Introduction
Myelodysplastic syndromes (MDS) are hematopoietic
stem cell disorders characterized by ineffective hematopoiesis and
peripheral blood cytopenia. Degrees of cytopenia, increasing bone
marrow blasts and cytogenetic abnormalities reflect the risk of
progression to acute myelogenous leukemia, and are incorporated
into prognostic scoring systems (1).
The median age of diagnosis is 70–79 years (2). Therefore, patients in this age group
are mostly ineligible for transplantation. Introduction of a novel
hypomethylating agent, azacitidine (AZA), caused a stir in MDS
treatment strategy. High-risk MDS patients have shown improved
overall survival (OS) when treated with the hypomethylating agent
AZA compared with conventional therapies (1). A phase 1/2 study of AZA-7 in Japan
demonstrated that AZA was effective, safe, and well tolerated in
MDS patients (2). Based on these
results, AZA was approved for MDS in all-risk groups in Japan in
2011, and has since become the first-line treatment for
transplant-ineligible high-risk MDS patients. However, Japanese
post-marketing data assessing the safety and efficacy of AZA in
real-world settings are currently limited. We here report the
results for patients with MDS patients treated with AZA in a
real-world setting.
Patients and methods
Patients
In this retrospective study, transplant-ineligible
patients with MDS were treated with AZA at two hematology centers,
Kansai Medical University Hospital and Kansai Medical University
Medical Center, between June 2012 and August 2018. MDS subtypes
were defined according to the World Health Organization (WHO) 2016
criteria (3). Risks were assessed
using the revised international prognosis scoring system (IPSS-R)
(4). Patients who received a
transplant were excluded. In our facility, indication for
transplant is patient with high risk in IPSS-R, under 60 years old,
without serious complications. This study was conducted in
accordance with the Declaration of Helsinki and the requirements of
the institution's review board.
Treatment regimens
Patients received one cycle of AZA treatment
consisting of 75 mg/m2/day for 5 or 7 days per month
without any chemotherapy, according to phase III study1.
Two types of alternative scheduling were used at the treatment
sites because weekend treatment was not possible: a 5-day regimen
consisting of 5 days of AZA treatment with no weekend treatment,
and a 7-day regimen consisting of 5 days of AZA treatment with no
weekend treatment, followed by 2 further days of AZA treatment.
Patients with neutropenia received antimicrobial prophylaxis. The
red blood cell and platelet transfusion thresholds were as follows:
Hemoglobin level <7 g/dl and platelet count
<20×109/l, respectively. We did not use
granulocyte-colony stimulating factor (G-CSF) and other stimulating
factors as a standard therapy.
Response criteria
Efficacy was measured using complete remission (CR),
hematologic improvement (HI), stable disease (SD), and failure, as
defined by the International Working Group (IWG) 2006 criteria
(5).
However, not all patients in our cohort underwent
bone marrow examination to evaluate response. We confirmed CR was
patients who compatible to IMG criteria with evaluation by bone
marrow exam, and HI included patients who compatible to IMG
criteria without bone marrow evaluation. The timing of evaluation
was various, so we evaluated best response.
Evaluation of safety
Toxicity was evaluated according to the Common
Terminology Criteria for Adverse Events (AEs) (CTCAE4.0)
[http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40].
Statistical analysis
The primary end point is OS. OS was calculated from
the start of AZA treatment until the time of death or the last
clinical follow-up. Survival curves were generated using the
Kaplan-Meier method, and differences were evaluated using the
log-rank test. Multivariate Cox proportional hazards models were
used to determine whether baseline characteristics were associated
with OS. Associations between baseline characteristics and response
were analyzed using Fisher's exact test. To determine if correction
of cytopenia improved OS, a time-dependent Cox model was used to
assess the prognostic impact of achieving CR and HI.
All statistical tests were two-sided, statistical
significance was defined as P<0.05, and 95% confidence intervals
(CI) were calculated. All statistical analyses were performed using
EZR (Saitama Medical Center, Jichi Medical University, Saitama,
Japan), which is a graphical user interface for R version 2.13.0
(The R Foundation). Specifically, EZR is a modified version of R
Commander (version 1.6–3), which adds statistical functions
frequently used in biostatistics (6).
