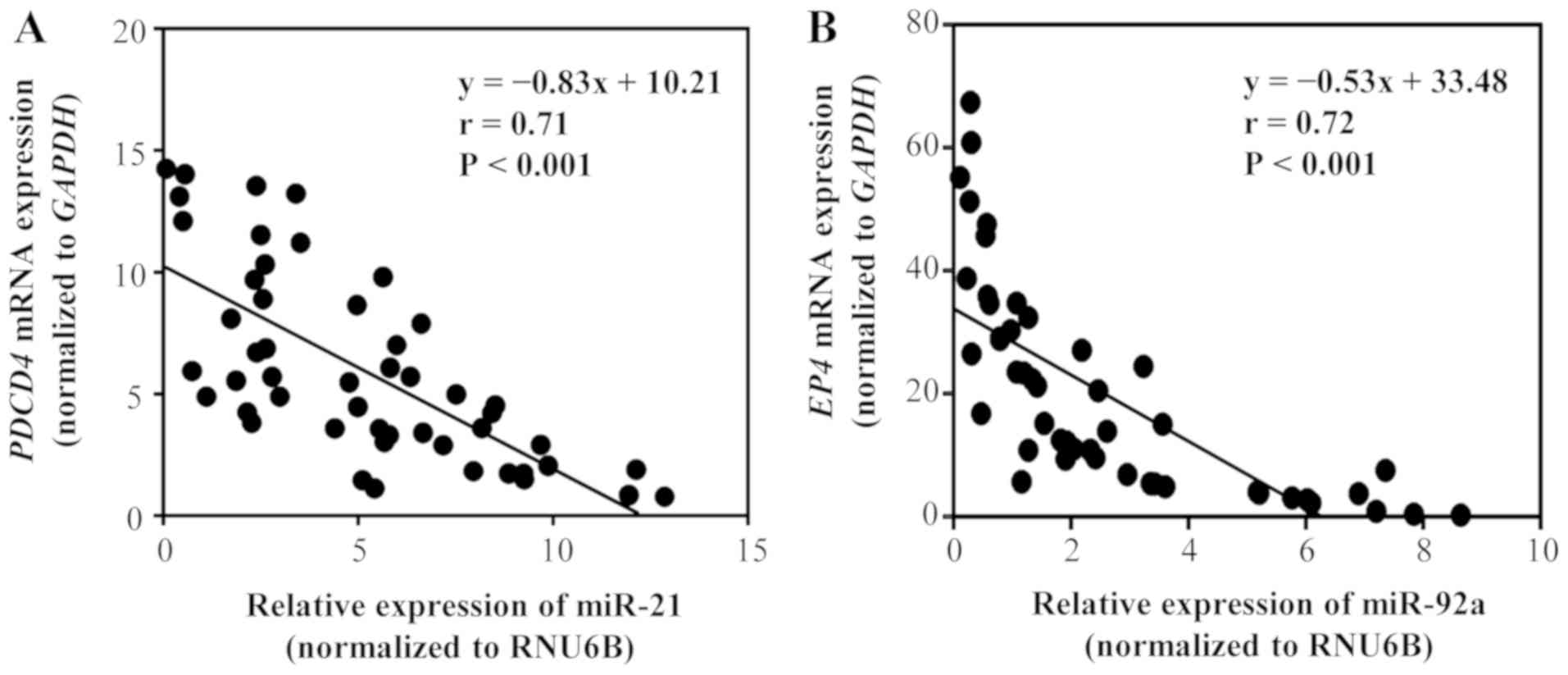
Correlation between target genes, and
miR-21 and miR-92a levels in GC tissues
We investigated the correlation between miR-21 and
PDCD4 mRNA expression, and miR-92a and EP4 mRNA
expression in GC tissues (Fig. 9).
PDCD4 for miR-21 and EP4 for miR-92a were selected as potential
target genes, based on the results of searches using miRBase and
miRWalk databases (Table SII).
Primary tumor tissues from 48 patients with stage II GC (n=24) and
stage III GC (n=24) were examined. A significant negative
correlation was identified between miR-21 and PDCD4 mRNA
expression (P<0.01), as well as between miR-92a and EP4
mRNA expression (P<0.01). These results indicated that
PDCD4 expression may be negatively regulated by miR-21,
whereas EP4 mRNA expression may be negatively regulated by
miR-92a.
Discussion
The present study demonstrated the potential role of
ex-miR-21 and ex-miR-92a as non-invasive biomarkers for the
prediction of peritoneal recurrence and prognosis in patients with
stage II/III GC that underwent R0 resection.
