Introduction
Lung cancer is one of the most rapidly growing types
of cancer and exhibits a high cancer-associated morbidity rate
worldwide (1). Non-small cell lung
cancer (NSCLC) accounts for ~80% of lung cancer cases (2). Treatment methods, including surgery,
radiotherapy, chemotherapy and molecular targeted therapy, have
improved the overall survival rate, but prognosis for patients
diagnosed at an advanced stage remains poor (3,4).
Therefore, it is urgent to investigate novel biomarkers for
diagnosis and prediction of prognosis for patients with NSCLC.
MicroRNAs (miRNAs) are a class of small non-coding
RNAs involved in post-transcriptional regulation of gene expression
through interactions with the 3′ untranslated regions (3′UTRs) of
target mRNAs (5,6). In NSCLC, certain miRNAs have been
identified as biomarkers or therapeutic targets; for example, high
expression levels of miRNA (miR)-18a, miR-20a and miR-92a correlate
with poor prognosis in patients with NSCLC (7). High expression of miR-493-5p may
improve clinical prognosis of NSCLC by targeting the oncogene
integrin subunit b1 (8). miR-410
acts as an oncogene in NSCLC by downregulating solute carrier
family 34 member through the activation of the Wnt/β-catenin
pathway (9). However, the functional
effects and underlying role of miR-1296 in NSCLC remain unknown.
Therefore, the present study investigated the function of miR-1296
in NSCLC.
The results of the present study demonstrated that
miR-1296 expression was significantly downregulated in NSCLC
tissues and cells. In addition, survival analysis revealed that
reduced miR-1296 expression was associated with a poor prognosis in
patients with NSCLC. Multivariate Cox analysis demonstrated that
reduced miR-1296 expression was an independent risk factor of NSCLC
prognosis. Overexpression of miR-1296 inhibited cell proliferation,
invasion and Wnt signaling in NSCLC. In conclusion, these results
indicated that miR-1296 expression may be a potential biomarker of
NSCLC prognosis and potential target of NSCLC treatment.
Materials and methods
Patients and tissue samples
NSCLC and adjacent normal tissue samples were
collected from 106 NSCLC patients (54 male and 52 female) who
underwent surgical resection at the Department of Cardiothoracic
Surgery, The Second People's Hospital of Qinzhou (Qinzhou, China)
between December 2010 and December 2014. Following surgical
resection, the tissue samples were immediately frozen and stored at
−80°C until RNA extraction. The age of the patients ranged between
26 and 80 years (mean age, 50.5 years). The experiments were
approved by the Ethics Committee of The Second People's Hospital of
Qinzhou. Written informed consent was obtained from all patients.
Clinical stages were classified according to the World Health
Organization Tumor-Node-Metastasis (TNM) criteria (10).
Cell culture and transfection
Four human NSCLC cell lines: A549, H1299, H460 and
SK-MES-1, and an immortalized and non-tumorigenic human bronchial
epithelial cell line NL20 were purchased from American Type Culture
Collection. The cell lines were cultured in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc.) medium supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2. A total of 1×106 cells were transfected
with 100 nM miRNA-negative control (miR-NC), miR-1296 mimic (100
nM) or miR-1296 inhibitor (100 nM; Chang Jing Bio-Tech, Ltd.) using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
cells were harvested for RT-qPCR or western blot analysis to assess
the mRNA and protein expression 48 h following transfection.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The RNA was reverse
transcribed to generate cDNA using Prime Script RT-PCR kit (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol
The thermocycling conditions were as follows: 95°C for 5 min,
followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. U6
small nuclear RNA was used as an internal control. U6 forward,
5′-CTCGCTTCGGCAGCACA-3′, and reverse, 5′-AAACGCTTCACGAATTTGCGT-3′.
miR-1296 primers were purchased from Takara Biotechnology Co., Ltd.
The mRNA expression fold changes were calculated using the
2−ΔΔCq method (11).
Cell proliferation assay
Cell proliferative ability was evaluated by using
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.)
according to the manufacturer's instructions. Briefly, transfected
cells (3×103 cells/well) were seeded in 96-well plates.