Results
Patient characteristics
The clinical characteristics of the patients (n=85;
median age, 73 years, range, 50–95 years; 59% male) included in
this study are shown in Table I.
Patients were classified according to the 2016 WHO Classification
as follows: Acute myeloid leukemia (AML) with
myelodysplasia-related changes [27%; the 2016 WHO classification
for AML notably includes the French American British classification
of MDS as refractory anemia with excess blasts in transformation
(RAEB-T)], MDS with excess blasts type 1 (18%), MDS with excess
blasts type 2 (8%), myelodysplastic/myeloproliferative neoplasm
(16%), therapy-related myeloid neoplasms (5%), and others (2%). The
median number of AZA cycles was seven [standard deviation (SD):
2–54]. Forty-five percent of patients were treated using the 5-day
regimen. The median follow-up period was 12.0 (SD: 1.2–73.6)
months.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Value (n=85) |
|---|
| Median age, years
(range) | 73 (50–95) |
| Male, % | 59 |
| PS 0/1, % | 86 |
| Diagnosis, % |
|
|
MDS-RS | 18 |
|
MDS-EB-1 | 24 |
|
MDS-EB-2 | 8 |
|
MDS/MPN | 16 |
| t-MN | 5 |
| AML with
myelodysplasia-related changes (RAEB-T in FAB) | 27 |
|
Others | 2 |
| IPSS-R, % |
|
| Very
low | 2 |
| Low | 11 |
|
Intermediate | 29 |
| High | 31 |
| Very
high | 27 |
| Median
cycle, n (range) | 7 (2–54) |
| 5 days regimen,
% | 45 |
| Median follow-up
periods, months (range) | 12.0 (1.2–73.6) |
Treatment outcomes
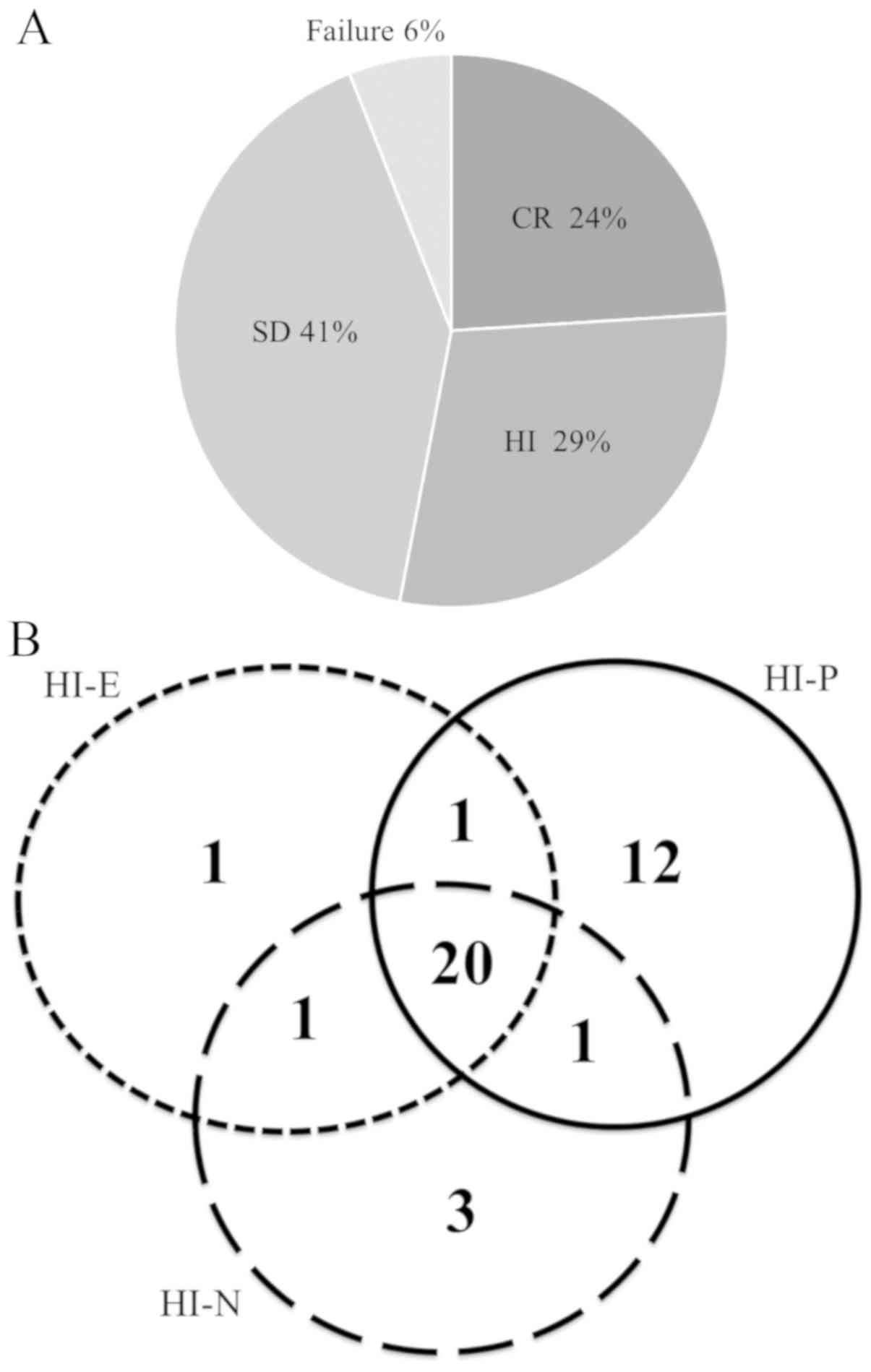
CR was achieved in 24% of patients, HI in 29%, and
SD in 41 and 6% of patients had treatment failure according to the
IWG criteria (Fig. 1A). In patients
with HI, the specific responses of cytopenia in the three lineages
were as follows: Erythroid (HI-E) in one patient, platelet response
(HI-P) in 12 patients, and neutrophil response (HI-N) in three
