Introduction
Liver cancer is the second leading cause of
cancer-associated mortality worldwide, resulting in >500,000
deaths per year (1). Despite recent
advances in surgical resection and liver transplantation, the
5-year survival rate of patients with liver cancer remains <17%
(2). In the majority of cases, liver
cancer is diagnosed at an advanced stage with limited therapeutic
options. Only 10–20% of tumors are considered surgically resectable
at the time of diagnosis (3), and
the long-term survival of the patients remains unsatisfactory due
to postsurgical recurrence. Thus, an improved understanding of the
molecular mechanisms, as well as identification of prognostic
biomarkers and potential therapeutic targets of liver cancer are
desirable.
Annexins (ANX) are a superfamily of
calcium-dependent phospholipid-binding proteins with a structural
homology of 40–60% (4). In humans,
annexins comprise 13 members (ANXA1-A11, A13 and A8L1; A12 is
unassigned) (4), the nomenclature of
which is summarized in Table I. Each
ANXA is composed of two major domains; the amino-terminus allows
interactions with cytoplasmic proteins, whereas the
carboxyl-terminus contains the calcium- and membrane-binding sites
(4). ANXAs are involved in a wide
range of biological processes such as vesicle trafficking (5), anti-inflammation (6), anti-coagulation (7), calcium signaling (8), cell differentiation, apoptosis and
proliferation (4). A number of
studies have reported aberrant expression levels of ANXAs in
various types of cancer, and that ANXAs may function as tumor
suppressors or promoters depending on the cancer type. For example,
ANXA1 serves a tumor-promoting role in colorectal and lung cancer
(9,10), but functions as a tumor suppressor in
esophageal and gastric cancer (11).
The role of ANXA1 has also been investigated in hepatocellular
carcinoma (HCC), but the results are controversial (12). In addition, other ANXAs, including
ANXA2, A3, A4, A6, A7 and A10, have been reported to be
dysregulated in liver cancer (13–18).
However, the majority of these studies only reported the expression
levels of ANXAs and lacked any prognosis data. The expression
patterns, prognostic roles and functions of ANXAs in liver cancer
remain unclear.
 | Table I.Nomenclature and characterization of
the ANXAs. |
Table I.
Nomenclature and characterization of
the ANXAs.
| ANXA | Symbol | Synonyms | Chromosomal
location | Mass, Da (length,
a.a.) |
|---|
| Annexin A1 | ANXA1 | ANX1, LPC1 | 9q21.13 | 38,714 (346) |
| Annexin A2 | ANXA2 | ANX2, ANX2L4,
CAL1H, LPC2, LIP2 | 15q22.2 | 38,694 (339) |
| Annexin A3 | ANXA3 | ANX3 | 4q21.21 | 36,375 (323) |
| Annexin A4 | ANXA4 | ANX4 | 2p13.3 | 35,883 (319) |
| Annexin A5 | ANXA5 | ANX5, ENX2 | 4q27 | 35,937 (320) |
| Annexin A6 | ANXA6 | ANX6 | 5q33.1 | 75,873 (673) |
| Annexin A7 | ANXA7 | ANX7 | 10q22.2 | 52,739 (488) |
| Annexin A8 | ANXA8 | ANX8 | 10q11.22 | 30,693 (276) |
| Annexin A8 Like
1 | ANXA8L1 | ANXA8L2 | 10q11.22 | 36,879 (327) |
| Annexin A9 | ANXA9 | ANX31 | 1q21.3 | 38,364 (345) |
| Annexin A10 | ANXA10 | ANX14 | 4q32.3 | 37,278 (324) |
| Annexin A11 | ANXA11 | ANX11 | 10q22.3 | 54,390 (505) |
| Annexin A13 | ANXA13 | ANX13 | 8q24.13 | 35,463 (316) |
In the present study, the distinct expression
patterns, prognostic values and potential functions of ANXAs in
liver cancer were investigated by analyzing the gene expression,
copy number variation and survival data published online.
Materials and methods
Ethics statement
The present study was approved by the Academic
Committee of Zhengzhou University (Zhengzhou, Henan, China) and
performed in accordance with the principles of the Declaration of
Helsinki. Online databases were used to retrieve the datasets.
ANXA mRNA expression level
evaluation
The Oncomine database (https://www.oncomine.org) was used to evaluate the
mRNA expression levels of ANXAs in different types of cancer. The
mRNA levels of ANXAs in cancer tissues and normal controls were
compared using a Student's t-test, with P≤0.01 and fold change
(FC)>2.
GEPIA (http://gepia.cancer-pku.cn) is an online database for
analyzing tumor and normal sample RNA sequencing data from The
Cancer Genome Atlas (TCGA; http://portal.gdc.cancer.gov/) and the Genotype-Tissue
Expression (GTEx) projects (http://commonfund.nih.gov/GTEx/). In the present
study, the GEPIA database was used to analyze the mRNA expression
levels of ANXAs in liver cancer and normal liver samples. Each ANXA
was entered into the database separately and analyzed with the
settings |log2(FC)|≥1 and P≤0.01. The method for
differential gene expression analysis is one-way ANOVA.
Kaplan-Meier survival analysis
The Kaplan-Meier (KM) plotter (http://kmplot.com) database was used to analyze the
associations between ANXA mRNA expression levels and OS of patients
with liver cancer and the log-rank test was used to obtain the
P-values. Briefly, ANXAs were entered into the database and
analyzed using different settings of clinical parameters [e.g. sex
and pathological stage (19)]. The
cases were divided into high or low expression groups based on the
median expression level of each gene. Differences in OS were tested
by Cox proportional hazards regression. KM survival plots were
obtained with the number-at-risk, hazard ratio (HR), 95% confidence
intervals (CI) and P-value displayed on the webpage. P<0.05 was
considered to indicate a statistically significant difference.
cBioPortal for cancer genomics
The cBioPortal for Cancer Genomics (www.cbioportal.org) provides visualization, analysis
and downloads of large-scale cancer genomics datasets (20). The liver HCC (TCGA, provisional)
dataset (21), which contained 442
patients with HCC, was selected for analyses of ANXAs. The
alterations of ANXAs, as well as the network between ANXAs and the
50 most frequently altered neighboring genes were obtained
according to the instructions on the cBioPortal.
Gene function and pathway enrichment
analysis
Gene Ontology (GO; http://geneontology.org/) analysis was used to predict
the enriched biological functions of ANXAs and genes associated
with ANXA alterations (neighboring genes). A Kyoto Encyclopedia of
Genes and Genomes (KEGG; http://www.kegg.jp/) pathway analysis was used to
determine the enriched biological pathways of ANXAs and their
neighboring genes. The Database for Annotation, Visualization and
Integrated Discovery (DAVID) online tool (https://david.ncifcrf.gov/) was used to perform the GO
functional annotation and KEGG pathway enrichment analysis.
Results
mRNA expression levels of ANXAs in
patients with liver cancer
The mRNA expression levels of 13 ANXAs in liver
cancer were compared with those in normal samples using the
Oncomine database. As presented in (Fig.
