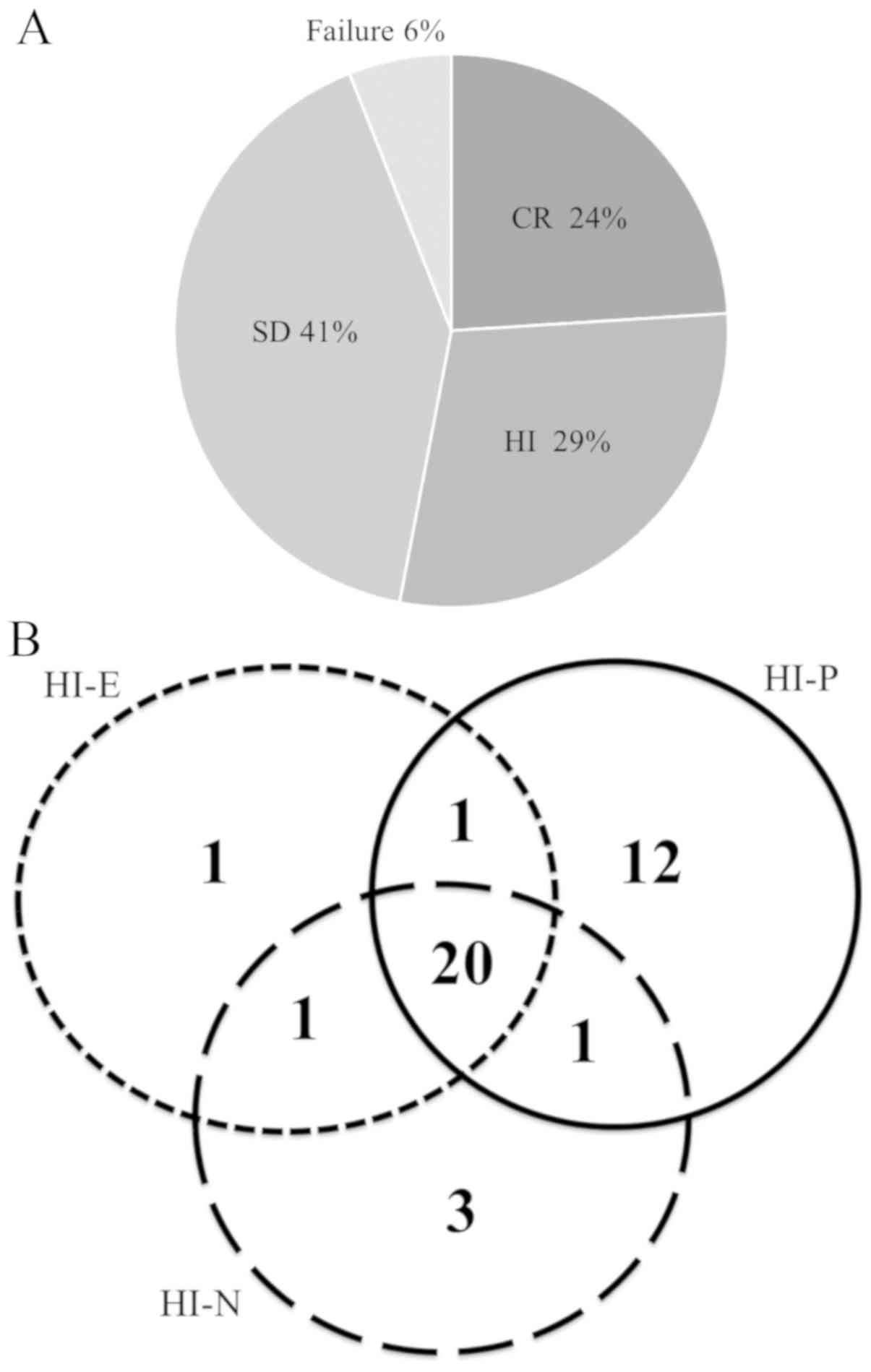
Spandidos Publications style
Nakaya A, Fujita S, Satake A, Nakanishi T, Azuma Y, Tsubokura Y, Saito R, Konishi A, Hotta M, Yoshimura H, Yoshimura H, et al: Evaluation of azacitidine in patients with transplant‑ineligible myelodysplastic syndromes and acute myeloid leukemia with myelodysplasia‑related changes in a Japanese clinical setting. Oncol Lett 19: 1317-1321, 2020.
APA
Nakaya, A., Fujita, S., Satake, A., Nakanishi, T., Azuma, Y., Tsubokura, Y. ... Nomura, S. (2020). Evaluation of azacitidine in patients with transplant‑ineligible myelodysplastic syndromes and acute myeloid leukemia with myelodysplasia‑related changes in a Japanese clinical setting. Oncology Letters, 19, 1317-1321. https://doi.org/10.3892/ol.2019.11225
MLA
Nakaya, A., Fujita, S., Satake, A., Nakanishi, T., Azuma, Y., Tsubokura, Y., Saito, R., Konishi, A., Hotta, M., Yoshimura, H., Ishii, K., Ito, T., Nomura, S."Evaluation of azacitidine in patients with transplant‑ineligible myelodysplastic syndromes and acute myeloid leukemia with myelodysplasia‑related changes in a Japanese clinical setting". Oncology Letters 19.2 (2020): 1317-1321.
Chicago
Nakaya, A., Fujita, S., Satake, A., Nakanishi, T., Azuma, Y., Tsubokura, Y., Saito, R., Konishi, A., Hotta, M., Yoshimura, H., Ishii, K., Ito, T., Nomura, S."Evaluation of azacitidine in patients with transplant‑ineligible myelodysplastic syndromes and acute myeloid leukemia with myelodysplasia‑related changes in a Japanese clinical setting". Oncology Letters 19, no. 2 (2020): 1317-1321. https://doi.org/10.3892/ol.2019.11225