Introduction
Gastrointestinal stromal tumors (GISTs) are the most
commonly reported primary mesenchymal tumors of the
gastrointestinal tract (1) and 30%
of GISTs are highly malignant, with a high risk of abdominal and
liver metastases (2). Therefore, it
is important to improve the risk prediction for patients with a
GIST prior to clinical treatment, to aid in the identification of
patients who may require adjuvant imatinib therapy post-surgery
(3). Patients with tumors classified
as high risk have a poor prognosis and a high risk of recurrence
and metastasis (4). The current risk
classification criteria of the National Comprehensive Cancer
Network (NCCN) are based on tumor size, mitotic activity and
primary lesion localization, whereas tumor site and
gastrointestinal bleeding are independent risk factors of a poor
prognosis for patients with GISTs (5,6).
Identifying biomarkers associated with clinical risk may aid
diagnosis and improve the prognostic prediction of patients with
GISTs.
As an auxiliary transcriptional regulator, ski is
involved in nervous system development (7), proliferation and differentiation of
hematopoietic cells (8), muscle
differentiation (9), regeneration
and wound healing (10).
Furthermore, ski is overexpressed in a number of different types of
cancer, including colorectal, gastric, pancreatic and lung cancer
(11–14). Our previous study demonstrated that
high ski expression levels in fibrosarcoma were associated with
poor tumor cell differentiation and a less favorable prognosis, and
that ski significantly promoted fibrosarcoma cell proliferation
(data not yet published). Fibrosarcoma and GISTs are both primary
mesenchymal tumors, therefore the potential value of ski expression
levels as a prognostic biomarker and risk classifier in GISTs was
investigated in the present study.
Materials and methods
Patient and tissue samples
The present study was approved by The Clinical
Ethics Committee of Daping Hospital and Research Institute of
Surgery, Army Medical University (Chongqing, China). Cases newly
diagnosed as GIST by pathologists from January 2013 to December