1), ANXA1, A2, A3 and A4 were upregulated, whereas ANXA10 was
downregulated in liver cancer compared with normal tissues. Among
these, ANXA2 was upregulated in four datasets [Mas (22), Roessler2 (23), Roessler (23) and Wurmbach (24)] with fold-changes of 2.409, 3.506,
3.528 and 2.350, respectively (Table
II). In addition, the Mas dataset exhibited higher mRNA levels
of ANXA1, A3 and A4 in HCC compared with those in normal liver,
with fold-changes of 5.649, 5.253 and 2.270, respectively. ANXA10
was the only downregulated ANXA gene in liver cancer, with
fold-changes of −6.349, −5.586 and −10.711 in the Roessler,
Roessler2 and Wurmbach datasets, respectively. However, for the
transcription levels of ANXA5, A6, A7, A8, A8L1, A9, A11 and A13,
no significant difference was identified between tumors and normal
liver samples (data not shown).
 | Table II.Significant changes of ANXA
expression at the transcriptional level between liver cancer and
normal liver tissues using the Oncomine database. |
Table II.
Significant changes of ANXA
expression at the transcriptional level between liver cancer and
normal liver tissues using the Oncomine database.
| ANXA | Comparison | Fold change | P-value | t-test | Database | (Refs.) |
|---|
| ANXA1 | HCC vs. Normal | 5.649 |
1.60×10−13 | 9.880 | Mas | (22) |
| ANXA2 | HCC vs. Normal | 3.506 |
1.10×10−65 | 20.365 | Roessler2 | (23) |
|
| HCC vs. Normal | 2.409 |
1.28×10−10 | 8.629 | Mas | (22) |
|
| HCC vs. Normal | 3.528 |
2.69×10−7 | 6.044 | Roessler | (23) |
|
| HCC vs. Normal | 2.350 |
2.78×10−4 | 4.128 | Wurmbach | (24) |
| ANXA3 | HCC vs. Normal | 5.253 |
2.18×10−28 | 17.617 | Mas | (22) |
| ANXA4 | HCC vs. Normal | 2.270 |
1.03×10−11 | 8.673 | Mas | (22) |
| ANXA10 | HCC vs. Normal | −10.711 |
1.36×10−10 | −8.183 | Wurmbach | (24) |
|
| HCC vs. Normal | −5.586 |
2.50×10−69 | −22.733 | Roessler2 | (23) |
|
| HCC vs. Normal | −6.439 |
8.55×10−8 | −7.275 | Roessler | (23) |
ANXA mRNA levels are associated with
clinicopathological parameters of patients with liver cancer
The mRNA expression levels of 13 ANXAs in liver
cancer were accessed from the GEPIA database. As demonstrated in
(Fig. 2), four dysregulated members
were identified. The expression levels of ANXA2, A5 and A11 were
significantly higher (P<0.01), whereas ANXA10 was significantly
lower in liver cancer compared with those in normal liver tissues
(P<0.01). In addition, the ANXA mRNA levels were compared
between groups of patients with different pathological stages
[classified according to American Joint Committee on Cancer TNM
(19)] of liver cancer. The results
revealed that the expression levels of ANXA3, A8, A8L1 and A10 were
significantly different between patients with different
pathological stages (Fig. 3).
However, the results of ANXA3, A8 and 8L1 levels need to be
interpreted with caution due to their very low expression
levels.
Prognostic values of ANXAs in patients
with liver cancer
The prognostic values of 13 ANXAs in liver cancer
were determined using the KM plotter database. The results
demonstrated that four members were significantly associated with
prognosis. As presented in (Fig. 4),
the survival curves revealed that high mRNA expression levels of
ANXA2 and A5 were significantly associated with poor prognosis (HR,
1.45; 95% CI, 1.02–2.05; P=0.035 and HR, 1.67; 95% CI, 1.18–2.37;
P=0.0035, respectively), whereas high mRNA expression levels of
ANXA7 and A10 were significantly associated with improved prognosis
compared with the respective low expression groups (HR, 0.56; 95%
CI, 0.39–0.80; P=0.0012 and HR, 0.55;95% CI,0.39–0.78; P=0.00078,
respectively), although the high expression of ANXA10 resulted in
decreased survival time. The other ANXAs, including ANXA1 (HR,
0.84; 95% CI, 0.59–1.18; P=0.31), A3 (HR, 0.84; 95% CI, 0.59–1.19;
P=0.33), A4 (HR, 0.89; 95% CI, 0.63–1.26; P=0.53), A6 (HR, 0.82;
95% CI, 0.58–1.16; P=0.26), A8 (HR, 1.30; 95% CI, 0.92–1.84;
P=0.14), A8L1 (HR, 1.16; 95% CI, 0.82–1.64; P=0.39), A9 (HR, 1.29;
95% CI, 0.91–1.83; P=0.14), A11 (HR, 0.91; 95% CI, 0.64–1.28;
P=0.59) and A13 (HR, 1.15; 95% CI, 0.81–1.62; P=0.43) were not
associated with OS.
Prognostic values of ANXAs in patients
with liver cancer according to clinicopathological features
The association between individual ANXAs and other
clinicopathological features such as sex and clinical stages were
further analyzed. As presented in Table III, in male patients with liver
cancer, high mRNA expression levels of ANXA2 and A5 were
significantly associated with poor OS (HR, 1.95; 95% CI, 1.23–3.18;
P=0.036 and HR, 1.93;95% CI, 1.22–3.05; P=0.004, respectively),
whereas high mRNA expression levels of ANXA7 and A10 were
significantly associated with improved OS compared with the low
expression group (HR, 0.53; 95% CI, 0.33–0.83; P=0.0051 and HR,
0.49; 95% CI, 0.31–0.79; P=0.0023, respectively), which was similar
to that in all patients with liver cancer. None of the ANXAs were
identified as associated with OS in female patients. High
expression of ANXA5 was significantly associated with poor OS in
patients with stage I (HR, 2.21; 95% CI, 1.17–4.18; P=0.012) and
stage III (HR, 2.06; 95% CI, 1.10–3.84; P=0.021) liver cancer; high
expression of ANXA7 (HR, 0.41; 95% CI, 0.22–0.78; P=0.0048) and A10
(HR, 0.37;95% CI, 0.20–0.70; P=0.0017) were significantly
associated with improved OS in patients with stage III liver cancer
(Table IV). The expression levels
of other ANXAs were not significantly associated with OS in
patients at different clinical stages, although the expression of
ANXA2 (HR, 1.71; 95% CI, 0.92–3.18; P=0.088) was modestly
associated with poor OS in patients with stage I liver cancer.
 | Table III.Association between ANXAs and the sex
of patients with liver cancer. |
Table III.