Several previous studies have revealed the potential
of ex-miRNAs as stable biomarkers for patients with cancer
(30,31). Additionally, our previous studies
demonstrated the usefulness of ex-miR-451a as a biomarker to
predict recurrence and prognosis in patients with non-small cell
lung cancer and pancreatic cancer (25,26).
Furthermore, ex-miR-21 serves as an independent predictive
biomarker for recurrence and prognosis in patients with colorectal
cancer (24). However, to the best
of our knowledge, no studies have examined the clinical
significance of ex-miRNAs in the prediction of peritoneal
recurrence in patients with GC.
The present study evaluated the ex-miRNAs specific
for peritoneal recurrence using a miRNA microarray. In this
analysis, miR-21 exhibited significant upregulation, whereas
miR-92a was the most downregulated miRNA in samples from patients
with stage II GC that exhibited peritoneal recurrence after R0
resection compared with in samples from patients with stage II GC
that did not show any recurrence after R0 resection and healthy
controls. Based on these results, miR-21 and miR-92a were selected
as biomarkers for the prediction of peritoneal recurrence.
Furthermore, the results revealed that ex-miR-21 expression was
significantly higher and ex-miR-92a expression was significantly
lower in patients with GC compared with in healthy controls. Our
previous studies demonstrated that ex-miR-21 levels were
significantly higher in patients with colorectal cancer and
non-small cell lung cancer than in healthy controls (24,25).
Additionally, Huang et al (32) reported significantly lower levels of
serum miR-92a in patients with GC than in healthy controls. By
contrast, Zhu et al (33)
reported that plasma miR-92a, but not ex-miR-92a, is elevated in
patients with GC compared with in healthy controls. While the
reason underlying this discrepancy remains unknown, it may be
associated with the instability of miRNAs in plasma samples
(33). Furthermore, the present
study examined the association between ex-miR-21 and ex-miR-92a
levels and clinicopathological characteristics, and revealed that
high ex-miR-21 expression was significantly associated with tumor
stage. Zhao et al (34)
reported a significant association between plasma miR-21 levels and
tumor size, lymph node metastasis and progression of tumor stage.
The present study failed to observe any association between
ex-miR-92a expression and clinicopathological characteristics.
While studies have examined the diagnostic and
prognostic values of circulating plasma/serum free-miRNAs in
patients with GC, few reports have focused on the plasma/serum
ex-miRNAs (32–35). The present study demonstrated that
high ex-miR-21 levels and low ex-miR-92a levels were significantly
associated with poor OS and PRFS in patients with stage II and III
GC that underwent R0 resection. Cox multivariate analysis revealed
that ex-miR-21 and ex-miR-92a were independent prognostic factors.
Although the prognostic value of plasma/serum ex-miR-21 in patients
with GC has been reported previously (36,37), the
value of ex-miR-21 as a predictive biomarker for peritoneal
recurrence, to the best of our knowledge, has not been examined.
Only a few studies have reported plasma/serum miR-92a as a
biomarker for diagnosis in patients with GC (38). To the best of our knowledge, the
present study is the first report to clarify the predictive value
of ex-miR-21 and ex-miR-92a for peritoneal recurrence and prognosis
in patients with GC.
Interestingly, Chen et al (5) reported the importance of the
exosome-dependent molecular transfer or signaling pathway
activation in the four stages (exfoliation, survival, adhesion and
invasion and angiogenesis) of peritoneal dissemination in GC. The
present study demonstrated that ex-miR-21 and ex-miR-92a levels
differ significantly between patients with peritoneal recurrence
and patients without peritoneal recurrence. The mechanism
underlying the peritoneal metastasis may be mediated by alterations
in the levels of ex-miR-21 and ex-miR-92a. The results of our
preliminary experiments confirmed that the alterations in ex-miRNA
levels in the peritoneal cavity affect the ex-miRNA levels in the
peripheral blood (data not shown). A previous study reported that
the tumor-derived exosomes containing miRNAs can initiate
pre-metastatic niche formation by inducing metastasis of host cells
(39). In particular, ex-miR-21 is
known to affect the growth and metastasis of tumor cells via the
activation of Toll-like receptors on the surrounding immune cells
(40). These results indicated that
ex-miR-21 may contribute to the formation of a pre-metastatic niche
in the peritoneum. In addition, the phenotype of cancer stem cells
is enhanced following overexpression of miR-21, leading to the
promotion of invasion, migration and tumorigenesis (41). miR-92a, a member of the miR-17-92
cluster, may be closely linked to the functions of the E2F family
of transcription factors, which are important regulators of the
cell cycle and apoptosis (42).
