Introduction
Multiple myeloma (MM), a common malignancy that
accounts for ~1% of all human malignant tumors and 10–15% of all
cases of hematological malignancy globally, is characterized by the
abnormal proliferation of plasma cells in the bone marrow (1). The majority of patients with MM develop
myeloma bone disease (MBD), which is characterized by severe bone
pain, fractures, osteoporosis and hypercalcemia (2,3). Bone
destruction in MBD is usually attributed to an imbalance between
enhanced osteoclast activity and the attenuation of osteoblast
function (4). Osteogenesis is
mediated by the recruitment of bone marrow mesenchymal stem cells
(BMMSCs), which have the potential for osteogenic differentiation
(5). Previous study has indicated
that the osteogenic differentiation of BMMSCs from MM-mesenchymal
stem cells (MSCs) in patients with MM is impaired (6). Therefore, improving the osteogenic
differentiation ability of MM-MSCs is a key issue that may promote
bone regeneration and repair.
MicroRNAs (miRNAs/miRs) are highly conserved, short
non-coding RNA molecules that have been identified as regulators
involved in cell proliferation, differentiation, organ development
and metabolism (7). A number of
studies have reported that numerous miRNAs, including miR-133
(8), miR-141 (9) and miR-34a (10), promote or inhibit the differentiation
of preosteoblasts and osteoblasts by regulating key transcription
factors and osteogenic markers in the osteoblast signaling pathway
(11,12). However, to the best of our knowledge,
the role of miR-221-5p in the osteogenic differentiation of BMMSCs
from MBD-MSCs in patients with MBD remains unclear.
The present study aimed to examine the role of
miR-221-5p in the osteogenic differentiation of MBD-MSCs, and to
investigate the molecular mechanism of miR-221-5p-regulated
osteogenic differentiation. It was identified that miR-221-5p was
downregulated in MBD-MSCs, and participated in osteogenic
differentiation. Inhibition of miR-221-5p resulted in an increase
in the osteogenic potential of MBD-MSCs via the upregulation of
smad family member 3 (smad3). The potential mechanism may involve
the excessive phosphorylation of PI3K/AKT/mTOR due to the action of
miR-221-5p.
Materials and methods
Patients
The present study was approved by the General
Hospital of Western Theater Command (Chengdu, China), and written
informed consent was obtained from all participants. Bone marrow
(BM) samples were obtained from 5 patients with MBD (age range,
36–57 years; 2 males and 3 females) who underwent surgery at The
General Hospital of Western Theater Command (Chengdu, China)
between April 2016 and April 2017. Additionally, five healthy
controls (age range, 32–46 years; 3 males, 2 females) were included
in the present study to obtain N-MSCs. The inclusion criteria were
as follows: i) Patients who were diagnosed with MM at first
admission and who had one or more osteolytic lesions on imaging
examination; ii) patients who were willing to participate in the
study; iii) patients without comorbid diseases that may result in
osteolytic destruction, including prostate, breast, thyroid and
lung cancer. The exclusion criteria were as follows: i) Patients
who received chemotherapy prior to the study; and ii) patients with
MM who developed plasma cell leukemia.
Isolation and propagation of
BMMSC
N-MSCs and MBD-MSCs were isolated from BM samples
using Ficoll (Sigma-Aldrich; Merck KGaA). Following centrifugation
at 450 × g for 20 min at room temperature, the mononuclear cells
were resuspended and seeded in a T25 cell culture bottle at a
density of 5,000 cells/cm2 with DMEM (HyClone; GE
Healthcare Life Sciences) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and maintained at 37°C in a humidified
atmosphere (5% CO2). The medium was replaced twice per
week until the cultures attained 80–90% confluence.
MSC identification by flow
cytometry
The immunophenotype of the N-MSCs and MBD-MSCs were
evaluated at the third passage. The collected cells were
resuspended in 100 µl PBS (1×106 cells) with 5 µl of the
following antibodies: Mouse anti-human CD44-PE (cat. no. 338807;
1:20), CD90-FITC (cat. no. 328107; 1:20), CD105-PC5.5 (cat. no.
323215; 1:20), CD34-FITC (cat. no. 343603; 1:20) and CD45-PE (cat.
no. 368509; 1:20; all BioLegend, Inc.) for 30 min at 4°C.
Subsequently, N-MSCs and MBD-MSCs were analyzed using a FACSCalibur
flow cytometer and Cell Quest software (version 3.3; BD
Biosciences).
