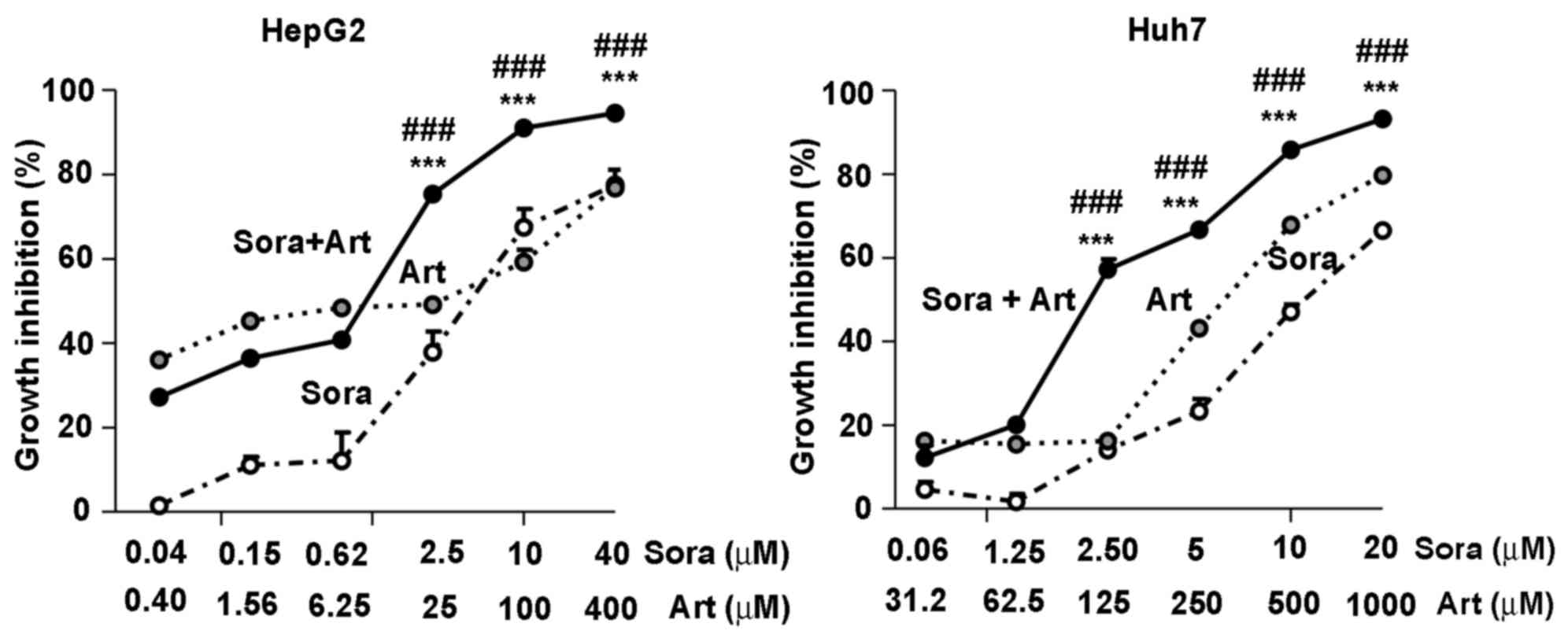
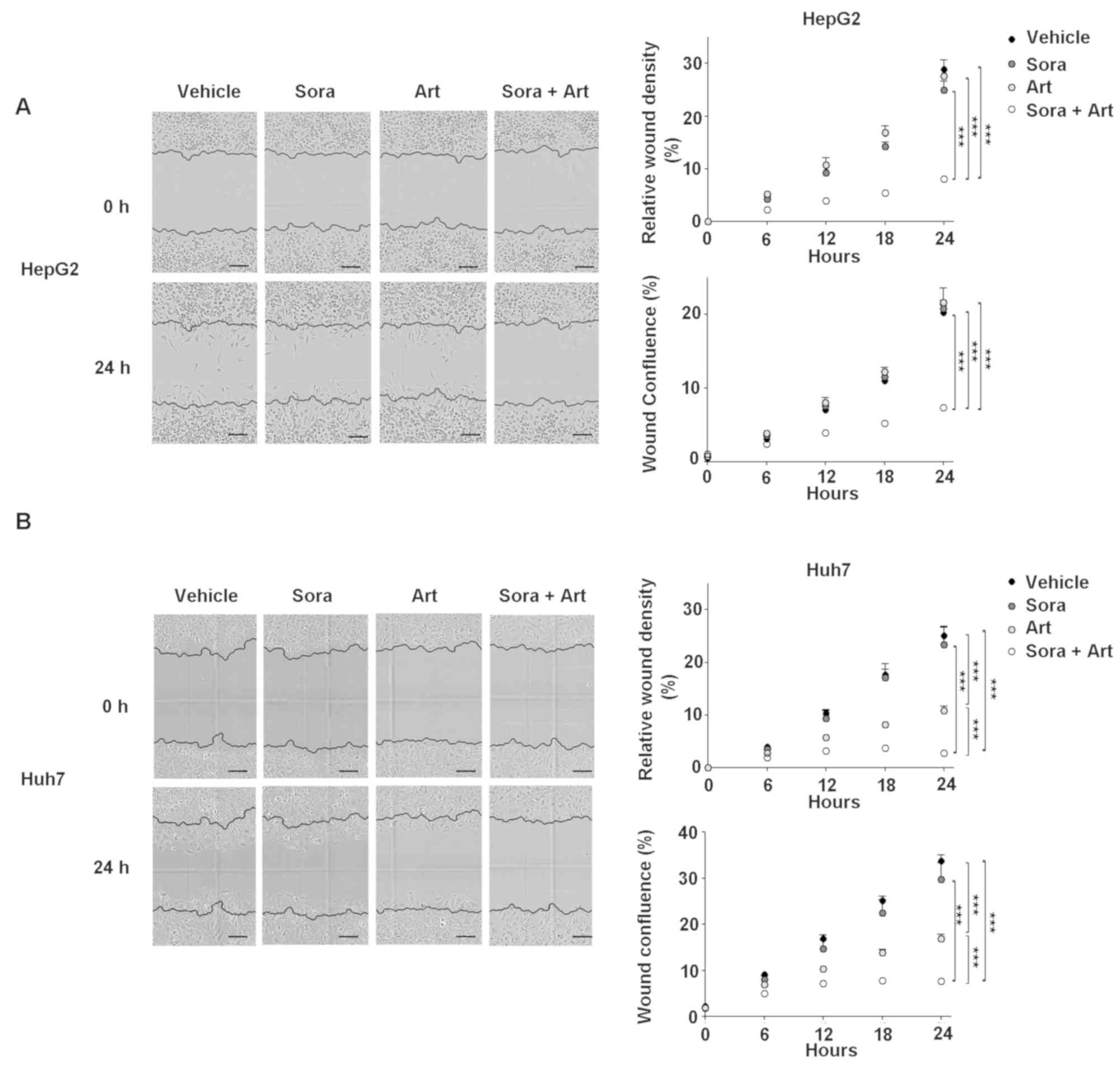
|
1
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun W and Cabrera R: Systemic treatment of
patients with advanced, unresectable hepatocellular carcinoma:
Emergence of therapies. J Gastrointest Cancer. 49:107–115. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dondorp A, Nosten F, Stepniewska K, Day N
and White N; South East Asian Quinine Artesunate Malaria
(SEAQUAMAT) Group, : Artesunate versus quinine for treatment of
severe falciparum malaria: A randomised trial. Lancet. 366:717–725.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
5
|
Crespo-Ortiz MP and Wei MQ: Antitumor
activity of artemisinin and its derivatives: From a well-known
antimalarial agent to a potential anticancer drug. J Biomed
Biotechnol. 2012:2475972012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar B, Kalvala A, Chu S, Rosen S, Forman
SJ, Marcucci G, Chen CC and Pullarkat V: Antileukemic activity and
cellular effects of the antimalarial agent artesunate in acute
myeloid leukemia. Leuk Res. 59:124–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krishna S, Ganapathi S, Ster IC, Saeed ME,
Cowan M, Finlayson C, Kovacsevics H, Jansen H, Kremsner PG, Efferth
T and Kumar D: A randomised, double blind, placebo-controlled pilot
study of oral artesunate therapy for colorectal cancer.
EBioMedicine. 2:82–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chauhan AK, Min KJ and Kwon TK:
RIP1-dependent reactive oxygen species production executes
artesunate-induced cell death in renal carcinoma Caki cells. Mol
Cell Biochem. 435:15–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tong Y, Liu Y, Zheng H, Zheng L, Liu W, Wu
J, Ou R, Zhang G, Li F, Hu M, et al: Artemisinin and its
derivatives can significantly inhibit lung tumorigenesis and tumor
metastasis through Wnt/beta-catenin signaling. Oncotarget.
7:31413–31428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang CZ, Zhang H, Yun J, Chen GG and Lai
PB: Dihydroartemisinin exhibits antitumor activity toward
hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol.