ex-miR-21 and ex-miR-92a may support peritoneal tumor invasion and
recurrence following curative resection of GC. Further studies are
required to elucidate these mechanisms.
miRNAs specifically target protein-coding mRNAs
either by direct cleavage of the target mRNA or through the
inhibition of protein synthesis (43). miRNAs regulate the gene expression at
the post-transcriptional level by binding to the 3′-untranslated
regions of specific mRNAs (44).
miR-21 drives tumorigenesis via the inhibition of negative
regulations of the RAS/mitogen-activated protein
kinase/extracellular signal-regulated kinase signaling pathway, and
miR-21 overexpression downregulates the expression of PDCD4,
PTEN and tropomyosin 1, thereby promoting cell proliferation and
cancer progression (45,46). miR-92a suppresses cell proliferation
and invasion via the EP4/Notch 1 signaling pathway, and the
restoration of miR-92a expression may result in the suppression of
cell proliferation and the induction of apoptosis through the
downregulation of EP4 receptor in GC (42). Furthermore, PDCD4 has been
reported as a target gene of miR-21, whereas EP4 is a known
target of miR-92a (24). The present
study observed a significant inverse correlation between miR-21 and
PDCD4 mRNA levels, and miR-92a and EP4 mRNA levels,
in GC tissues. These results suggest that PDCD4 mRNA
expression is regulated by miR-21, whereas miR-92a regulates
EP4 expression.
In conclusion, the present study demonstrated the
applications of plasma ex-miR-21 and ex-miR-92a for the prediction
of peritoneal recurrence in patients with GC. However, the small
sample size of the retrospective marker analyses was a limitation
of the present study. Therefore, a larger scale prospective study
is required to confirm the usefulness of ex-miR-21 and ex-miR-92a
as biomarkers. Furthermore, in our preliminary study, the ex-miR-21
and ex-miR-92a levels of some patients normalized following
surgery. These points will be considered in future studies.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Miss J. Tamura
(Department of Surgery, Teikyo University School of Medicine) for
her technical assistance.
Funding
This study is partly supported by Grants-in-Aid for
Scientific Research from the Japanese Society for the Promotion of
Science (grant nos. 15K10150, 17K10655 and 18K08716).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HI conceived and designed the study. NS and HI wrote
the manuscript. NS and HI performed the experiments. RF, TF, YS,
DT, HM, YI, YK, MH and TK collected the clinical data. HI, TF and
RF reviewed and edited the manuscript. All authors read and
approved the manuscript, and agree to be held accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work is appropriate investigated and
resolved.
Ethics approval and consent to
participate
The study protocol conformed to the guidelines of
the Teikyo University Ethics Committee, and was approved by the
review board of Teikyo University (approval no. 09-081-3). Written
informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
miRNA
|
microRNA
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Hundahl SA, Menck HR, Mansour EG and
Winchester DP: The National Cancer Data Base report on gastric
carcinoma. Cancer. 80:2333–2341. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bando E, Yonemura Y, Takeshita Y,
Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T,
Nishimura G and Miwa K: Intraoperative lavage for cytological
examination in 1,297 patients with gastric carcinoma. Am J Surg.
178:256–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen KB, Chen J, Jin XL, Huang Y, Su QM
and Chen L: Exosome-mediated peritoneal dissemination in gastric
cancer and its clinical applications. Biomed Rep. 8:503–509.
2018.PubMed/NCBI
|
|
6
|
Sugarbaker PH and Yonemura Y: Clinical
pathway for the management of resectable gastric cancer with
peritoneal seeding: Best palliation with a ray of hope for cure.
Oncology. 58:96–107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishigami H, Fujiwara Y, Fukushima R,
Nashimoto A, Yabusaki H, Imano M, Imamoto H, Kodera Y, Uenosono Y,
Amagai K, et al: Phase III trial comparing intraperitoneal and
intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in
patients with gastric cancer with peritoneal metastasis: PHOENIX-GC
trial. J Clin Oncol. 36:1922–1929. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeung HC, Rha SY, Jang WI, Noh SH and
Chung HC: Treatment of advanced gastric cancer by palliative
gastrectomy, cytoreductive therapy and postoperative
intraperitoneal chemotherapy. Br J Surg. 89:460–466. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuramoto M, Shimada S, Ikeshima S, Matsuo
A, Yagi Y, Matsuda M, Yonemura Y and Baba H: Extensive
intraoperative peritoneal lavage as a standard prophylactic
strategy for peritoneal recurrence in patients with gastric
carcinoma. Ann Surg. 250:242–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang P and Hu X: Strategies of diagnosis
and treatment for peritoneal metastasis of gastric cancer. Zhonghua
Wei Chang Wai Ke Za Zhi. 20:500–503. 2017.(In Chinese). PubMed/NCBI
|
|
11
|
La Torre M, Rossi Del Monte S, Ferri M,
Cosenza G, Mercantini P and Ziparo V: Peritoneal washing cytology
in gastric cancer. How, when and who will get a benefit? A review.