MSC differentiation
The third-generation N-MSCs and MBD-MSCs were seeded
at a density of 1×105 cells/well in 6-well plates. Once
the cultures attained 60–70% confluence, the medium was discarded
and 2 ml fresh DMEM containing 10% FBS, 1×10−8 mol/l
dexamethasone, 10 mmol/ml sodium β-glycerophosphate and 50 µg/ml
vitamin C was added to incubate the N-MSCs and MBD-MSCs for 2
weeks, and the media were replaced twice a week. The osteogenic
differentiation of N-MSCs and MBD-MSCs was evaluated by using
Alizarin Red S staining (1%; pH 4.2; Novon Scientific) for 5 min at
room temperature. Images of the N-MSCs and MBD-MSCs were captured
using a light microscope (magnification, ×40).
Cell transfection
N-MSCs were seeded into a 6-well plate at a density
of 1×105 cells/well 1 day prior to transfection. Cell
transfection was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol with 50 pmol/ml miR-221-5p inhibitor or
mimic (Guangzhou RiboBio Co., Ltd.). The medium was changed after 6
h. Following culture for 48 h at 37°C in a humidified atmosphere
(5% CO2), the transfected cells were collected for
western blotting and reverse transcription-quantitative PCR
(RT-qPCR) analyses.
Lentiviruses overexpressing smad3 (lv-smad3; Smad3
mRNA sequence, NM_001145102; vector name, GV358) and the
corresponding control lentiviruses (lv-green fluorescent protein)
were purchased from Shanghai GeneChem Co., Ltd. N-MSCs were seeded
into a 6-well plate at a density of 1×105 cells/well.
Lentivirus (1×108 TU/ml) infection was performed when
the cells reached 20–40% confluence. The lentiviruses were
transfected into N-MSCs with a multiplicity of infection of 30.
After transfection for 8–12 h, fresh medium was added for further
incubation at 37°C in a humidified atmosphere (5% CO2).
Cells were collected for RT-qPCR or western blot analysis 72 h
after transfection.
RT-qPCR assay
Total RNA was extracted from N-MSCs and MBD-MSCs
(1×105 cells/well) using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and converted to cDNA
using a PrimeScript™ RT reagent kit (Takara Biotechnology Co.,
Ltd.) according to the manufacturer's protocol at 37°C for 15 min
and 85°C for 5 sec. Subsequently, primers and SYBR®
Premix Ex Taq II (Takara Biotechnology Co., Ltd.) were used to
detect the expression of alkaline phosphatase (ALP), osteopontin
(OPN), osteocalcin (OC), and smad3 in an Applied Biosystems 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: 3 min at 95°C;
40 cycles of 95°C for 5 sec and 60°C for 30 sec followed by 72°C
for 30 sec. For the quantification of miRNA expression, RT was
performed at 42°C for 60 min and 70°C for 10 min using Bulge-Loop™
miRNA RT-qPCR Primer and Bulge-Loop™ miRNA RT-qPCR Starter kit
(cat. no. C10211-1; Guangzhou RiboBio Co., Ltd.). miR-221-5p
expression levels were quantified at 95°C for 10 min, followed by
40 cycles of 95°C for 2 sec, 60°C for 20 sec and 70°C for 10 sec
using Bulge-Loop™ miRNA RT-qPCR Starter kit (cat. no. C10211-1).
The 2−∆∆Cq method was used for comparative quantitation
(13). β-actin and U6 small nuclear
RNA genes were used as endogenous normalization controls. The data
were analyzed using Bio-Rad CFX Manager software (version 3.0;
Bio-Rad Laboratories, Inc.). The primer sequences (Sangon Biotech
Co., Ltd.) are presented in Table I.
The catalog number of miR-221-5p primer is MQPS0000852-1 and the
catalog number of U6 is miRAN0002-1.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| ALP |
GACCTCCTCGGAAGACACTCTG |
CGCCTGGTAGTTGTTGTGAGC |
| OPN |
GCCGACCAAGGAAAACTCACT |
GGCACAGGTGATGCCTAGGA |
| OC |
CCAGGCGCTACCTGTATCAATG |
ATGTGGTCAGCCAACTCGTCA |
| Smad3 |
AGGGCTTTGAGGCTGTCTACC |
GTCCACGCTGGCATCTTCTG |
| β-actin |
TGGCACCCAGCACAATGAA |
CTAAGTCATAGTCCGCCTAGAAGCA |
Western blotting
Cell specimens were lysed using
radioimmunoprecipitation assay lysis buffer (Wuhan Boster
Biological Technology, Ltd.) and quantified using a Bicinchoninic
Acid Protein assay kit (Wuhan Boster Biological Technology, Ltd.).