patients. Two-lineage responses were as follows: HI-E and HI-P in
one patient, HI-P and HI-N in seven patients, and HI-E and HI-N in
one patient (Fig. 1B). The median OS
was 22.7 months (12.5–28.7). The median OS according to response
was as follows: 24.9 months (12.4-not achieved) for both CR and HI
and 15.9 (8.9–25.2) months for both SD and failure (P=0.146). The
prognostic classification of MDS regarding survival was made using
IPSS-R, and the median OS was as follows: Not available for very
low and low, 31.6 months for intermediate, 23.0 months for high,
and 12.0 months for very high (P=0.09) (Fig. 2).
Toxicity
Severe AEs (grade ≥3) were neutropenia (n=16) and
infection (n=16). Five patients died from severe infection. Other
hematological AEs were thrombocytopenia (n=8) and anemia (n=5).
Non-hematological AEs were grade 3 renal failure (n=1) and grade 3
febrile neutropenia (n=2).
Predictors for survival
We carried out multivariate analysis of OS to
identify the clinical factors that defined patients who achieved
improved outcomes after AZA treatment (Table II). We evaluated age >75 years,
male sex, 7-day regimen, RAEB, and IPSS-R ≥high. Male sex and
IPSS-R ≥high were significantly associated with OS [hazard ratio
(HR): 1.97 (95% CI: 1.20–3.81], P=0.043; HR: 2.71 (95% CI:
1.21–5.30), P=0.005; respectively) (Table II).
 | Table II.Multivariate analysis for overall
survival. |
Table II.
Multivariate analysis for overall
survival.
| Variables | Hazard ratio | 95% CI | P-value |
|---|
| Male | 1.97 | 1.20–3.81 | 0.043 |
| Female | 1 |
|
|
| IPSS-R (≥high) | 2.71 | 1.21–5.30 | 0.005 |
| IPSS-R
(<high) | 1 |
|
|
Other factors including AEs dropped out after
backward stepwise selection. We also performed Fisher's test to
identify the basic characteristics of patients who responded.