Minerva Gastroenterol Dietol. 57:43–51. 2011.PubMed/NCBI
|
|
12
|
Abe S, Yoshiwara H, Tabata H, Tachibana M,
Monden N, Nakamura T and Nagaoka S: Curative resection of gastric
cancer: Limitation of peritoneal lavage cytolology in predicating
the outcome. J Surg Oncol. 59:226–229. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Wang L, Wu Z, Sun R, Jin H, Ma J,
Liu L, Ling R, Yi J, Wang L, et al: Three dysregulated microRNAs in
serum as novel biomarkers for gastric cancer screening. Med Oncol.
31:2982014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma GJ, Gu RM, Zhu M, Wen X, Li JT, Zhang
YY, Zhang XM and Chen SQ: Plasma post-operative miR-21 expression
in the prognosis of gastric cancers. Asian Pac J Cancer Prev.
14:7551–7554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng D, Liu Z and Du Y: Epigenetic
alterations as cancer diagnostic, prognostic, and predictive
biomarkers. Adv Genet. 71:125–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grady WM and Tewai M: The next thing in
prognostic molecular markers: MicroRNA signatures of cancer. Gut.
59:706–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kowal J, Tkach M and Thery C: Biogenesis
and secretion of exosome. Curr Opin Cell Biol. 29:116–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palma J, Yaddannapudi SC, Pigati L, Havens
MA, Jeong S, Weiner GA, Weimer KM, Stern B, Hastings ML and Duelli
DM: MicroRNAs are exported from malignant cells in customized
particles. Nucleic Acids Res. 40:9125–9138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silva M and Melo SA: Non-coding RNAs in
exosomes: New players in cancer biology. Curr Genomics. 16:295–303.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simons M and Raposo G: Exosomes-vesicular
carriers for intercelluar communication. Curr Opin Cell Biol.
21:575–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ge Q, Zhou Y, Lu J, Bai Y, Xie X and Lu Z:
MiRNA in plasma exosome is stable under different storage
conditions. Molecules. 19:1567–1575. 2014. View Article : Google Scholar
|
|
22
|
Hannafon BN and Ding WQ: Intercellular
communication by exosome-derived microRNAs in cancer. Int J Mol
Sci. 14:14240–14269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dejima H, Iinuma H, Kanaoka R, Matsutani N
and Kawamura K: Exosomal microRNA in plasma as a non-invasive
biomarker for the recurrence of non-small cell lung cancer. Oncol
Lett. 13:1256–1263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsukamoto M, Iinuma H, Yagi T, Matsuda K
and Hashiguchi Y: Circulating exosomal microRNA-21 as a biomarker
in each tumor stage of colorectal cancer. Oncology. 92:360–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanaoka R, Iinuma H, Dejima H, Sakai T,
Uehara H, Matsutani N and Kawamura M: Usefulness of plasma exosomal
microRNA-451a as a noninvasive biomarker for early prediction of
recurrence and prognosis of non-small cell lung cancer. Oncology.
94:311–323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takahasi K, Iinuma H, Wada K, Minezaki S,
Kawamura S, Kainuma M, Ikeda Y, Shibuya M, Miura F and Sano K:
Usefulness of exosome-encapsulated microRNA-451a as a minimally
invasive biomarker for prediction of recurrence and prognosis in
pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci.
25:155–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumata Y, Iinuma H, Suzuki Y, Tsukahara D,
Midorikawa H, Igarashi Y, Soeda N, Kiyokawa T, Horikawa M and
Fukushima R: Exosome-encapsulated microRNA-23b as a minimally
invasive liquid biomarker for the prediction of recurrence and
prognosis of gastric cancer patients in each tumor stage. Oncol
Rep. 40:319–330. 2018.PubMed/NCBI
|
|
28
|
Bertero L, Massa F, Metovic J, Zanetti R,
Castellano I, Ricardi U, Papotti M and Cassoni P: Eighth edition of
the UICC classification of malignant tumours: An overview of the
changes in the pathological TNM classification criteria-what has
changed and why? Virchows Arch. 472:519–531. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng L, Sharples RA, Scicluna BJ and Hill
AF: Exosomes provide a protective and enriched source of miRNA for
biomarker profiling compared to intracellular and cell-free blood.