The cellular lysate (20 µg/lane) was separated by 10% SDS-PAGE and
then transferred onto PVDF membranes (EMD Millipore). Membranes
were blocked with 5% skimmed milk powder at 37°C for 1 h and
incubated with the following primary antibodies: Anti-smad3 (cat.
no. 9523; 1:1,000), anti-PI3K (cat. no. 4249; 1:1,000),
anti-phosphorylated (p)-PI3K (cat. no. 4228; 1:1,000), anti-AKT
(cat. no. 4685; 1:1,000), anti-p-AKT (cat. no. 4060; 1:1,000),
anti-mTOR (cat. no. 2983; 1:1,000), anti-p-mTOR (cat. no. 5536;
1:1,000) and anti-β-actin (cat. no. 4970; 1:1,000 dilution; all
Cell Signaling Technology, Inc.), anti-ALP (cat. no. ab83259;
1:500), anti-OPN (cat. no. ab8448; 1:1,000) and anti-OC (cat. no.
ab93876; 1:500; all Abcam), at 4°C overnight. Subsequently, the
membranes were incubated with HRP-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (cat. no. BA1054; 1:5,000
dilution; Wuhan Boster Biological Technology, Ltd.) for 1 h at room
temperature. Protein bands were detected using an ECL
chemiluminescence kit (EMD Millipore) according to the
manufacturer's protocol. Protein levels were calculated relative to
β-actin. The protein bands were visualized using the ChemiDoc™ MP
imaging system (Bio-Rad Laboratories, Inc.). Image-ProPlus software
(version 6.0; Media Cybernetics, Inc.) was used for densitometry
analysis.
Luciferase reporter assay
miR-221-5p targets were predicted using
bioinformatics software, including TargetScan (www.targetscan.org), mirDB (mirdb.org),
Diana Tools (diana.imis.athena-innovation.gr) and venny2.1.0
(bioinfogp.cnb.csic.es/tools/venny/index.html).
N-MSCs (4×104/well) were seeded into 24-well plates,
followed by transfection with the Renilla luciferase pRL-TK
plasmid (100 ng/ml; Shanghai GenePharma Co., Ltd.) with the
recombinant firefly luciferase pGL3 reporters containing the
3′-untranslated region (3′-UTR) region of human smad3 (2 µg/ml;
Shanghai GenePharma Co., Ltd.) in combination with miR-221-5p
mimic, miR-221-5p inhibitor and controls using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.). At
48 h after transfection, cells were collected and lysed. Luciferase
and Renilla signals were measured using a Dual-Luciferase
Reporter assay kit (cat. no. E1910; Promega Corporation), according
to the manufacturer's protocol. Firefly luciferase activity was
normalized to Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using SPSS
version 20.0 (IBM Corp.). Data are presented as the means ±
standard error and derived from three independent experiments. The
unpaired Student's t-test (for parametric data) or Mann-Whitney U
test (for non-parametric data) were used to compare two independent
samples. Differences among multiple groups were compared by one-way
ANOVA with Dunnett's post hoc test or two-way ANOVA with
Bonferroni's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. P<0.01 was considered to
indicate a statistically highly significant difference.
Results
Osteogenic differentiation capacity is
reduced in MBD-MSCs
After 7 days of primary culture, partial cell
colonies were identified, and the cells were fusiform and
pleomorphic. After 14 days of primary culture, the N-MSCs and
MBD-MSCs proliferated rapidly and attained 80–90% confluence
(Fig. 1A). MBD-MSCs exhibited slower
cell proliferation and higher morphological variation compared with
normal (N)-MSCs. Flow cytometry analysis revealed that the cells
were negative for CD34 and CD45 but positive for CD44, CD90 and
CD105 (Fig. 1B). These results
suggested that the cultured cells were MSCs. The osteogenic
induction of N-MSCs and MBD-MSCs was visualized by staining with
Alizarin Red S, and the results revealed that the calcium
deposition of N-MSCs was markedly higher compared with MBD-MSCs
(Fig. 1C). Furthermore, the RT-qPCR
and western blotting results demonstrated that the mRNA and protein
expression levels of ALP, OPN and OC were significantly lower in
MBD-MSCs compared with in N-MSCs (Fig.
1D and E). These data indicated that the osteogenic
differentiation ability of MBD-MSCs was inhibited.