Following cell were culture for 1, 2, 3 and 4 days, CCK-8 solution
was added to each well and then incubated for 2 h at 37°C in a
humidified atmosphere with 5% CO2. Cell proliferation
was detected by a VICTOR microplate reader (BioTek Instruments,
Inc.) and absorbance was measured at 450 nm.
Transwell assay
Transwell cell invasion assay was performed using a
24-well Transwell chamber (Costar; Corning, Inc.) with Matrigel (BD
Biosciences). Transfected cells (1×105 cells/well) in
serum-free medium were seeded in the upper chamber. Medium with 10%
FBS was added to the lower chamber. The cells were maintained at
37°C in a humidified atmosphere with 5% CO2 for 48 h.
The cells in the lower chamber were fixed with 100% methanol for 20
min at 4°C, stained using 1% crystal violet for 15 min 4°C and
counted using a light microscope (Olympus Corporation).
Western blot analysis
Transfected cells were lysed in
radioimmunoprecipitation assay buffer according to the
manufacturer's instructions. Protein concentrations were determined
using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific,
Inc) by measuring optical density at a wavelength of 280 nm.
Protein (30 µg/lane) was separated by SDS-PAGE on 10% gels and
transferred onto polyvinylidene fluoride membrane. The membranes
were blocked with 5% skimmed milk at room temperature for 1.5 h and
incubated with specific primary antibodies against transcription
factor 4 (TCF4; 1:1,000; cat. no. sc-8631, Santa Cruz
Biotechnology, Inc., CA, USA), β-catenin (1:500; cat. no. sc-16512,
Santa Cruz Biotechnology, Inc., CA, USA) and GAPDH (1:1,000; cat.
no. sc-16512 2118S, Cell Signaling Technology, Inc., CA, USA)
overnight at 4°C. Primary antibody incubation was followed by
incubation with horseradish peroxidase-conjugated secondary
antibodies (1:1,000; cat. no. sc-2357; Santa Cruz Biotechnology,
Inc.) at room temperature for 1 h. The proteins were detected using
an enhanced chemiluminescence (ECL) detection system (Bio-Rad
Laboratories, Inc.) and an ECL kit (cat. no. 32106; Thermo Fisher
Scientific, Inc.). ImageJ software (version 1.48; National
Institutes of Health Bethesda) was used to measure the band
density. GAPDH was used as a loading control.
Statistical analysis
The data were analyzed using SPSS 18.0 software
(SPSS, Inc.). The results are presented as the mean ± standard
deviation. Differences between two groups were analyzed using
Student's t-test; differences among ≥3 groups were analyzed using
one-way analysis of variance, followed by multiple comparisons by
the Student-Newman-Keuls test. The χ2 test was used to
evaluate the association between miR-1296 and clinical factors. The
Kaplan-Meier method was used to plot the survival curves, and the
log-rank test was used for overall survival analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-1296 expression is downregulated
in patients with NSCLC and in NSCLC cells
To analyze the role of miR-1296 expression in NSCLC,
the expression levels of miR-1296 in NSCLC tissues and adjacent
normal tissues were evaluated by RT-qPCR. The results demonstrated
that miR-1296 expression levels in NSCLC tissues were significantly
lower compared with in adjacent normal tissues (P<0.05; Fig. 1A). In addition, four human NSCLC cell
lines exhibited significantly downregulated miR-1296 expression
compared with the normal control NL20 cell line (Fig. 1B). Thus, these results indicated that
low miR-1296 expression levels may potentially serve as a
prognostic marker for patients with NSCLC.
Downregulation of miR-1296 expression
is associated with TNM stage and lymph-node metastasis of patients
with NSCLC
Patient with NSCLC were classified into two groups
by miR-1296 expression (high and low) based on the median
expression of miR-1296 in all NSCLC tissue samples.
Clinicopathological characteristic analysis demonstrated that low
miR-1296 expression was closely associated with lymph-node
metastasis (P=0.001; Table I) and
advanced TNM stage (P=0.006; Table
I). By contrast, no association was observed between miR-1296
expression and other clinicopathological features, including sex,
age, tumor differentiation and tumor size (Table I).
 | Table I.Association between
clinicopathological characteristics of 106 patients and miR-1296
expression levels in non-small cell lung cancer. |
Table I.