However, we failed to find any significant associations between
patient characteristics and response to AZA (Table III).
 | Table III.Results of Fisher's exact test
investigating the association of variables with the response to
AZA. |
Table III.
Results of Fisher's exact test
investigating the association of variables with the response to
AZA.
| Variables | Odds ratio | 95% CI | P-value |
|---|
| Age ≥75 years | 1.09 | 0.44–2.72 | 0.854 |
| Age <75 years | 1 |
|
|
| Male | 1.22 | 0.50–3.01 | 0.667 |
| Female | 1 |
|
|
| 7 day regimen | 0.70 | 0.28–1.74 | 0.437 |
| 5 day regimen | 1 |
|
|
| IPSS-R (≥high) | 0.57 | 0.20–1.63 | 0.291 |
| IPSS-R
(<high) | 1 |
|
|
| RAEB | 2.41 | 0.85–6.79 | 0.097 |
| Not RAEB | 1 |
|
|
| AE positive | 0.56 | 0.23–1.38 | 0.207 |
| AE negative | 1 |
|
|
Influence of HI on OS
To determine if correction of cytopenia improved OS,
we assessed the prognostic impact of achieving CR and HI using a
time-dependent Cox model (Table
IV). However, we failed to find any significant associations
between response and OS.
 | Table IV.Time-dependent Cox model of overall
survival according to the achievement of CR and HI. |
Table IV.
Time-dependent Cox model of overall
survival according to the achievement of CR and HI.
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| CR | 0.70 | 0.27–1.83 | 0.465 |
| HI | 2.58 | 1.19–5.58 | 0.016 |
Discussion
In this retrospective study, we analyzed the
outcomes of patients with all-risk transplant-ineligible MDS
following treatment with AZA in a clinical setting. The results
suggested that AZA administration was effective, with an overall
response rate of >50%.
AZA has been studied in patients with higher-risk
MDS in two major randomized multicenter trials, with median
survivals of 21 months in CALGB9221 (7) and 24.5 months in AZA-001 (1). The median survival in the current study
was comparable (22.7 months), even though our cohort included
all-risk patients and more elderly patients than the trials. No
reports to date have evaluated the effect of AZA using IPSS-R. Most
patients in the current cohort were high- or intermediate-risk
according to IPSS-R, and OS according to IPSS-R classification
revealed that high-risk patients had poorer OS, though IPSS-R
classification showed no difference in response to AZA using
Fisher's exact analysis. This suggests that IPSS-R reflects the
outcome of MDS but not the efficacy of AZA treatment, i.e., the
prognosis of MDS is largely related to chromosomal abnormalities,
but treatments are not.
More patients who achieved HI also achieved platelet
recovery, suggesting that the effects of AZA might be predicted by
platelet recovery. van der Helm et al (8) reported that platelet doubling after the
first cycle of AZA might be a useful indicator of AZA efficacy. In
our study, the median number of AZA cycles required to achieve HI
was three, suggesting that early platelet recovery may be a useful
predictor of response. However, there were few responders in terms
of erythroid recovery. AZA was approved for all-risk MDS in Japan
in 2011, before the approval of erythropoiesis-stimulating agents
(ESAs) for low-risk MDS. Unlike in western countries, some patients
therefore initially received AZA without ESA, which might affect
the erythroid recovery response in Japan. If ESAs were used prior
to AZA, the erythroid recovery may have been different. We assessed
the influence of HI on OS but failed to find any significant
associations between response and OS. However, we think the most
benefit of using AZA is reduction of number of transfusions. We did
not assess the quality of life (QOL) improvement, however,
reduction of number of transfusions might have improved patient's
QOL.