Association between ANXAs and the sex
of patients with liver cancer.
| ANXAs | Sex | Cases, n | HR (95% CI) | P-value |
|---|
| ANXA1 | Male | 246 | 0.72
(0.46–1.12) | 0.1390 |
|
| Female | 118 | 0.97
(0.56–1.68) | 0.8994 |
| ANXA2 | Male | 246 | 1.95
(1.23–3.18) | 0.0036 |
|
| Female | 118 | 1.28
(0.74–2.24) | 0.3754 |
| ANXA3 | Male | 246 | 0.81
(0.52–1.26) | 0.3489 |
|
| Female | 118 | 0.80
(0.45–1.41) | 0.4426 |
| ANXA4 | Male | 246 | 1.02
(0.66–1.59) | 0.9253 |
|
| Female | 118 | 0.93
(0.53–1.63) | 0.7996 |
| ANXA5 | Male | 246 | 1.93
(1.22–3.05) | 0.0040 |
|
| Female | 118 | 1.42
(0.81–2.47) | 0.2176 |
| ANXA6 | Male | 246 | 0.72
(0.46–1.12) | 0.1458 |
|
| Female | 118 | 0.88
(0.50–1.53) | 0.6474 |
| ANXA7 | Male | 246 | 0.53
(0.33–0.83) | 0.0051 |
|
| Female | 118 | 0.97
(0.55–1.69) | 0.9079 |
| ANXA8 | Male | 246 | 1.28
(0.82–1.99) | 0.2792 |
|
| Female | 118 | 1.33
(0.76–2.33) | 0.3218 |
| ANXA8L1 | Male | 246 | 1.30
(0.83–2.02) | 0.2463 |
|
| Female | 118 |
0.96(0.55–1.67) | 0.8717 |
| ANXA9 | Male | 246 | 1.44
(0.92–2.26) | 0.1061 |
|
| Female | 118 | 1.15
(0.66–2.01) | 0.6123 |
| ANXA10 | Male | 246 | 0.49
(0.31–0.79) | 0.0023 |
|
| Female | 118 | 0.61
(0.35–1.07) | 0.0817 |
| ANXA11 | Male | 246 | 0.84
(0.54–1.31) | 0.4366 |
|
| Female | 118 | 0.88
(0.51–1.53) | 0.6490 |
| ANXA13 | Male | 246 | 1.50
(0.96–2.35) | 0.0748 |
|
| Female | 118 | 0.83
(0.48–1.45) | 0.5123 |
 | Table IV.Association between ANXAs and the
clinical stage of patients with liver cancer. |
Table IV.
Association between ANXAs and the
clinical stage of patients with liver cancer.
| ANXAs | Clinical
stagea | Cases, n | HR (95% CI) | P-value |
|---|
| ANXA1 | I | 170 | 0.82
(0.45–1.50) | 0.5175 |
|
| II | 83 | 0.87
(0.4–1.90) | 0.7345 |
|
| III | 83 | 0.74
(0.41–1.33) | 0.3048 |
| ANXA2 | I | 170 | 1.71
(0.92–3.18) | 0.0884 |
|
| II | 83 | 0.99
(0.45–2.14) | 0.9710 |
|
| III | 83 | 1.46
(0.81–2.64) | 0.2051 |
| ANXA3 | I | 170 | 0.93
(0.50–1.7) | 0.8034 |
|
| II | 83 | 0.70
(0.32–1.56) | 0.3842 |
|
| III | 83 | 0.6
(0.33–1.11) | 0.1002 |
| ANXA4 | I | 170 | 0.84
(0.46–1.54) | 0.5760 |
|
| II | 83 | 0.74
(0.34–1.6) | 0.4393 |
|
| III | 83 | 1.43
(0.78–2.62) | 0.2393 |
| ANXA5 | I | 170 | 2.21
(1.17–4.18) | 0.0122 |
|
| II | 83 | 0.81
(0.37–1.76) | 0.5909 |
|
| III | 83 | 2.06
(1.1–3.84) | 0.0209 |
| ANXA6 | I | 170 | 1.04
(0.57–1.91) | 0.8932 |
|
| II | 83 | 0.94
(0.43–2.06) | 0.8786 |
|
| III | 83 | 0.58
(0.31–1.07) | 0.0761 |
| ANXA7 | I | 170 | 0.60
(0.32–1.11) | 0.0988 |
|
| II | 83 | 0.70
(0.31–1.56) | 0.3820 |
|
| III | 83 | 0.41
(0.22–0.78) | 0.0048 |
| ANXA8 | I | 170 | 0.76
(0.41–1.41) | 0.3879 |
|
| II | 83 | 1.87
(0.84–4.17) | 0.1216 |
|
| III | 83 | 1.10
(0.61–2.01) | 0.7468 |
| ANXA8L1 | I | 170 | 0.98
(0.54–1.80) | 0.9551 |
|
| II | 83 | 1.65
(0.74–3.68) | 0.2183 |
|
| III | 83 | 0.65
(0.35–1.19) | 0.1583 |
| ANXA9 | I | 170 | 1.29
(0.70–2.39) | 0.4065 |
|
| II | 83 | 1.78
(0.80–3.96) | 0.1532 |
|
| III | 83 | 1.47
(0.81–2.68) | 0.2039 |
| ANXA10 | I | 170 | 0.60
(0.32–1.12) | 0.1019 |
|
| II | 83 | 0.76
(0.35–1.65) | 0.4800 |
|
| III | 83 | 0.37
(0.20–0.70) | 0.0017 |
| ANXA11 | I | 170 | 0.97
(0.53–1.77) | 0.9131 |
|
| II | 83 | 0.48
(0.21–1.08) | 0.0689 |
|
| III | 83 | 1.01
(0.56–1.81) | 0.9758 |
| ANXA13 | I | 170 | 1.17
(0.63–2.14) | 0.6189 |
|
| II | 83 | 1.30
(0.59–2.84) | 0.5122 |
|
| III | 83 | 1.59
(0.87–2.89) | 0.1273 |
Predicted functions and pathways of
ANXAs in liver cancer
Using the cBioPortal online tool, alterations (copy
number variation, mutations and mRNA expression change of ANXAs)
were identified in 205 out of 442 patients with liver cancer
(46.4%) in the selected dataset (Fig.
5A). An interaction network for ANXAs and the 50 most
frequently altered neighboring genes was constructed. The results
demonstrated that signal transduction-associated genes, such as
EGFR, PRKCA, HCRT, GNAI1 and GNBs, were associated with ANXA
alterations (Fig. 5B).