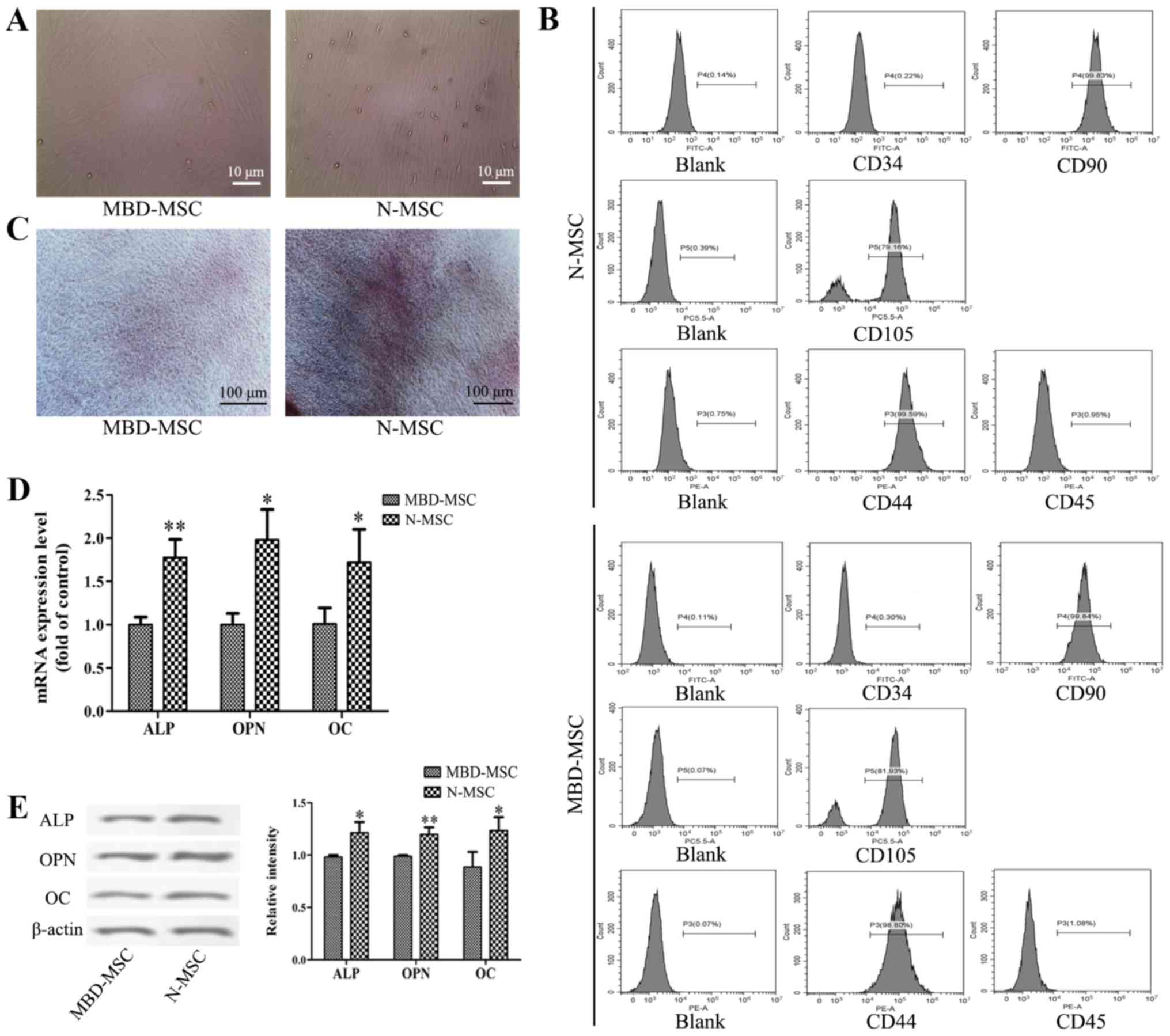 | Figure 1.Osteogenic differentiation ability of
MBD-MSCs is inhibited compared with that of N-MSCs. (A) Images
captured using a light microscope showing MSCs in primary culture
on the 14th day. Scale bar, 10 µm. (B) Surface markers of the third
generation MSCs were identified by flow cytometry. (C) Images of
Alizarin Red staining were captured using a light microscope
following osteogenic induction of N-MSCs and MBD-MSCs. Scale bar,
100 µm. (D) Reverse transcription-quantitative PCR was performed to
detect the mRNA expression levels of ALP, OPN and OC following
osteogenic induction of N-MSCs and MBD-MSCs. (E) Western blotting
was performed to detect the protein expression levels of ALP, OPN
and OC following osteogenic induction of N-MSCs and MBD-MSCs.