2014 were included. Patients whose specimens did not qualify for
immunohistochemistry, had missing clinical data or contact
information were excluded. Patients provided informed written
consent and were informed that their clinical data may be used in
publications. A total of 81 tissue samples were obtained from
patients diagnosed with pathologically verified GISTs from the
Department of Gastrointestinal Surgery of Daping Hospital
(Chongqing, China), including 38 samples from male patients and 43
samples from female patients (mean age, 61 years; age range, 35–80
years). Patient clinical and pathological data were obtained from
patient medical records. Follow-up was performed by telephone every
2 months, starting from the date of primary diagnosis after the
surgery and ending at any observable relapse (local recurrence
and/or distant metastasis).
Immunohistochemistry (IHC)
Rabbit polyclonal ski antibody (cat. no. sc-9140;
Santa Cruz Biotechnology, Inc.) was used to determine ski
expression levels. Positive and negative fibrosarcoma samples from
our previous study (data not yet published) were used as positive
and negative controls. Formalin-fixed and paraffin-embedded (4%
neutral formalin for 6–12 h at room temperature) tissues were
sliced into 4-µm-thick sections. The tissues were subsequently
deparaffinized and pretreated with 1 mmol/l EDTA at pH 9.0 in a
high-pressure cooker for 3 min and then treated with 3%
H2O2 for 10 min. Subsequently, washing was
performed for 3 min three times using 0.01 M PBS at room
temperature, and the slides were incubated with an anti-ski
antibody (1:500) in the humidified chamber at 4°C overnight. Slides
were washed the next day and incubated with 3,3′-diaminobenzidine
stain for 5 min at room temperature, viewed under a bright-field
microscope (BX41; Olympus) and images were captured by Cell Sens
Standard software (version 1.16; Olympus) and analyzed blinded by
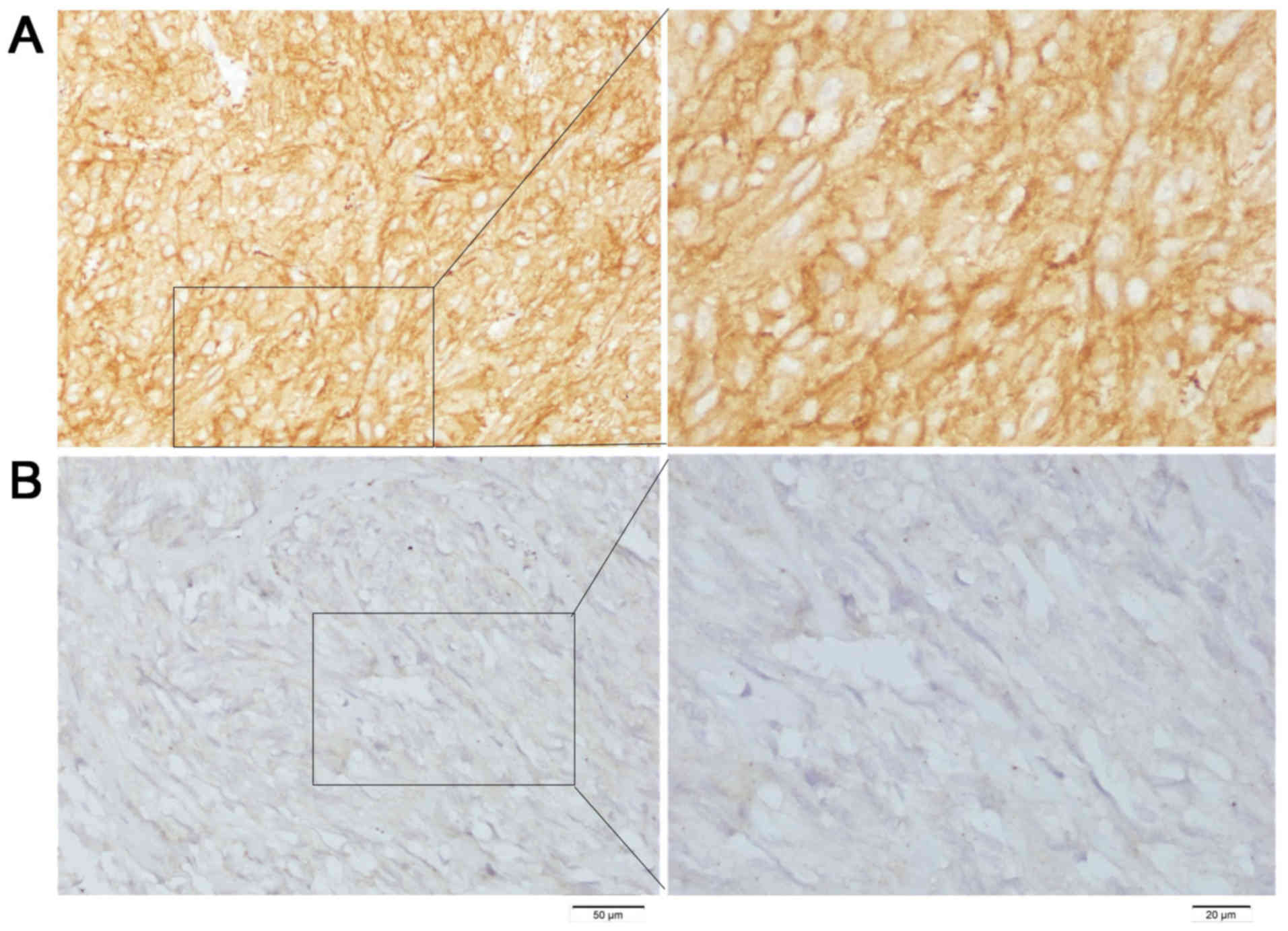
two pathologists. Ski staining was scored as: Strong staining, 3+;
moderate staining, 2+; faint staining, 1+; or absence of staining,
0.