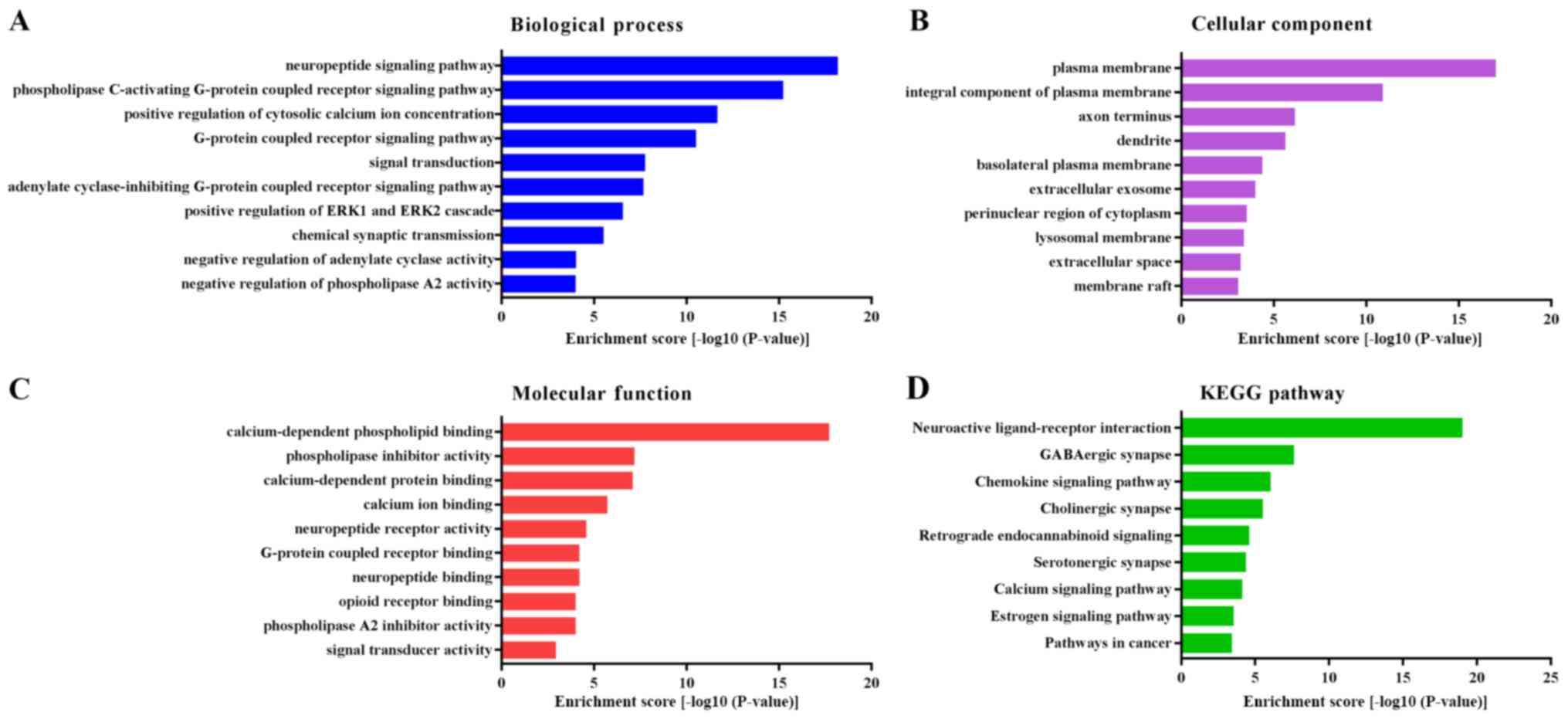
In order to investigate the potential biological
functions of ANXAs in liver cancer, GO functional annotation and
KEGG pathway enrichment analyses were performed for ANXAs, and the
50 most frequently altered neighboring genes using the DAVID. The
results of the GO analysis revealed that ANXAs and their
neighboring genes were involved in several biological processes,
such as ‘neuropeptide signaling pathway’, ‘phospholipase
C-activating G-protein coupled receptor signaling pathway’,
‘positive regulation of cytosolic calcium ion concentration’,
‘G-protein coupled receptor signaling pathway’ and ‘signal
transduction’ (Fig. 6A). The
cellular component analysis revealed that ANXAs and their
neighboring genes were primarily located in ‘plasma membrane’,
‘axon terminus’, ‘dendrite’ and ‘extracellular exosome’ (Fig. 6B). The molecular function analysis
suggested that ANXAs and the neighboring genes were primarily
enriched in ‘calcium-dependent phospholipid binding’,
‘calcium-dependent protein binding’, ‘calcium ion binding’,
‘G-protein coupled receptor binding’ and ‘neuropeptide receptor
activity’ (Fig. 6C). In addition,
the results of the KEGG pathway analysis demonstrated that ANXAs
and the neighboring genes were primarily enriched in ‘neuroactive
ligand-receptor interaction’, ‘GABAergic synapse’, ‘chemokine
signaling pathway’, ‘calcium signaling pathway’ and ‘pathways in
cancer’ (Fig. 6D).
Discussion
Aberrant expression of ANXAs is common in various
types of cancer such as melanoma, pancreatic cancer, gastric
cancer, lung cancer and breast cancer (8). ANXAs have been demonstrated to be
involved in carcinogenesis and progression of liver cancer
according to previous studies (12–18).
However, the complex roles of ANXAs in the carcinogenesis,
progression and prognosis of liver cancer remain to be elucidated.
In the present study, the mRNA expression and prognostic values of
different ANXAs in liver cancer were investigated by bioinformatics
analysis.
ANXA1 is the first known member of ANXAs, which has
been reported to be involved in a wide range of cell signaling
pathways including inflammatory, cell differentiation,
proliferation and apoptosis (25–27).
ANXA1 enhances growth and migration in breast cancer by mediating
alternative macrophage polarization in the tumor microenvironment
(28). In addition, ANXA1 regulates
TGF-β signaling and promotes metastasis of basal-like breast cancer
cells (29). In liver cancer, the
expression pattern of ANXA1 in previous studies is controversial.
Suo et al (30) reported that
ANXA1 was upregulated in liver cancer compared with nontumor
tissues, and that high levels of ANXA1 expression were
significantly associated with tumor grade. Lin et al
(12) reported that high ANXA1
expression predicted poor prognosis and enhanced the malignant
phenotype of tumor cells in liver cancer. However, Hongsrichan
et al (31) identified no
ANXA1 protein expression in liver cancer by immunohistochemistry,
and Xue et al (32) reported
decreased ANXA1 expression levels in liver cancer using tissue
microarray analysis. In the present study, the expression of ANXA1
was increased in liver cancer compared with normal tissues, which
was consistent with Suo's and Lin's studies. However, the present
study did not observe a significant association between ANXA1
expression and OS of patients with liver cancer. The prognostic
value of ANXA1 in liver cancer requires further investigation.