*P<0.05, **P<0.01 vs. MBD-MSC. ALP, alkaline phosphatase;
MBD, myeloma bone disease; MSC, mesenchymal stem cell; N, normal;
OC, osteocalcin; OPN, osteopontin. |
Inhibition of miR-221-5p promotes
osteogenic differentiation of MBD-MSCs
To investigate whether miR-221-5p is dysregulated in
the osteogenic differentiation of MSCs, the expression levels of
miR-221-5p were detected in N-MSCs and MBD-MSCs prior to and
following osteogenic induction. As presented in Fig. 2A, the mRNA expression levels of
miR-221-5p in MBD-MSCs were significantly lower compared with those
in N-MSCs. Following osteoblast induction, the miR-221-5p mRNA
level in N-MSCs was significantly decreased, while no obvious
change was detected in MBD-MSCs. These data suggested that
decreased expression levels of miR-221-5p may contribute to
osteogenic differentiation. Furthermore, to verify the role of
miR-221-5p in the osteogenic differentiation of MBD-MSCs, MBD-MSCs
were transfected with miR-221-5p mimic or inhibitor. RT-qPCR
revealed that the expression levels of miR-221-5p were
significantly increased in the mimic group and decreased in the
inhibitor group compared with the respective control groups, which
indicated a high transfection efficiency in MBD-MSCs (Fig. 2B). Subsequently, the expression
levels of marker genes of osteogenic differentiation were detected
following transfection. As presented in Fig. 2C-E, the mRNA and protein expression
levels of ALP, OPN and OC were significantly increased in the
inhibitor group and decreased in the mimic group compared with the
respective control groups. These data demonstrated that the
inhibition of miR-221-5p can effectively promote the osteogenic
differentiation of MBD-MSCs.
 | Figure 2.Effects of miR-221-5p inhibition on
the osteogenic differentiation of MBD-MSCs. (A) RT-qPCR was used to
quantify the expression levels of miR-221-5p in N-MSCs and MBD-MSCs
prior to and following osteoblast induction. (B) RT-qPCR was used
to quantify the expression levels of miR-221-5p in MBD-MSCs
following transfection with miR-221-5p mimic or inhibitor. (C)
Expression levels of marker genes of osteogenic differentiation
were detected by RT-qPCR following transfection. (D) Western
blotting was performed to detect the protein expression levels of
ALP, OPN and OC following transfection. (E) Relative band intensity
of ALP, OPN and OC protein. *P<0.05, **P<0.01 vs. NC-mimic,
unless indicated otherwise. #P<0.05,
##P<0.01 vs. NC-inhibitor. ALP, alkaline phosphatase;
MBD, myeloma bone disease; miR-221-5p, microRNA-221-5p; MSC,
mesenchymal stem cell; N, normal; NC, negative control; OC,
osteocalcin; OPN, osteopontin; RT-qPCR, reverse
transcription-quantitative PCR. |
Smad3 is a direct target of
miR-221-5p
Using bioinformatics analysis, it was identified
that the 3′-UTR of smad3 contains a conserved putative target site
for miR-221-5p (Fig. 3A). A
luciferase reporter assay was performed to validate the association
between miR-221-5p and smad3. As presented in Fig. 3B and C, miR-221-5p inhibitor
significantly increased luciferase activity, while miR-221-5p mimic
significantly inhibited the luciferase activity compared with the
controls. Furthermore, the present study revealed that
overexpression of miR-221-5p significantly decreased smad3
expression, whereas transfection with miR-221-5p inhibitor
significantly increased smad3 expression at the mRNA and protein
levels (Fig. 3D-F). In summary, it
may be suggested that miR-221-5p targets smad3 and negatively
regulate smad3 expression.
Overexpression of smad3 enhances the
effect of miR-221-5p inhibitor on the osteogenic differentiation of
MBD-MSCs
To determine whether smad3 is involved in
miR-221-5p-regulated osteogenic differentiation of MBD-MSCs, the
present study used a lentiviral vector overexpressing smad3.
Western blotting and RT-qPCR results demonstrated that the protein
and mRNA expression levels of smad3 were significantly increased
following transduction with lentiviral vector, which indicated high
transduction efficiency in MBD-MSCs (Fig. 4A and B). Furthermore, it was
identified that the overexpression of smad3 could not only promote
calcium deposition and the expression of osteogenic markers, but it
could also enhance the effect of miR-221-5p inhibitor on osteogenic
differentiation (Fig. 4C and D).
These results indicated that miR-221-5p regulates osteogenic
differentiation by upregulating smad3.
 | Figure 4.Effects of smad3 overexpression on
miR-221-5p inhibitor-mediated osteogenic differentiation. (A)
Protein expression levels of smad3 were detected by western
blotting following transduction with lv-smad3 lentiviral vector.
**P<0.01 vs. NC. (B) mRNA expression levels of smad3 were
detected by RT-qPCR following transfection with lentiviral vector.
*P<0.05 vs. NC. (C) N-mesenchymal stem cells transfected with
miR-221-5p inhibitors were subsequently infected with the
lentiviral vector. Images of Alizarin Red staining were captured
using a light microscope. Scale bar, 100 µm. (D) RT-qPCR was
performed to detect the mRNA expression levels of ALP, OPN and OC
following transfection with miR-221-5p inhibitor and/or infection
with lentiviral vector. *P<0.05, **P<0.01 vs. control.