The recommended schedule for AZA administration is
75 mg/m2 for 7 consecutive days every 28 days. However,
a 7-consecutiv day regimen is difficult to administer in the clinic
because weekend administration is often not possible. Our study
sites thus adopted alternative schedules of either 5- or 7-day
regimens. There was no significant difference in efficacy between
these two regimens according to univariate analysis, multivariate
analysis for survival, or Fisher's exact test for response.
Despite numerous studies, the optimal schedule of
AZA administration is currently unclear. Fujimaki et al
(9) reported a retrospective study
in which 52% of patients (high-risk MDS) achieved HI with the 5-day
schedule. Morita et al (10)
reported a phase 2 study in low-risk MDS patients in which 47.1% of
patients achieved HI and 21.6% achieved CR with the 5-day regimen.
Lyons et al (11) compared
three different regimens (5 day, 5-2-2 days, and 5-2-5 day), and
reported no significant difference in response. Although it is too
early to draw conclusions regarding the optimal treatment regimen,
current data suggest that the regimen length may not significantly
affect prognosis.
We analyzed the basic characteristics of the
patients in relation to response to AZA using Fisher's exact test,
but failed to find any significant associations between patient
characteristics and AZA treatment response. The patient
characteristics predicting response to AZA thus remain unknown. We
also identified the baseline characteristics associated with
survival by multivariate analysis. It revealed that male sex and a
high-risk IPSS-R classification had high HRs for survival. As we
described above, this suggests that IPSS-R reflects the prognosis
of MDS but not the efficacy of AZA treatment. Lifestyle and habits
such as tobacco and alcohol have been reported to increase the
prevalence of MDS, which may contribute to the worse prognosis of
men (12).
In our study, five patients died of infection after
AZA treatment. None of these patients showed a response, all had
stable disease, and all developed neutropenia due to AZA. In
practice, some patients continue AZA even if they do not show a
response. The guidelines recommend that, in the absence of any
alternative, transplant-ineligible patients should continue AZA
until progression, potentially resulting in unplanned treatment and
subsequent neutropenia. We suggest that this mortality rate may
thus reflect the real-world setting. Beguin et al (13) reported that six of 99 patients died
of AEs, though they did not describe the AEs in detail.
This study had some limitations, including its
retrospective design, the small number of study sites, and small
sample size. In this study, we excluded transplant eligible
patients, considering that it might be difficult to evaluate the
effectiveness of AZA alone. Conversely, it may include worse
conditioned patients who cannot undertake transplant, that might
become the selection bias.
According to the National Comprehensive Cancer
Network guidelines for low-risk MDS (14), AZA treatment is recommended after
failure of ESA treatment. As previously stated however, AZA was
approved prior to ESA in Japan, and some patients in our study thus
received AZA without prior ESA treatment. The 2016 WHO
classification of AML with myelodysplasia-related changes includes
MDS as RAEB-T in the FAB classification. In clinical practice,
patients belonging to this category are treated with AZA, and we
therefore included this group in our study. Not all patients in our
cohort underwent bone marrow examination to evaluate response, and
we could therefore not judge ‘partial remission’ or ‘marrow CR’,
which require precise bone marrow blast counts. We therefore used
CR, HI, SD, and failure to evaluate outcomes in this study.
Furthermore, the timing of evaluation was various, and it depended
on physicians' decision. We picked up the best response
retrospectively. This is because there is no criteria of evaluation
of AZA. We are not certain when AZA shows the effectiveness. This
point needs to be clarified in the prospective trials.