ANXA2 is one of the most abundant ANXAs, and is
widely distributed in the nucleus, cytoplasm, endosomes and
extracellular space. The potential role of ANXA2 in cancer has been
widely investigated. Previous studies have reported that ANXA2 is
upregulated in various types of cancer including lung (33), breast (34), gastric (35), pancreatic (36), colorectal (37) and liver (38) cancers. Increased expression of ANXA2
is associated with cancer development and poor prognosis (39,40). In
addition, knockdown of ANXA2 effectively suppresses tumor
progression in vitro and in vivo; studies that
focused on the underlying molecular mechanisms have demonstrated
that ANXA2 either promotes tumor cell invasion by forming a
heterotetramer with S100A10 (41),
contributing to heterotypic cell-cell interactions between tumor
cells and microvascular endothelial cells, or facilitates tumor
cell proliferation and chemoresistance by inhibiting p53 expression
and activating the transcription factors STAT3 and NFκB (37,42,43). In
liver cancer, protein and mRNA expression levels of ANXA2 were
identified as upregulated and associated with poor prognosis
(38,44). Knockdown of ANXA2 suppressed liver
cancer cell migration and invasion by regulating the trafficking of
CD147-harboring membrane microvesicles (45). In addition, the expression of ANXA2
was also elevated in the serum of patients with liver cancer
compared with healthy controls (46). The results of the present study
confirmed that ANXA2 was upregulated in liver cancer and that high
expression of ANXA2 was significantly associated with poor OS.
ANXA3 has been demonstrated to function either as a
tumor suppressor or promoter candidate in different types of
cancer. Upregulation of ANXA3 enhances drug resistance and promotes
tumor metastasis in breast cancer (47) and promotes tumor growth and predicts
poor prognosis in gastric cancer (48), whereas in prostate cancer, ANXA3
protein expression is downregulated, which is associated with the
tumor stage and Gleason score (49).
In liver cancer, ANXA3 is upregulated and preferentially expressed
in cancer stem cells. High levels of ANXA3 maintains cancer cell
stemness via hypoxia-inducible factor α/Notch and JNK signaling
pathways (50,51). ANXA3 promotes tumorigenesis and drug
resistance in liver cancer, which makes it a potential therapeutic
target (18). In addition, serum
ANXA3 is also increased in liver cancer compared with normal liver
tissues; therefore, ANXA3 is a promising biomarker for the
diagnosis, prognosis and therapeutic response evaluation of liver
cancer (52). In the present study,
ANXA3 was upregulated in liver cancer compared with healthy
tissues, but the mRNA level of ANXA3 was not associated with
OS.
ANXA4 is primarily expressed in epithelial cells
(53). Recent studies have
demonstrated that ANXA4 is upregulated and acts as an oncogene in
multiple types of cancer, including lung (54), colorectal (55), cervical (56) and gallbladder cancer (57). In liver cancer, ANXA4 has been
reported as upregulated, particularly in patients with early
recurrence or metastasis (58). High
expression levels of ANXA4 predicted early recurrence or metastasis
and poor OS of patients with liver cancer (58). In addition, inhibition of ANXA4
suppresses liver cancer cell proliferation, migration and invasion
both in vivo and in vitro (15,58).
Consistent with these results, the results of the present study
demonstrated that the expression of ANXA4 was higher in liver
cancer compared with normal tissues, but it was not associated with
OS.
ANXA5 exhibits tumor promoter activity in the
majority of different types of tumor, including liver cancer
(59). Guo et al (60) analyzed protein expression in five
pairs of matched primary tumor and tumor thrombus samples using
two-dimensional gel electrophoresis, which revealed that ANXA5 was
upregulated in tumor thrombus samples. Sun et al (61) reported that the expression of ANXA5
was positively associated with the progression and metastasis of
liver cancer, and that ANXA5 promoted carcinogenesis via the
integrin and mitogen-activated protein kinase kinase-extracellular
signal-regulated kinase pathways. Consistent with these studies,
the results of the present study demonstrated that ANXA5 expression
was increased in liver cancer compared with normal liver tissues.
Furthermore, to the best of our knowledge, the prognostic role of
ANXA5 in liver cancer has not yet been reported. In the present
study, it was revealed that high expression of ANXA5 was
significantly associated with poor OS.
There are limited studies available that focus on
ANXA6 in liver cancer. The protein level of ANXA6 is decreased,
whereas the mRNA level is increased in liver cancer compared with
non-tumorous tissues, suggesting post-transcriptional regulation of
ANXA6 (16). To the best of our
knowledge, there are currently no reports stating the prognostic
value of ANXA6 in liver cancer. In the present study, no
significant differences were observed in the mRNA level of ANXA6
between liver cancer and normal tissues, and ANXA6 expression was
not associated with OS. In addition, the roles of ANXA8, A8L1, A9,
A11 and A13 in liver cancer have rarely been reported. The present
study demonstrated that these ANXAs were not associated with OS in
patients with liver cancer.
ANXA7 has been reported to be upregulated in liver
cancer and to promote tumor cell migration and invasion by
interacting with galectin-3 or receptor of activated protein C
kinase 1 (62–64), whereas the inhibition of ANXA7
decreases tumor cell invasion and migration (65). Consistent with these studies, the
present study revealed modestly increased expression levels of
ANXA7 in liver cancer compared with normal liver tissues. In
contrast, high expression levels of ANXA7 were associated with
improved OS. This contradictory result was also reported by Wang
et al (66), who demonstrated
that upregulation of ANXA7 increased liver cancer cell migration
in vitro, but decreased lymph node metastasis in
vivo. In addition, the aforementioned study reported a dynamic
change of ANXA7 expression during liver cancer progression
(66). Therefore, the exact role of
ANXA7 in liver cancer remains unclear and requires further
investigation.
ANXA10 is the latest ANXA member to be identified
(67). In previous studies, aberrant
expression of ANXA10 was associated with carcinogenesis and
progression of various types of cancer (68–70),
which suggested a possible tumor promoter or suppressor role,
although its functional role remains to be clarified. In liver
cancer, downregulation of ANXA10 is associated with the malignant
phenotype of tumor cells and poor prognosis (14). Overexpression of ANXA10 inhibits
proliferation and promotes apoptosis of HepG2 cells (71). Consistent with these studies, the
results of the present study confirmed that ANXA10 was decreased
and associated with clinical stage in patients with liver cancer.
In addition, high expression of ANXA10 was associated with an
improved prognosis compared with patients with low ANXA10
expression.
Previous studies have suggested that the risk of
developing liver cancer in males is higher compared with that in
females (72–75). Irrespective of the etiology, the
morbidity of liver cancer in males is 2–4-fold higher compared with
that in females (3). A number of
studies have suggested that the androgen receptor (AR) may be
responsible for the sex disparity observed in liver cancer
(76,77). Of note, a study has reported that the
transcription and splicing of ANXA7 is regulated by AR-signaling in
prostate cancer (78). However, to
the best of our knowledge, the role of ANXAs in the sex disparity
of liver cancer has rarely been studied. In the present study, the
mRNA expression levels of ANXA2, A5, A7 and A10 were significantly
associated with OS in male, but not in female patients, which
suggested a possible role for these ANXAs in sex disparity.