##P<0.01 vs. lv-smad3 group. ALP, alkaline
phosphatase; lv-smad3, lentiviral vector overexpressing smad3;
miR-221-5p, microRNA-221-5p; NC, negative control; OC, osteocalcin;
OPN, osteopontin; smad3, smad family member 3; RT-qPCR, reverse
transcription-quantitative PCR. |
Inhibition of miR-221-5p may promote
the osteogenic differentiation of MBD-MSCs by activating the
PI3K/AKT/mTOR pathway
Western blotting was used to further investigate the
potential signaling pathway associated with miR-221-5p regulated
osteogenic differentiation. The results demonstrated that the
protein expression levels of AKT, p-AKT, mTOR and p-mTOR were
significantly increased following transfection with miR-221-5p
inhibitor compared with the control, while no significant changes
in the expression levels of PI3K and p-PI3K were observed. In
addition, there were no significant difference in the ratios of
p-PI3K to PI3K, p-AKT to AKT and p-mTOR to mTOR between groups was
identified (Fig. 5). These results
suggested that the inhibition of miR-221-5p may increase the
expression levels of phosphorylated proteins by inducing total
protein expression, thus activating the PI3K/AKT/mTOR signaling
pathway and promoting osteogenic differentiation.
Discussion
A prominent clinical feature of MBD is progressive
bone destruction, including osteoporosis, osteolytic lesions,
pathological fracture, hypercalcemia and bone pain (14). Increased bone resorption and
decreased bone formation result in bone damage in patients with MM.
MSCs are ideal seed cells for bone tissue engineering due to their
strong renewal ability and multidirectional differentiation
potentiality (15). Therefore,
osteogenic defects in patients with MBD may be treated by improving
the osteogenic capability of MSCs. Osteoblasts act as the major
cells that contribute to bone formation by secreting ALP and bone
matrix proteins that induce bone matrix mineralization (16). The present study demonstrated that
calcium deposition and the expression levels of bone formation
indicators were decreased in MBD-MSCs following osteoblast
induction compared with N-MSCs, which indicates that the osteogenic
differentiation ability of MSCs is reduced in patients with
MBD.
miRNAs are essential regulatory molecules that can
regulate the expression levels of genes associated with bone
homeostasis via transcriptional inhibition or mRNA cleavage
(17). A number of studies (11,18,19) have
highlighted the biological roles of dysregulated miRNAs in
osteoblast differentiation. For example, it has been suggested that
miR-21 promotes the osteogenic differentiation of MSCs by
regulating the PI3K/β-catenin pathway (20). In another study, miR-208a-3p has been
reported to suppress osteogenic differentiation and inhibit bone
formation by targeting activin A receptor type 1 (21). Recently, miR-221 was reported to
regulate the osteoblast differentiation of MSCs in patients with
osteoporosis by targeting runt-related transcription factor 2
(22). Despite these findings,
further investigations are required to identify additional
mechanisms underlying the effects of miR-221-5p in osteogenic
differentiation. The present study identified that the expression
levels of miR-221-5p were significantly lower in MBD-MSCs compared
with in N-MSCs. Following osteoblast induction, the expression
levels of miR-221-5p in N-MSCs were significantly decreased, while
no notable alteration in expression was detected in MBD-MSCs. These
results suggested that miR-221-5p may act as an inhibitor in the
osteogenic differentiation of MSCs. Furthermore, it was identified
that knockdown of miR-221-5p could induce osteogenic
differentiation in MBD-MSCs, which was indicated by the increased
mRNA expression levels of typical osteoblast differentiation
markers, including ALP, OPN and OC. In addition, the overexpression
of miR-221-5p inhibited the expression of osteogenic
differentiation markers. These data suggested that miR-221-5p
serves as a negative regulator in the osteogenic differentiation of
MBD-MSCs. In future studies, ALP staining and Alizarin Red staining
should be used to verify this result.
miRNA regulation in the osteogenic differentiation
of MSCs is achieved by miRNAs acting on specific target genes and
signal transduction pathways. Smad3 is an important member of the
transforming growth factor-β1/smad signaling pathway, as it can
form complexes with smad4 and translocate into the nucleus, which
results in the activation or suppression of downstream target
genes, and the regulation of cell function and metabolism (23). A number of studies have indicated
that smad3 serves an important role in bone formation, remodeling
and maintenance (24–26). Loss of smad3 decreases bone formation
and induces osteopenia via the dysregulation of osteoblast
differentiation and apoptosis (27).