Association between
clinicopathological characteristics of 106 patients and miR-1296
expression levels in non-small cell lung cancer.
| Characteristics | Total patients
(n=106) | High miR-1296
(n=54) | Low miR-1296
(n=52) | P-value |
|---|
| Age (years) |
|
|
| 0.849 |
| ≤50 | 54 | 28 | 26 |
|
|
>50 | 52 | 26 | 26 |
|
| Sex |
|
|
| 0.301 |
| Male | 64 | 30 | 34 |
|
|
Female | 42 | 24 | 18 |
|
| Tumor
differentiation |
|
|
| 0.079 |
|
High-middle | 66 | 38 | 28 |
|
| Poor | 40 | 16 | 24 |
|
| Tumor size (cm) |
|
|
| 0.248 |
|
<3 | 57 | 32 | 25 |
|
| ≥3 | 49 | 22 | 27 |
|
| Lymph node
metastasis |
|
|
| 0.001a |
| No | 62 | 40 | 22 |
|
| Yes | 44 | 14 | 30 |
|
| Tumor-node-metastasis
stage |
|
|
| 0.006a |
| I/II | 61 | 38 | 23 |
|
|
III/IV | 45 | 16 | 29 |
|
Association of miR-1296 expression and
prognosis of patients with NSCLC
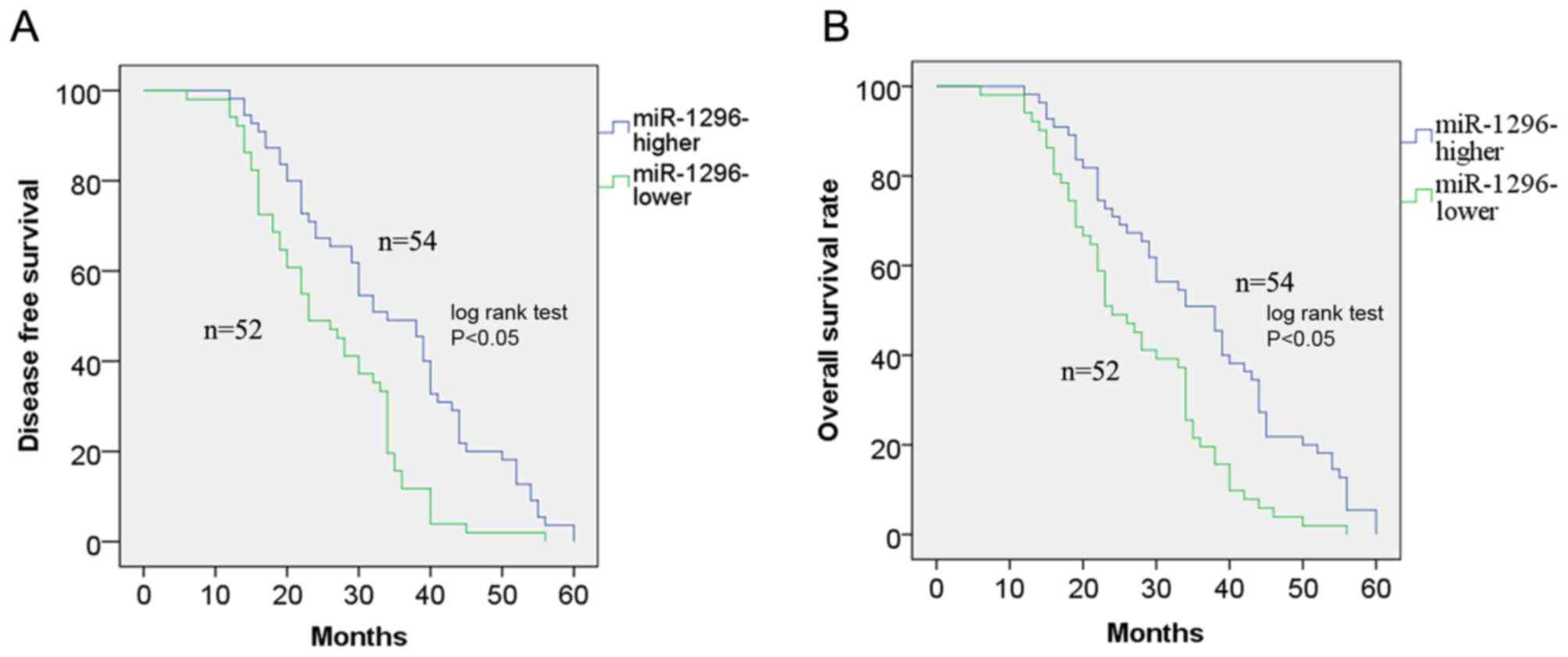
The Kaplan-Meier method was used to plot the
survival curves, which were further analyzed by the log-rank test.