J Extracell Vesicles. 3:237432014. View Article : Google Scholar
|
|
31
|
Sun Z, Chen C, Su Y, Wang W, Yang S, Zhou
Q, Wang G, Li Z, Song J, Zhang Z, et al: Regulatory mechanisms and
clinical perspectives of circRNA in digestive system neoplasms. J
Cancer. 10:2885–2891. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang S, Wang J, Li J, Luo Q, Zhao M,
Zheng L, Dong X, Chen C, Che Y, Liu P, et al: Serum microRNA
expression profile as a diagnostic panel for gastric cancer. Jpn J
Clin Oncol. 46:811–818. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu C, Ren C, Han J, Ding Y, Du J, Dai N,
Dai J, Ma H, Hu Z, Shen H, et al: A five-microRNA panel in plasma
was identified as a potential biomarker for early detection of
gastric cancer. Br J Cancer. 110:2291–2299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao G, Jiang T, Liu Y, Huai G, Lan C, Li
G, Jia G, Wang K and Yang M: Droplet digital PCR-based circulating
microRNA detection serves as a promising diagnostic method for
gastric cancer. BMC Cancer. 18:6762018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Z, Zhu D, Wu L, He M, Zhou X, Zhang
L, Zhang H, Wang W, Zhu J, Cheng W, et al: Six serum-based miRNAs
as potential diagnostic biomarkers for gastric cancer. Cancer
Epidemiol Biomarkers Prev. 26:188–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Komatsu S, Ichikawa D, Tsujiura M, Konishi
H, Takeshita H, Nagata H, Kawaguchi T, Hirajima S, Arita T,
Shiozaki A, et al: Prognostic impact of circulating miR-21 in the
plasma of patients with gastric carcinoma. Anticancer Res.
33:271–276. 2013.PubMed/NCBI
|
|
37
|
Zheng Y, Cui L, Sun W, Zhou H, Yuan X, Huo
M, Chen J, Lou Y and Guo J: MicroRNA-21 is a new marker of
circulating tumor cells in gastric cancer patients. Cancer Biomark.
10:71–77. 2011-2012. View Article : Google Scholar
|
|
38
|
Liu HN, Wu H, Tseng YJ, Chen YJ, Zhang DY,
Zhu L, Dong L and Shen XZ: Serum microRNA signatures and
metabolomics have high diagnostic value in gastric cancer. BMC
Cancer. 18:4152018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rana S, Malinowska K and Zöller M:
Exosomal tumor microRNA modulates premetastatic organ cells.
Neoplasia. 15:281–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu Y, Luo F, Liu Y, Shi L, Lu X, Xu W and
Liu Q: Exosomal miR-21 derived from arsenite-transformed human
bronchial epithelial cells promotes cell proliferation associated
with arsenite carcinogenesis. Arch Toxicol. 89:1071–1082. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang J, Yang P, Guo Z, Yang R, Yang H,
Yang F, Li L and Xiang B: Overexpression of microRNA-21 strengthens
stem cell-like characteristics in a hepatocellular carcinoma cell
line. World J Surg Oncol. 14:2782016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shin VY, Siu MT, Liu X, Ng EKO, Kwong A
and Chu KM: MiR-92 suppresses proliferation and induces apoptosis
by targeting EP4/Notch1 axis in gastric cancer. Oncotarget.
9:24209–24220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:R17–R29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Szafranska AE, Davison TS, John J, Cannon
T, Sipos B, Maghnouj A, Labourier E and Hahn SA: MicroRNA
expression alterations are linked to tumorigenesis and
non-neoplastic processes in pancreatic ductal adenocarcinoma.
Oncogene. 26:4442–4452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Motoyama K, Inoue H, Mimori K, Tanaka F,
Kojima K, Uetake H, Sugihara K and Mori M: Clinicopathological and
prognostic significance of PDCD4 and microRNA-21 in human gastric
cancer. Int J Oncol. 36:1089–1095. 2010.PubMed/NCBI
|
|
46
|
Cao Z, Yoon JH, Nam SW, Lee JY and Park
WS: PDCD4 expression inversely correlated with miR-21 levels in
gastric cancers. J Cancer Res Clin Oncol. 138:611–619. 2012.
View Article : Google Scholar : PubMed/NCBI
|