The potential biological functions of ANXAs in liver
cancer were also investigated in the present study through GO
functional annotation and KEGG pathway enrichment analyses. The GO
analysis revealed that ANXAs and their neighboring genes primarily
participated in ‘neuropeptide signaling pathway’, ‘G-protein
coupled receptor signaling pathway’, ‘positive regulation of
cytosolic calcium ion concentration’ and ‘signal transduction’,
which were consistent with previous studies reporting that ANXAs
serve important roles in calcium signaling (7,79). In
addition, the KEGG pathway analysis demonstrated that ANXAs and
their neighboring genes were primarily involved in ‘neuroactive
ligand-receptor interaction’, ‘GABAergic synapse’, ‘chemokine
signaling pathway’, ‘calcium signaling pathway’ and ‘pathways in
cancer’. Among them, the most significantly enriched pathway was
the ‘neuroactive ligand-receptor interaction’, suggesting that
ANXAs may function in liver cancer through the neuroactive
ligand-receptor interaction pathway.
In the present study, the expression levels,
prognostic roles and potential biological functions of 13 ANXAs in
liver cancer were systemically investigated. The results indicated
that ANXA1, A2, A3, A4, A5 and A10 may be potential therapeutic
targets for liver cancer treatment, whereas ANXA2, A5, A7 and A10
may be potential prognostic biomarkers of liver cancer. However,
functional experiments are required in order to confirm the role of
ANXAs in liver cancer progression as well as their specificity and
sensitivity as biomarkers. The results of the present study
introduced ANXA2/5/10 as good candidates for future experimental
works.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81700514) and the Key
Scientific Research Projects in Colleges and Universities of Henan
Province (grant no. 19A320064).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available in the (TCGA) and (Oncomine)
repositories, (https://portal.gdc.cancer.gov/, http://www.oncomine.org/).
Authors' contributions
CZ and LM conceived and designed the study. CZ, PW,
TS and LZ collected and analyzed the data. CZ and PW wrote the
initial draft of the manuscript.. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Academic
Committee of Zhengzhou University (Zhengzhou, Henan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enrich C, Rentero C and Grewal T: Annexin
A6 in the liver: From the endocytic compartment to cellular
physiology. Biochim Biophys Acta Mol Cell Res. 1864:933–946. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugimoto MA, Vago JP, Teixeira MM and
Sousa LP: Annexin A1 and the resolution of inflammation: Modulation
of neutrophil recruitment, apoptosis, and clearance. J Immunol Res.
2016:82392582016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rand JH, Wu XX, Quinn AS and Taatjes DJ:
Resistance to annexin A5 anticoagulant activity: A thrombogenic
mechanism for the antiphospholipid syndrome. Lupus. 17:922–930.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gerke V, Creutz CE and Moss SE: Annexins:
linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell
Biol. 6:449–461. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mussunoor S and Murray GI: The role of
annexins in tumour development and progression. J Pathol.
216:131–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ydy LR, do Espirito Santo GF, de Menezes
I, Martins MS, Ignotti E and Damazo AS: Study of the annexin A1 and
its associations with carcinoembryonic antigen and mismatch repair
proteins in colorectal cancer. J Gastrointest Cancer. 47:61–68.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biaoxue R, Xiling J, Shuanying Y, Wei Z,
Xiguang C, Jinsui W and Min Z: Upregulation of Hsp90-beta and
annexin A1 correlates with poor survival and lymphatic metastasis
in lung cancer patients. J Exp Clin Cancer Res. 31:702012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Chen Y, Xu D, Wang J and Yu G:
Differential expression of ANXA1 in benign human gastrointestinal
tissues and cancers. BMC Cancer. 14:5202014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin Y, Lin G, Fang W, Zhu H and Chu K:
Increased expression of annexin A1 predicts poor prognosis in human
hepatocellular carcinoma and enhances cell malignant phenotype. Med
Oncol. 31:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ibrahim MM, Sun MZ, Huang Y, Jun M, Jin Y,
Yue D, Jiasheng W, Zhang J, Qazi AS, Sagoe K and Tang J:
Down-regulation of ANXA7 decreases metastatic potential of human
hepatocellular carcinoma cells in vitro. Biomed Pharmacother.
67:285–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu SH, Lin CY, Peng SY, Jeng YM, Pan HW,
Lai PL, Liu CL and Hsu HC: Down-regulation of annexin A10 in
hepatocellular carcinoma is associated with vascular invasion,
early recurrence, and poor prognosis in synergy with p53 mutation.
Am J Pathol. 160:1831–1837. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YY, Ge C, Tian H, Jiang JY, Zhao FY,
Li H, Chen TY, Yao M and Li JJ: The transcription factor Ikaros
inhibits cell proliferation by downregulating ANXA4 expression in
hepatocellular carcinoma. Am J Cancer Res. 7:1285–1297.
2017.PubMed/NCBI
|
|
16
|
Meier EM, Rein-Fischboeck L, Pohl R,
Wanninger J, Hoy AJ, Grewal T, Eisinger K, Krautbauer S, Liebisch
G, Weiss TS and Buechler C: Annexin A6 protein is downregulated in
human hepatocellular carcinoma. Mol Cell Biochem. 418:81–90. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mohammad HS, Kurokohchi K, Yoneyama H,
Tokuda M, Morishita A, Jian G, Shi L, Murota M, Tani J, Kato K, et
al: Annexin A2 expression and phosphorylation are up-regulated in
hepatocellular carcinoma. Int J Oncol. 33:1157–1163.
2008.PubMed/NCBI
|
|
18
|
Pan QZ, Pan K, Weng DS, Zhao JJ, Zhang XF,
Wang DD, Lv L, Jiang SS, Zheng HX and Xia JC: Annexin A3 promotes
tumorigenesis and resistance to chemotherapy in hepatocellular
carcinoma. Mol Carcinog. 54:598–607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cancer Genome Atlas Research Network.
Electronic address, . simplewheeler@bcm.edu; Cancer
Genome Atlas Research Network: Comprehensive and integrative
genomic characterization of hepatocellular carcinoma. Cell.
169:1327–1341.e23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mas VR, Maluf DG, Archer KJ, Yanek K, Kong
X, Kulik L, Freise CE, Olthoff KM, Ghobrial RM, McIver P and Fisher
R: Genes involved in viral carcinogenesis and tumor initiation in
hepatitis C virus-induced hepatocellular carcinoma. Mol Med.
15:85–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roessler S, Jia HL, Budhu A, Forgues M, Ye
QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX and Wang XW: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wurmbach E, Chen YB, Khitrov G, Zhang W,
Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J, et
al: Genome-wide molecular profiles of HCV-induced dysplasia and
hepatocellular carcinoma. Hepatology. 45:938–947. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheikh MH and Solito E: Annexin A1:
Uncovering the many talents of an old protein. Int J Mol Sci.
19(pii): E10452018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
D'Acquisto F, Piras G and Rattazzi L:
Pro-inflammatory and pathogenic properties of Annexin-A1: The whole
is greater than the sum of its parts. Biochem Pharmacol.
85:1213–1218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Locatelli I, Sutti S, Jindal A, Vacchiano
M, Bozzola C, Reutelingsperger C, Kusters D, Bena S, Parola M,
Paternostro C, et al: Endogenous annexin A1 is a novel protective
determinant in nonalcoholic steatohepatitis in mice. Hepatology.