The results demonstrated that low miR-1296 expression was
associated with poor disease-free survival (DFS; P<0.05;
Fig. 2A) and overall survival (OS;
P<0.05; Fig. 2B) of patients with
NSCLC compared with high miR-1296 expression. Univariate and
multivariate Cox proportional hazards regression model analysis
revealed that lymph node metastasis [P=0.001; hazard ratio
(HR)=2.038; 95% confidence interval (CI), 0.712–3.664], advanced
TNM stage (P=0.001; HR=2.113; 95% CI, 0.812–3.544) and low miR-1296
expression (P=0.001; HR=2.263; 95% CI, 1.125–3.732) were
independent predictors of poor DFS in NSCLC (Table II). In addition, lymph node
metastasis (P=0.001; HR=1.932; 95% CI, 0.872–3.145), advanced TNM
stage (P=0.001; HR=2.063; 95% CI, 0.995–3.448) and low miR-1296
expression (P=0.001; HR=2.138; 95% CI, 1.042–4.349) were
independent predictors of poor overall survival rate in NSCLC
(Table III). These results
suggested that low miR-1296 expression may be an independent
predictor for poor prognosis in patients with NSCLC.
 | Table II.Univariate and multivariate Cox
analysis of disease-free survival in 106 patients with non-small
cell lung cancer. |
Table II.
Univariate and multivariate Cox
analysis of disease-free survival in 106 patients with non-small
cell lung cancer.
|
| Univariate Cox
analysis | Multivariate Cox
analysis |
|---|
|
|
|
|
|---|
| Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | 0.766
(0.544–1.245) | 0.612 |
|
|
| Sex | 0.644
(0.352–1.446) | 0.794 |
|
|
| Tumor
differentiation | 0.993
(0.764–1.544) | 0.446 |
|
|
| Tumor size (cm) | 1.019
(0.688–1.836) | 0.278 |
|
|
| Lymph node
metastasis | 2.234
(0.885–3.864) | 0.001a | 2.038
(0.712–3.664) | 0.001a |
| TNM stage | 2.543
(1.255–4.222) | 0.001a | 2.013
(0.812–3.544) | 0.001a |
| Low miR-1296
levels | 2.688
(1.644–4.388) | 0.001a | 2.013
(1.125–3.732) | 0.001a |
 | Table III.Univariate and multivariate Cox
analysis of overall survival in 106 patients with non-small cell
lung cancer. |
Table III.