83:1278–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenshields AL, Shepherd TG and Hoskin
DW: Contribution of reactive oxygen species to ovarian cancer cell
growth arrest and killing by the anti-malarial drug artesunate. Mol
Carcinog. 56:75–93. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morris CA, Duparc S, Borghini-Fuhrer I,
Jung D, Shin CS and Fleckenstein L: Review of the clinical
pharmacokinetics of artesunate and its active metabolite
dihydroartemisinin following intravenous, intramuscular, oral or
rectal administration. Malar J. 10:2632011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Efferth T, Giaisi M, Merling A, Krammer PH
and Li-Weber M: Artesunate induces ROS-mediated apoptosis in
doxorubicin-resistant T leukemia cells. PLoS One. 2:e6932007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang B, Hou D, Liu Q, Wu T, Guo H, Zhang
X, Zou Y, Liu Z, Liu J, Wei J, et al: Artesunate sensitizes ovarian
cancer cells to cisplatin by downregulating RAD51. Cancer Biol
Ther. 16:1548–1556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Efferth T: Cancer combination therapy of
the sesquiterpenoid artesunate and the selective EGFR-tyrosine
kinase inhibitor erlotinib. Phytomedicine. 37:58–61. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nunes JJ, Pandey SK, Yadav A, Goel S and
Ateeq B: Targeting NF-kappa B signaling by artesunate restores
sensitivity of castrate-resistant prostate cancer cells to
antiandrogens. Neoplasia. 19:333–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vandewynckel YP, Laukens D, Geerts A,
Vanhove C, Descamps B, Colle I, Devisscher L, Bogaerts E, Paridaens
A, Verhelst X, et al: Therapeutic effects of artesunate in
hepatocellular carcinoma: Repurposing an ancient antimalarial
agent. Eur J Gastroenterol Hepatol. 26:861–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ilamathi M, Santhosh S and
Sivaramakrishnan V: Artesunate as an anti-cancer agent targets
stat-3 and favorably suppresses hepatocellular carcinoma. Curr Top
Med Chem. 16:2453–2463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HH, Zhou HJ, Wu GD and Lou XE:
Inhibitory effects of artesunate on angiogenesis and on expressions
of vascular endothelial growth factor and VEGF receptor KDR/flk-1.
Pharmacology. 71:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cervello M, Bachvarov D, Lampiasi N,
Cusimano A, Azzolina A, McCubrey JA and Montalto G: Molecular
mechanisms of sorafenib action in liver cancer cells. Cell Cycle.
11:2843–2855. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Liu Y, Meng L, Ji B and Yang D:
Synergistic antitumor effect of sorafenib in combination with ATM
inhibitor in hepatocellular carcinoma cells. Int J Med Sci.
14:523–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Byakika-Kibwika P, Lamorde M, Mayito J,
Nabukeera L, Mayanja-Kizza H, Katabira E, Hanpithakpong W, Obua C,
Pakker N, Lindegardh N, et al: Pharmacokinetics and
pharmacodynamics of intravenous artesunate during severe malaria
treatment in Ugandan adults. Malar J. 11:1322012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hubbard JM, Mahoney MR, Loui WS, Roberts
LR, Smyrk TC, Gatalica Z, Borad M, Kumar S and Alberts SR: Phase
I/II randomized trial of sorafenib and bevacizumab as first-line
therapy in patients with locally advanced or metastatic
hepatocellular carcinoma: North central cancer treatment group
trial N0745 (Alliance). Target Oncol. 12:201–209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finn RS, Merle P, Granito A, Huang YH,
Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Gerolami R, Caparello
C, et al: Outcomes of sequential treatment with sorafenib followed
by regorafenib for HCC: Additional analyses from the phase III
RESORCE trial. J Hepatol. 69:353–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tran BN, Nguyen HT, Kim JO, Yong CS and
Nguyen CN: Developing combination of artesunate with paclitaxel
loaded into poly-d,l-lactic-co-glycolic acid nanoparticle for
systemic delivery to exhibit synergic chemotherapeutic response.
Drug Dev Ind Pharm. 43:1952–1962. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goswami U, Kandimalla R, Kalita S,
Chattopadhyay A and Ghosh SS: Polyethylene glycol-encapsulated
histone deacetylase inhibitor drug-composite nanoparticles for
combination therapy with artesunate. ACS Omega. 3:11504–11516.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Efferth T: Cancer combination therapies
with artemisinin-type drugs. Biochem Pharmacol. 139:56–70. 2017.
View Article : Google Scholar : PubMed/NCBI
|