60:531–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moraes LA, Kar S, Foo SL, Gu T, Toh YQ,
Ampomah PB, Sachaphibulkij K, Yap G, Zharkova O, Lukman HM, et al:
Annexin-A1 enhances breast cancer growth and migration by promoting
alternative macrophage polarization in the tumour microenvironment.
Sci Rep. 7:179252017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Graauw M, van Miltenburg MH, Schmidt
MK, Pont C, Lalai R, Kartopawiro J, Pardali E, Le Dévédec SE, Smit
VT, van der Wal A, et al: Annexin A1 regulates TGF-beta signaling
and promotes metastasis formation of basal-like breast cancer
cells. Proc Natl Acad Sci USA. 107:6340–6345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suo A, Zhang M, Yao Y, Zhang L, Huang C,
Nan K and Zhang W: Proteome analysis of the effects of sorafenib on
human hepatocellular carcinoma cell line HepG2. Med Oncol.
29:1827–1836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hongsrichan N, Rucksaken R, Chamgramol Y,
Pinlaor P, Techasen A, Yongvanit P, Khuntikeo N, Pairojkul C and
Pinlaor S: Annexin A1: A new immunohistological marker of
cholangiocarcinoma. World J Gastroenterol. 19:2456–2465. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xue LY, Teng LH, Zou SM, Ren LQ, Zheng S,
Luo W, Bi R and Lü N: Expression of annexin I in different
histological types of carcinomas. Zhonghua Zhong Liu Za Zhi.
29:444–448. 2007.(In Chinese). PubMed/NCBI
|
|
33
|
Luo CH, Liu QQ, Zhang PF, Li MY, Chen ZC
and Liu YF: Prognostic significance of annexin II expression in
non-small cell lung cancer. Clin Transl Oncol. 15:938–946. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang T, Yuan J, Zhang J, Tian R, Ji W,
Zhou Y, Yang Y, Song W, Zhang F and Niu R: Anxa2 binds to STAT3 and
promotes epithelial to mesenchymal transition in breast cancer
cells. Oncotarget. 6:30975–30992. 2015.PubMed/NCBI
|
|
35
|
Han Y, Ye J, Dong Y, Xu Z and Du Q:
Expression and significance of annexin A2 in patients with gastric
adenocarcinoma and the association with E-cadherin. Exp Ther Med.
10:549–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang YK, Liu H, Wang XZ and Zhu S:
Annexin A2 and CD105 expression in pancreatic ductal adenocarcinoma
is associated with tumor recurrence and prognosis. Asian Pac J
Cancer Prev. 15:9921–9926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiu D, Liu L, Qiao F, Yang H, Cui L and
Liu G: Annexin A2 coordinates STAT3 to regulate the invasion and
migration of colorectal cancer cells in vitro. Gastroenterol Res
Pract. 2016:35214532016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H, Yao M, Wu W, Qiu L, Sai W, Yang
J, Zheng W, Huang J and Yao D: Up-regulation of annexin A2
expression predicates advanced clinicopathological features and
poor prognosis in hepatocellular carcinoma. Tumour Biol.
36:9373–9383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kling T, Ferrarese R, Ó hAilín D,
Johansson P, Heiland DH, Dai F, Vasilikos I, Weyerbrock A, Jörnsten
R, Carro MS and Nelander S: Integrative modeling reveals annexin
A2-mediated epigenetic control of mesenchymal glioblastoma.
EBioMedicine. 12:72–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo S, Xie C, Wu P, He J, Tang Y, Xu J and
Zhao S: Annexin A2 is an independent prognostic biomarker for
evaluating the malignant progression of laryngeal cancer. Exp Ther
Med. 14:6113–6118. 2017.PubMed/NCBI
|
|
41
|
Liu Y, Myrvang HK and Dekker LV: Annexin
A2 complexes with S100 proteins: Structure, function and
pharmacological manipulation. Br J Pharmacol. 172:1664–1676. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang CY, Chen CL, Tseng YL, Fang YT, Lin
YS, Su WC, Chen CC, Chang KC, Wang YC and Lin CF: Annexin A2
silencing induces G2 arrest of non-small cell lung cancer cells
through p53-dependent and -independent mechanisms. J Biol Chem.
287:32512–32524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jung H, Kim JS, Kim WK, Oh KJ, Kim JM, Lee
HJ, Han BS, Kim DS, Seo YS, Lee SC, et al: Intracellular annexin A2
regulates NF-κB signaling by binding to the p50 subunit:
Implications for gemcitabine resistance in pancreatic cancer. Cell
Death Dis. 6:e16062015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang HJ, Yao DF, Yao M, Huang H, Wu W,
Yan MJ, Yan XD and Chen J: Expression characteristics and
diagnostic value of annexin A2 in hepatocellular carcinoma. World J
Gastroenterol. 18:5897–5904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang W, Zhao P, Xu XL, Cai L, Song ZS,
Cao DY, Tao KS, Zhou WP, Chen ZN and Dou KF: Annexin A2 promotes
the migration and invasion of human hepatocellular carcinoma cells
in vitro by regulating the shedding of CD147-harboring
microvesicles from tumor cells. PLoS One. 8:e672682013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
El-Abd N, Fawzy A, Elbaz T and Hamdy S:
Evaluation of annexin A2 and as potential biomarkers for
hepatocellular carcinoma. Tumour Biol. 37:211–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Du R, Liu B, Zhou L, Wang D, He X, Xu X,
Zhang L, Niu C and Liu S: Downregulation of annexin A3 inhibits
tumor metastasis and decreases drug resistance in breast cancer.
Cell Death Dis. 9:1262018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang K and Li J: Overexpression of ANXA3
is an independent prognostic indicator in gastric cancer and its
depletion suppresses cell proliferation and tumor growth.
Oncotarget. 7:86972–86984. 2016.PubMed/NCBI
|
|
49
|
Köllermann J, Schlomm T, Bang H, Schwall
GP, von Eichel-Streiber C, Simon R, Schostak M, Huland H, Berg W,
Sauter G, et al: Expression and prognostic relevance of annexin A3
in prostate cancer. Eur Urol. 54:1314–1323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pan QZ, Pan K, Wang QJ, Weng DS, Zhao JJ,
Zheng HX, Zhang XF, Jiang SS, Lv L, Tang Y, et al: Annexin A3 as a
potential target for immunotherapy of liver cancer stem-like cells.
Stem Cells. 33:354–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tong M, Fung TM, Luk ST, Ng KY, Lee TK,
Lin CH, Yam JW, Chan KW, Ng F, Zheng BJ, et al: ANXA3/JNK signaling
promotes self-renewal and tumor growth, and its blockade provides a
therapeutic target for hepatocellular carcinoma. Stem Cell Reports.
5:45–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ma XL, Jiang M, Zhao Y, Wang BL, Shen MN,
Zhou Y, Zhang CY, Sun YF, Chen JW, Hu B, et al: Application of
serum annexin A3 in diagnosis, outcome prediction and therapeutic
response evaluation for patients with hepatocellular carcinoma. Ann
Surg Oncol. 25:1686–1694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dreier R, Schmid KW, Gerke V and Riehemann
K: Differential expression of annexins I, II and IV in human
tissues: An immunohistochemical study. Histochem Cell Biol.