Univariate and multivariate Cox
analysis of overall survival in 106 patients with non-small cell
lung cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | 0.665
(0.346–1.224) | 0.732 |
|
|
| Sex | 0.786
(0.422–1.411) | 0.694 |
|
|
| Tumor
differentiation | 1.115
(0.563–1.766) | 0.546 |
|
|
| Tumor size
(cm) | 1.203
(0.763–1.926) | 0.345 |
|
|
| Lymph node
metastasis | 2.096
(1.002–3.665) | 0.001a | 1.932
(0.872–3.145) | 0.001a |
| TNM stage | 2.315
(1.075–3.886) | 0.001a | 2.063
(0.995–3.448) | 0.001a |
| Low miR-1296
levels | 2.549
(1.393–4.504) | 0.001a | 2.138
(1.042–4.349) | 0.001a |
miR-1296 suppresses cell proliferation
and invasion in NSCLC cells
To further investigate the effects of miR-1296
expression in NSCLC cells, CCK-8 cell proliferation and Transwell
assays were performed. SK-MES-1 and A549 cells were transfected
with a miR-1296 mimic or miR-1296 inhibitor for upregulation or
downregulation of miR-1296 expression levels, as they exhibited
higher or lower expression of miR-1296 compared with the other two
cell lines (Fig. 3A and B). The
results of the CCK-8 assay indicated that miR-1296 overexpression
in SK-MES-1 and A549 cells inhibited cell proliferation, whereas
reduced miR-1296 expression enhanced cell proliferation compared
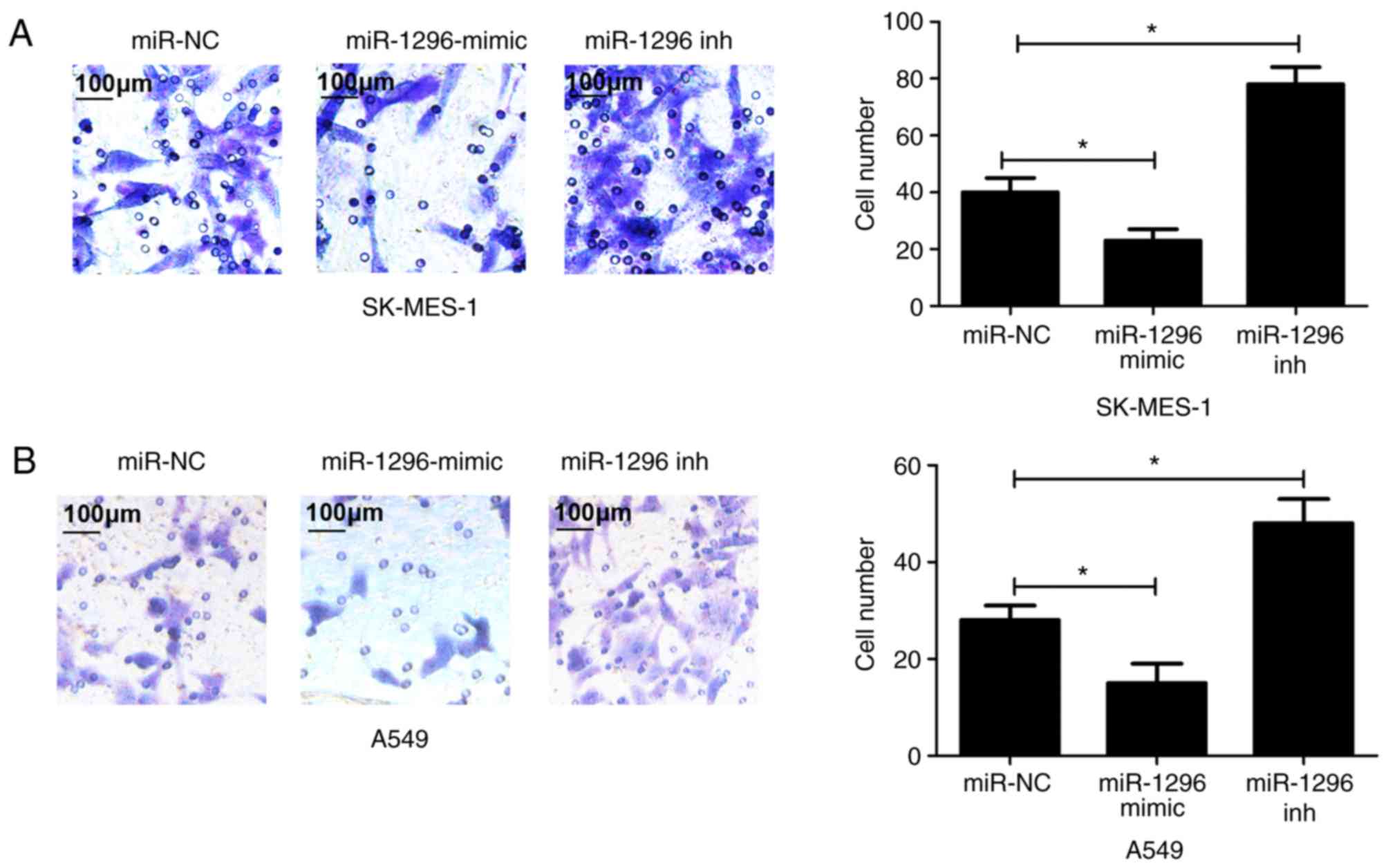
with respective negative control groups (Fig. 3C and D). Additionally, Transwell cell
invasion assay demonstrated that miR-1296 overexpression in
SK-MES-1 and A549 cells inhibited cell invasive ability, whereas
reduced miR-1296 expression enhanced cell invasive ability compared
with respective negative control groups (Fig. 4). Thus, these results indicated that
miR-1296 may suppress cell proliferation and invasion of NSCLC
cells.