Additionally, in a previous study, smad3 knockout mice developed
skeletal abnormalities shortly after weaning and this effect
worsened with age (28). The present
study used bioinformatics analysis to identify that the 3′-UTR of
smad3 contained a conserved putative target site for miR-221-5p in
MBD-MSCs. In the present study, a luciferase reporter assay,
RT-qPCR and western blotting demonstrated that smad3 is a direct
target of miR-221-5p and a negative association was revealed
between the expression levels of smad3 and miR-221-5p. Furthermore,
we demonstrated that overexpression of smad3 could enhance the
effect of miR-221-5p inhibitor on the osteogenic differentiation of
MBD-MSCs, indicating that miR-221-5p may be a negative regulator of
the osteogenic differentiation of MBD-MSCs by upregulating smad3.
In future studies, additional candidate targets of miR-221-5p
should be investigated to support the use of miR-221-5p as a
potential therapeutic target for MBD.
The PI3K/AKT/mTOR signaling pathway is closely
associated with the osteogenic differentiation of MSCs (29,30).
Under physiological conditions, the PI3K/AKT signaling pathway is
an important signaling pathway that serves a key role in inhibiting
apoptosis and inducing osteogenic differentiation (31). Zhang et al (32) reported that all-trans retinoic
acid induces the osteogenic differentiation of MSCs by activating
the PI3K/AKT/glycogen synthase kinase-3β signaling pathway. In
addition, miR-378 reduces glucose-suppressed osteogenic
differentiation by targeting caspase-3 and activating the PI3K/AKT
signaling pathway (33). The present
study revealed that inhibition of miR-221-5p increased the
expression levels of osteogenic differentiation markers and
significantly increased the levels of total proteins and
phosphorylated proteins associated with the PI3K/AKT/mTOR
pathway.
In conclusion, the present study demonstrated that
miR-221-5p was expressed at a low level in MBD-MSCs and may be a
negative regulator that participates in osteogenic differentiation.
Additionally, the inhibition of miR-221-5p increased osteoblast
differentiation by directly targeting smad3. The potential
mechanism may involve increased phosphorylation of PI3K/AKT/mTOR
via the activity of miR-221-5p. These results suggest that
miR-221-5p may be used as a promising therapeutic against MBD in
the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
Research Program ‘Stem Cell and Transformation Research’ Key
Project (grant. no. 2017YFA0105502).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FYF, RD, YS and XZ conceived and designed the
experiments. FYF, RD, SHL, QW, YZ and LG performed the experiments.
YL, PK and JZ analyzed the data and assisted with the experiments.
FYF, RD, YS and XZ wrote the paper. YS and XZ revised the
manuscript and supervised the study. All authors have read and
approved the final version of this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of General Hospital of Western Theater Command (Chengdu,
China) and written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kyle RA and Rajkumar SV: Criteria for
diagnosis, staging, risk stratification and response assessment of
multiple myeloma. Leukemia. 23:3–9. 2014. View Article : Google Scholar
|
|
2
|
Anderson KC: Progress and paradigms in
multiple myeloma. Clin Cancer Res. 22:5419–5427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yaccoby S: Advances in the understanding
of myeloma bone disease and tumour growth. Br J Haematol.
149:311–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heider U, Hofbauer LC, Zavrski I, Kaiser
M, Jakob C and Sezer O: Novel aspects of osteoclast activation and
osteoblast inhibition in myeloma bone disease. Biochem Biophys Res
Commun. 338:687–693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li H, Li T, Fan J, Li T, Fan L, Wang S,
Weng X, Han Q and Zhao RC: miR-216a rescues dexamethasone
suppression of osteogenesis, promotes osteoblast differentiation
and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT
pathway. Cell Death Differ. 22:1935–1945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raje N and Roodman GD: Advances in the
biology and treatment of bone disease in multiple myeloma. Clin
Cancer Res. 17:1278–1286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv H, Sun Y and Zhang Y: miR-133 is
involved in estrogen Deficiency-Induced osteoporosis through
modulating osteogenic differentiation of mesenchymal stem cells.
Med Sci Monit. 21:1527–1534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Q, Liu W, Sinha KM, Yasuda H and de
Crombrugghe B: Identification and characterization of microRNAs
controlled by the osteoblast-specific transcription factor osterix.
PLoS One. 8:e581042013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vimalraj S and Selvamurugan N: MicroRNAs:
Synthesis, gene regulation and osteoblast differentiation. Curr
Issues Mol Biol. 15:7–18. 2013.PubMed/NCBI
|
|
12
|
Zhou M, Li M and Yu X: Regulation of
microRNA in osteoblast differentiation and its clinical
significance. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 26:755–759.