In conclusion, the results of this study suggest
that all-risk transplant-ineligible MDS patients may benefit from
AZA treatment. IPSS-R risk category may not reflect the efficacy of
AZA, but may predict overall prognosis in patients with MDS. AZA
can be administered safely to MDS patients. However, further
studies are warranted to establish the criteria for identifying
patients likely to obtain maximum benefit from AZA treatment, and
to develop optimal treatment strategies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AN and SN designed the study. AN performed
statistical analysis and interpretation of data, and drafted the
article. AN, SF, AS, TN, YT, RS, YA, AK, MH, HY, KI, TI and SN
performed patient treatment, provided patient data, and analyzed
and interpreted it. AN and SN drafted the article and revised it
critically for important intellectual content. AN, SF, AS, TN, YT,
RS, YA, AK, MH, HY, KI, TI and SN provided final approval of the
version to be submitted. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and the requirements of the
institution's review board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fenaux P, Mufti GJ, Hellstrom-Lindberg E,
Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz
G, List A, et al: Efficacy of azacitidine compared with that of
conventional care regimens in the treatment of higher-risk
myelodysplastic syndromes: A randomised, open-label, phase III
study. Lancet Oncol. 10:223–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uchida T, Ogawa Y, Kobayashi Y, Ishikawa
T, Ohashi H, Hata T, Usui N, Taniwaki M, Ohnishi K, Akiyama H, et
al: Phase I and II study of azacitidine in Japanese patients with
myelodysplastic syndromes. Cancer Sci. 102:1680–1686. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2406.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greenberg PL, Tuechler H, Schanz J, Sanz
G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus
F, et al: Revised international prognostic scoring system for
myelodysplastic syndromes. Blood. 120:2454–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheson BD, Greenberg PL, Bennett JM,
Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM,
Stone RM, et al: Clinical application and proposal for modification
of the International Working Group (IWG) response criteria in
myelodysplasia. Blood. 108:419–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silverman LR, Demakos EP, Peterson BL,
Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D,
Powell BL, DeCastro CM, et al: Randomized controlled trial of
azacitidine in patients with the myelodysplastic syndrome: A study
of the cancer and leukemia group B. J Clin Oncol. 20:2429–2440.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Helm LH, Alhan C, Wijermans PW,
van Marwijk Kooy M, Schaafsma R, Biemond BJ, Beeker A, Hoogendoorn
M, van Rees BP, de Weerdt O, et al: Platelet doubling after the
first azacitidine cycle is a promising predictor for response in
myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia
(CMML) and acute myeloid leukaemia (AML) patients in the Dutch
azacitidine compassionate named patient programme. Br J Haematol.
155:599–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujimaki K, Miyashita K, Kawasaki R and
Tomita N: Efficacy and safety of a 5-day regimen of azacitidine for
patients with high-risk myelodysplastic syndromes. Eur J Haematol.
97:228–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morita Y, Maeda Y, Yamaguchi T, Urase F,
Kawata S, Hanamoto H, Tsubaki K, Ishikawa J, Shibayama H, Matsumura
I and Matsuda M: Five-day regimen of azacitidine for lower-risk
myelodysplastic syndromes (refractory anemia or refractory anemia
with ringed sideroblasts): A prospective single-arm phase 2 trial.
Cancer Sci. 109:3209–3215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lyons RM, Cosgriff TM, Modi SS, Gersh RH,
Hainsworth JD, Cohn AL, McIntyre HJ, Fernando IJ, Backstrom JT and
Beach CL: Hematologic response to three alternative dosing
schedules of azacitidine in patients with myelodysplastic
syndromes. J Clin Oncol. 27:1850–1856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ugai T, Matsuo K, Sawada N, Iwasaki M,
Yamaji T, Shimazu T, Sasazuki S, Inoue M, Kanda Y and Tsugane S;
Japan Public Health Centre-based Prospective Study Group, : Smoking
and alcohol and subsequent risk of myelodysplastic syndromes in
Japan: The Japan Public Health Centre-based Prospective Study. Br J
Haematol. 178:747–755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beguin Y, Selleslag D, Meers S, Graux C,
Bries G, Deeren D, Vrelust I, Ravoet C, Theunissen K, Voelter V, et
al: Safety and efficacy of azacitidine in Belgian patients with
high-risk myelodysplastic syndromes, acute myeloid leukaemia, or
chronic myelomonocytic leukaemia: results of a real-life,
non-interventional post-marketing survey. Acta Clin Belg. 70:34–43.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
The NCCN Clinical Practice Guidelines in
Oncology, Myelodysplastic Syndromes, Version2.20 2019-October18.
2018, https://www.nccn.org/professionals/physician_gls/pdf/mds.pdfNovember
11–2018
|