miR-1296 suppresses Wnt signaling in
NSCLC cells
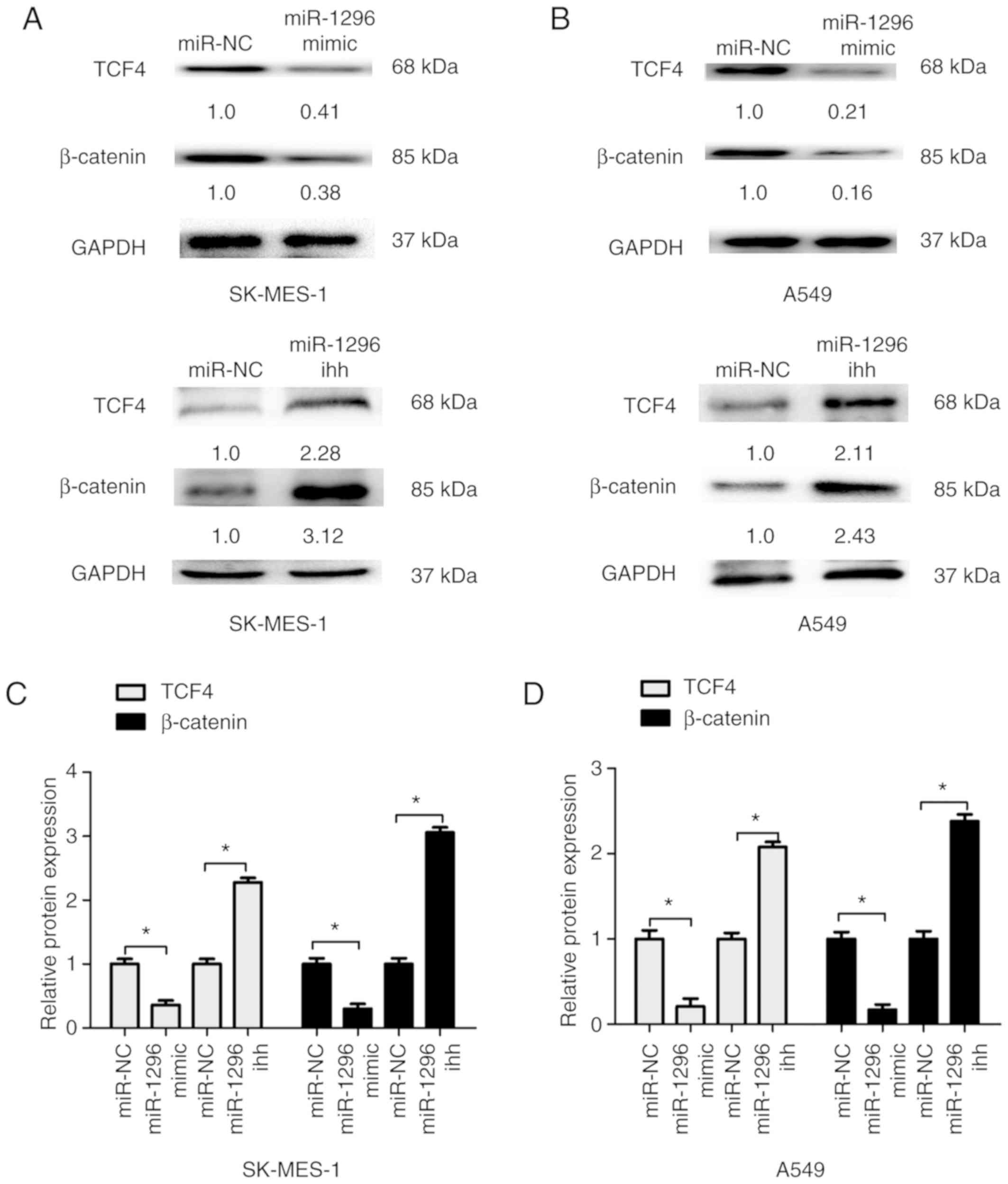
To investigate the effects of miR-1296 expression in
the Wnt signaling pathway, western blot analysis of downstream
factors β-catenin and TCF4 was performed. The results indicated
that miR-1296 overexpression inhibited Wnt signaling pathway by
reducing β-catenin and TCF4 expression in SK-MES-1 and A549 cells
compared with respective control groups. By contrast,
downregulation of miR-1296 significantly increased β-catenin and
TCF4 protein expression levels in SK-MES-1 and A549 cells compared
with the control groups (Fig. 5).