2012.(In Chinese). PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berenson JR: Myeloma bone disease. Best
Pract Res Clin Haematol. 18:653–672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diao Y, Ma Q, Cui F and Zhong Y: Human
umbilical cord mesenchymal stem cells: Osteogenesis in vivo as seed
cells for bone tissue engineering. J Biomed Mater Res A.
91:123–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu R, Li H, Liu W, Yang L, Tan YF and Luo
XH: Targeting miRNAs in osteoblast differentiation and bone
formation. Expert Opin Ther Targets. 14:1109–1120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inose H, Ochi H, Kimura A, Fujita K, Xu R,
Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, et al: A
microRNA regulatory mechanism of osteoblast differentiation. Proc
Natl Acad Sci USA. 106:20794–20799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng YB, Li X, Li ZY, Zhao J, Yuan XB, Ren
Y, Cui ZD, Liu YD and Yang XJ: microRNA-21 promotes osteogenic
differentiation of mesenchymal stem cells by the PI3K/β-catenin
pathway. J Orthop Res. 33:957–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arfat Y, Basra MAR, Shahzad M, Majeed K,
Mahmood N and Munir H: miR-208a-3p Suppresses osteoblast
differentiation and inhibits bone formation by targeting ACVR1. Mol
Ther Nucleic Acids. 11:323–336. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Gao Y, Cai L, Li F, Lou Y, Xu N,
Kang Y and Yang H: MicroRNA-221 is involved in the regulation of
osteoporosis through regulates RUNX2 protein expression and
osteoblast differentiation. Am J Transl Res. 9:126–135.
2017.PubMed/NCBI
|
|
23
|
Derynck R, Zhang Y and Feng XH: Smads:
Transcriptional activators of TGF-beta responses. Cell. 95:737–740.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hackinger S, Trajanoska K, Styrkarsdottir
U, Zengini E, Steinberg J, Ritchie GRS, Hatzikotoulas K, Gilly A,
Evangelou E, Kemp JP, et al: Evaluation of shared genetic aetiology
between osteoarthritis and bone mineral density identifies SMAD3 as
a novel osteoarthritis risk locus. Hum Mol Genet. 26:3850–3858.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Z, Greenblatt MB, Yan G, Feng H, Sun J,
Lotinun S, Brady N, Baron R, Glimcher LH and Zou W: SMURF2
regulates bone homeostasis by disrupting SMAD3 interaction with
vitamin D receptor in osteoblasts. Nat Commun. 8:145702017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu Q, Tu ML, Li CJ, Zhang L, Jiang TJ, Liu
T and Luo XH: GDF11 inhibits bone formation by activating Smad2/3
in bone marrow mesenchymal stem cells. Calcif Tissue Int.
99:500–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borton AJ, Frederick JP, Datto MB, Wang XF
and Weinstein RS: The loss of Smad3 results in a lower rate of bone
formation and osteopenia through dysregulation of osteoblast
differentiation and apoptosis. J Bone Miner Res. 16:1754–1764.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang X, Chen L, Xu X, Li C, Huang C and
Deng CX: TGF-beta/Smad3 signals repress chondrocyte hypertrophic
differentiation and are required for maintaining articular
cartilage. J Cell Biol. 153:35–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo G, Xu B and Huang Y: Icariside II
promotes the osteogenic differentiation of canine bone marrow
mesenchymal stem cells via the PI3K/AKT/mTOR/S6K1 signaling
pathways. Am J Transl Res. 9:2077–2087. 2017.PubMed/NCBI
|
|
30
|
Tong Y, Feng W, Wu Y, Lv H, Jia Y and
Jiang D: Mechano-growth factor accelerates the proliferation and
osteogenic differentiation of rabbit mesenchymal stem cells through
the PI3K/AKT pathway. BMC Biochem. 16:12015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu YX, Du J, Si MS, Mo JJ, Qiao SC and Lai
HC: The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1
preosteoblast proliferation and differentiation on SLA and SLActive
titanium surfaces. J Biomed Mater Res A. 101:748–754. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang S, Chen X, Hu Y, Wu J, Cao Q, Chen S
and Gao Y: All-trans retinoic acid modulates Wnt3A-induced
osteogenic differentiation of mesenchymal stem cells via activating
the PI3K/AKT/GSK3β signalling pathway. Mol Cell Endocrinol.
422:243–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
You L, Gu W, Chen L, Pan L, Chen J and
Peng Y: miR-378 overexpression attenuates high glucose-suppressed
osteogenic differentiation through targeting CASP3 and activating
PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 7:7249–7261.
2014.PubMed/NCBI
|



