110:137–148. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gaudio E, Paduano F, Ngankeu A, Ortuso F,
Lovat F, Pinton S, D'Agostino S, Zanesi N, Aqeilan RI, Campiglia P,
et al: A Fhit-mimetic peptide suppresses annexin A4-mediated
chemoresistance to paclitaxel in lung cancer cells. Oncotarget.
7:29927–29936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Duncan R, Carpenter B, Main LC, Telfer C
and Murray GI: Characterisation and protein expression profiling of
annexins in colorectal cancer. Br J Cancer. 98:426–433. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Choi CH, Chung JY, Chung EJ, Sears JD, Lee
JW, Bae DS and Hewitt SM: Prognostic significance of annexin A2 and
annexin A4 expression in patients with cervical cancer. BMC Cancer.
16:4482016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yao HS, Sun C, Li XX, Wang Y, Jin KZ,
Zhang XP and Hu ZQ: Annexin A4-nuclear factor-kappaB feedback
circuit regulates cell malignant behavior and tumor growth in
gallbladder cancer. Sci Rep. 6:310562016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen W, Chen L, Cai Z, Liang D, Zhao B,
Zeng Y, Liu X and Liu J: Overexpression of annexin A4 indicates
poor prognosis and promotes tumor metastasis of hepatocellular
carcinoma. Tumour Biol. 37:9343–9355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Peng B, Guo C, Guan H, Liu S and Sun MZ:
Annexin A5 as a potential marker in tumors. Clin Chim Acta.
427:42–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guo WX, Man XB, Yuan HX, Shi J, Xue J, Wu
MC and Cheng SQ: Proteomic analysis on portal vein tumor
thrombus-associated proteins for hepatocellular carcinoma. Zhonghua
Yi Xue Za Zhi. 87:2094–2097. 2007.(In Chinese). PubMed/NCBI
|
|
61
|
Sun X, Liu S, Wang J, Wei B, Guo C, Chen C
and Sun MZ: Annexin A5 regulates hepatocarcinoma malignancy via
CRKI/II-DOCK180-RAC1 integrin and MEK-ERK pathways. Cell Death Dis.
9:6372018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Srivastava M, Torosyan Y, Raffeld M,
Eidelman O, Pollard HB and Bubendorf L: ANXA7 expression represents
hormone-relevant tumor suppression in different cancers. Int J
Cancer. 121:2628–2636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Du Y, Meng J, Huang Y, Wu J, Wang B,
Ibrahim MM and Tang J: Guanine nucleotide-binding protein subunit
beta-2-like 1, a new Annexin A7 interacting protein. Biochem
Biophys Res Commun. 445:58–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Song L, Mao J, Zhang J, Ibrahim MM, Li LH
and Tang JW: Annexin A7 and its binding protein galectin-3
influence mouse hepatocellular carcinoma cell line in vitro. Biomed
Pharmacother. 68:377–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang Y, Wang Q, Du Y, Bai L, Jin F, Zhang
J, Fan S, Wang H, Song L, Gao Y, et al: Inhibition of annexin A7
gene and protein induces the apotosis and decreases the invasion,
migration of the hepatocarcinoma cell line. Biomed Pharmacother.
68:819–824. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang XY, Gao F, Sun YR, Bai LL, Ibrahim
MM, Wang B and Tang JW: In vivo and in vitro effect of
hepatocarcinoma lymph node metastasis by upregulation of Annexin A7
and relevant mechanisms. Tumour Biol. 37:911–924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Morgan RO, Jenkins NA, Gilbert DJ,
Copeland NG, Balsara BR, Testa JR and Fernandez MP: Novel human and
mouse annexin A10 are linked to the genome duplications during
early chordate evolution. Genomics. 60:40–49. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kim J, Kim MA, Jee CD, Jung EJ and Kim WH:
Reduced expression and homozygous deletion of annexin A10 in
gastric carcinoma. Int J Cancer. 125:1842–1850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
van der Heijden AG, Mengual L, Lozano JJ,
Ingelmo-Torres M, Ribal MJ, Fernández PL, Oosterwijk E, Schalken
JA, Alcaraz A and Witjes JA: A five-gene expression signature to
predict progression in T1G3 bladder cancer. Eur J Cancer.
64:127–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhu J, Wu J, Pei X, Tan Z, Shi J and
Lubman DM: Annexin A10 is a candidate marker associated with the
progression of pancreatic precursor lesions to adenocarcinoma. PLoS
One. 12:e01750392017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu X, Peng X, Hu Z, Zhao Q, He J, Li J
and Zhong X: Effects of over-expression of ANXA10 gene on
proliferation and apoptosis of hepatocellular carcinoma cell line
HepG2. J Huazhong Univ Sci Technolog Med Sci. 32:669–674. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li Y, Xu A, Jia S and Huang J: Recent
advances in the molecular mechanism of sex disparity in
hepatocellular carcinoma. Oncol Lett. 17:4222–4228. 2019.PubMed/NCBI
|
|
73
|
Liu C, Ren YF, Dong J, Ke MY, Ma F, Monga
SPS, Wu R, Lv Y and Zhang XF: Activation of SRY accounts for
male-specific hepatocarcinogenesis: Implication in gender disparity
of hepatocellular carcinoma. Cancer Lett. 410:20–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zheng B, Zhu YJ, Wang HY and Chen L:
Gender disparity in hepatocellular carcinoma (HCC): Multiple
underlying mechanisms. Sci China Life Sci. 60:575–584. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Naugler WE, Sakurai T, Kim S, Maeda S, Kim
K, Elsharkawy AM and Karin M: Gender disparity in liver cancer due
to sex differences in MyD88-dependent IL-6 production. Science.
317:121–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kanda T, Takahashi K, Nakamura M, Nakamoto
S, Wu S, Haga Y, Sasaki R, Jiang X and Yokosuka O: Androgen
receptor could be a potential therapeutic target in patients with
advanced hepatocellular carcinoma. Cancers (Basel). 9:pii432017.
View Article : Google Scholar
|
|
77
|
Yeh SH and Chen PJ: Gender disparity of
hepatocellular carcinoma: The roles of sex hormones. Oncology. 78
(Suppl 1):S172–S179. 2010. View Article : Google Scholar
|
|
78
|
Torosyan Y, Simakova O, Naga S, Mezhevaya
K, Leighton X, Diaz J, Huang W, Pollard H and Srivastava M:
Annexin-A7 protects normal prostate cells and induces distinct
patterns of RB-associated cytotoxicity in androgen-sensitive and
-resistant prostate cancer cells. Int J Cancer. 125:2528–2539.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Grewal T, Wason SJ, Enrich C and Rentero
C: Annexins-insights from knockout mice. Biol Chem. 397:1031–1053.
2016. View Article : Google Scholar : PubMed/NCBI
|