These results indicated that miR-1296 may suppress Wnt signaling in
NSCLC cells.
Discussion
Abnormal gene expression in cancer involves
inactivation of tumor suppressor genes and activation of oncogenes
(12). miRNAs are key regulators of
tumor progression in NSCLC, and certain miRNAs are valuable for
diagnostics and treatment of NSCLC (13). A previous study revealed that
dysregulation of miRNA expression may be used as sensitive and
accurate biomarkers or prognostic predictors of human NSCLC
(14). For example, miR-137 is
downregulated and its promoter is hypomethylated in lung cancer,
and high levels of miR-137 promoter methylation are associated with
poor disease-free survival in NSCLC (15); plasma exosomal microRNA-451a is a
noninvasive biomarker for early prediction of recurrence and
prognosis in non-small cell lung cancer (NSCLC) patients after
curative resection (16). Serum
miR-494 was significantly elevated in NSCLC patients and closely
correlated with poor clinical outcome (17).
miR-1296 has been identified as a tumor suppressor
in several types of cancer; for example, miR-1296-5p may be
involved in the regulation of migration and invasion of human
gastric cancer cells at least in part by targeting the erb-b2
receptor tyrosine kinase 2 (ERBB2)/Rac family small GTPase 1
signaling pathway (18). miR-1296-5p
is involved in the regulation of proliferation of breast cancer
cells by targeting the ERBB2/MTOR complex 1 signaling pathway
(19). Downregulation of miR-1296
may serve as a prognostic biomarker in hepatocellular carcinoma
(HCC) and miR-1296 inhibits metastasis and epithelial-mesenchymal
transition in HCC by targeting the SRSF protein kinase 1-mediated
PI3K/AKT pathway (20). In the
present study, miR-1296 expression was significantly downregulated
in NSCLC tissues compared to adjacent normal tissues. In addition,
miR-1296 expression was downregulated in NSCLC cell lines compared
with a normal lung cell line. Survival analysis demonstrated that
low miR-1296 expression predicted poor prognosis. Additionally,
multivariate Cox analysis revealed that low miR-1296 expression was
an independent risk factor of NSCLC prognosis. Thus, these results
indicated that miR-1296 expression level may be a potential
biomarker for NSCLC prognosis.
The present study demonstrated that miR-1296
overexpression inhibits cell proliferation and invasion, whereas
reduced miR-1296 expression enhanced cell proliferation and
invasion compared with the control groups. Wnt signaling pathway is
involved in tumor cell proliferation and invasion in NSCLC
(21); in the present study,
miR-1296 overexpression inhibited Wnt signaling by reducing the
expression levels of two key proteins, β-catenin and TCF4, in NSCLC
cells. By contrast, downregulation of miR-1296 significantly
promoted Wnt signaling by increasing β-catenin and TCF4 expression
in NSCLC cells.
In conclusion, the present study demonstrated that
miR-1296 expression was reduced in NSCLC tissues and cell lines
compared with healthy tissues and cells. In addition, low miR-1296
expression was associated with advanced TNM stage and lymph-node
metastasis of patients with NSCLC. miR-1296 expression level was
identified as a prognostic predictor of NSCLC. Overexpression of
miR-1296 inhibited cell proliferation, invasion and Wnt signaling
in NSCLC. These results indicated that miR-1296 expression may be a
potential biomarker for NSCLC prognosis, as well as a potential
target of NSCLC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD, YY and ZD conceived and designed the study, and
drafted the manuscript. HD, YY, CX and ZD collected, analyzed and
interpreted the data and critically revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Second People's Hospital of Qinzhou (Qinzhou, China). Written
informed consent was obtained from all patients in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carney DN and Hansen HH: Non-small-cell
lung cancer-stalemate or progress? N Engl J Med. 343:1261–1262.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sánchez de Cos J, Sojo González MA,
Montero MV, Pérez Calvo MC, Vicente MJ and Valle MH: Non-small cell
lung cancer and silent brain metastasis. Survival and prognostic
factors. Lung Cancer. 63:140–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suresh R, Ali S, Ahmad A, Philip PA and
Sarkar FH: The role of cancer stem cells in recurrent and
drug-resistant lung cancer. Adv Exp Med Biol. 890:57–74. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer. 96
(Suppl):R40–R44. 2007.PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu X, Zhu S, Tao Z and Ye S: High
circulating miR-18a, miR-20a, and miR-92a expression correlates
with poor prognosis in patients with non-small cell lung cancer.
Cancer Med. 7:21–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang Z, Kong R, He Z, Lin LY, Qin SS,
Chen CY, Xie ZQ, Yu F, Sun GQ, Li CG, et al: High expression of
miR-493-5p positively correlates with clinical prognosis of non
small cell lung cancer by targeting oncogene ITGB1. Oncotarget.
8:47389–47399. 2017.PubMed/NCBI
|
|
9
|
Zhang X, Ke X, Pu Q, Yuan Y, Yang W, Luo
X, Jiang Q, Hu X, Gong Y, Tang K, et al: MicroRNA-410 acts as
oncogene in NSCLC through downregulating SLC34A2 via activating
Wnt/β-catenin pathway. Oncotarget. 7:14569–14585. 2016.PubMed/NCBI
|
|
10
|
Okuyemi OT, Piccirillo JF and Spitznagel
E: TNM staging compared with a new clinicopathological model in
predicting oral tongue squamous cell carcinoma survival. Head Neck.
36:1481–1489. 2014.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Skrzypski M, Dziadziuszko R and Jassem J:
MicroRNA in lung cancer diagnostics and treatment. Mutat Res.
717:25–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu Y, Cheng Y, Song Y, Zhang Z, Deng M,
Wang C, Zheng G and He Z: MicroRNA-493 suppresses tumor growth,
invasion and metastasis of lung cancer by regulating E2F1. PLoS
One. 9:e1026022014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Min L, Wang F, Hu S, Chen Y, Yang J, Liang
S and Xu X: Aberrant microRNA-137 promoter methylation is
associated with lymph node metastasis and poor clinical outcomes in
non-small cell lung cancer. Oncol Lett. 15:7744–7750.
2018.PubMed/NCBI
|
|
16
|
Kanaoka R, Iinuma H, Dejima H, Sakai T,
Uehara H, Matsutani N and Kawamura M: Usefulness of plasma exosomal
MicroRNA-451a as a noninvasive biomarker for early prediction of
recurrence and prognosis of non-small cell lung cancer. Oncology.
94:311–323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Wang T, Zhang Y, Wang H, Wu Y,
Liu K and Pei C: Upregulation of serum miR-494 predicts poor
prognosis in non-small cell lung cancer patients. Cancer Biomark.
21:763–768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shan X, Wen W, Zhu D, Yan T, Cheng W,
Huang Z, Zhang L, Zhang H, Wang T, Zhu W, et al: miR 1296-5p
inhibits the migration and invasion of gastric cancer cells by
repressing ERBB2 expression. PLoS One. 12:e01702982017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen G, He M, Yin Y, Yan T, Cheng W, Huang
Z, Zhang L, Zhang H, Liu P, Zhu W and Zhu Y: miR-1296-5p decreases
ERBB2 expression to inhibit the cell proliferation in
ERBB2-positive breast cancer. Cancer Cell Int. 17:952017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Q, Liu X, Liu Z, Zhou Z, Wang Y, Tu J,
Li L, Bao H, Yang L and Tu K: MicroRNA-1296 inhibits metastasis and
epithelial-mesenchymal transition of hepatocellular carcinoma by
targeting SRPK1-mediated PI3K/AKT pathway. Mol Cancer. 16:1032017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|