Statistical analysis
All analyses were performed using SPSS software
(version 18.0; SPSS Inc.). The correlation between ski expression
levels with sex, age, tumor location and clinical risk
classification were determined using a two-tailed t-test, Pearson's
correlation analysis with Bonferroni's correction, ANOVA with a
post-hoc Tukey's test and Spearman's correlation analysis with
Bonferroni's correction, respectively. The Kaplan-Meier method was
used to assess the prognostic value of ski expression levels for
patients with GIST. A log-rank test was used to compare
disease-free survival (DFS) curves and a Cox proportional hazards
model was used to calculate univariate hazard ratios for the
variables. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients' characteristics
Primary GIST tissue samples from 81 patients were
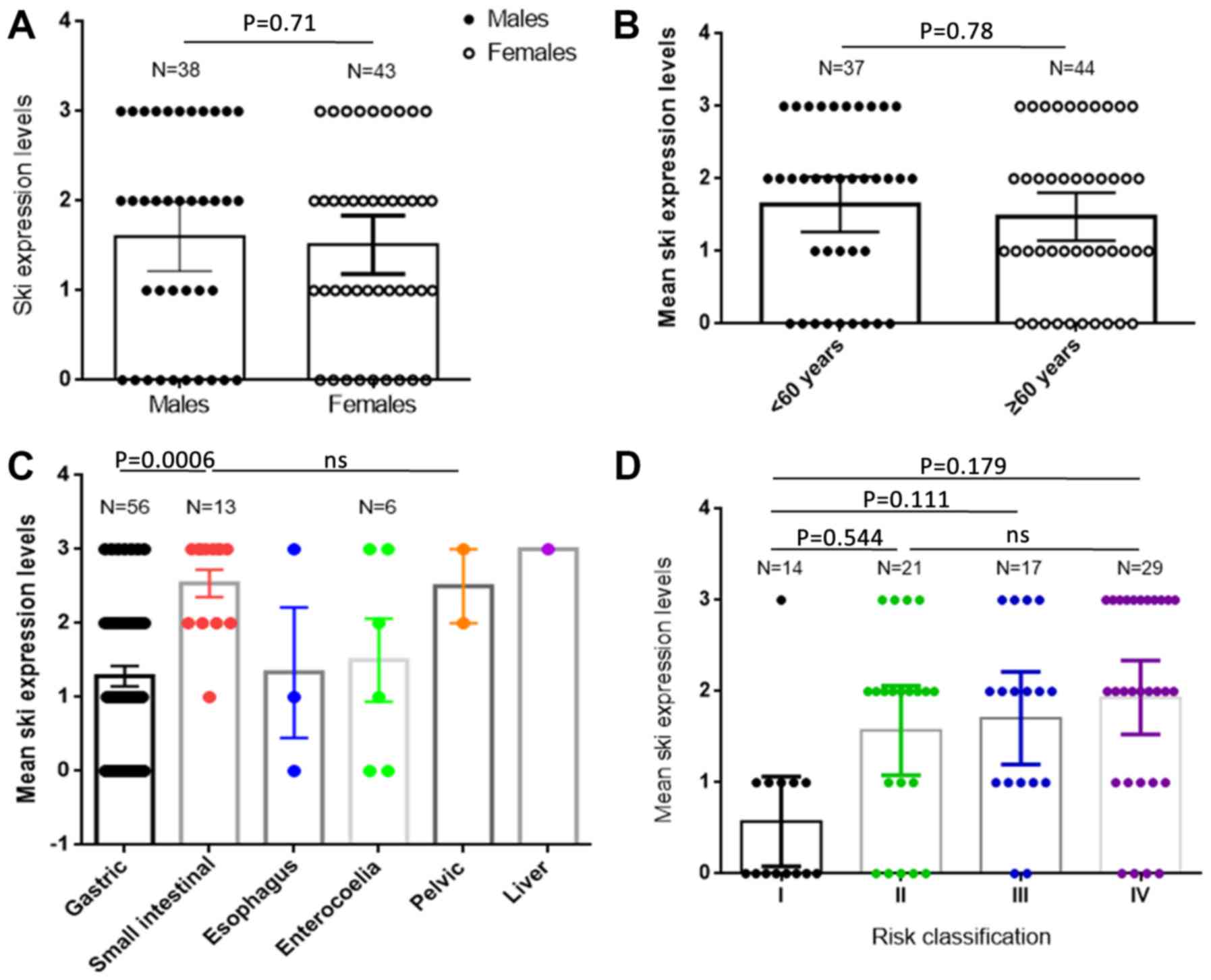
analyzed. Univariate survival analysis showed that sex and age were
not associated with patient prognosis (P=0.06 and P=0.8,
respectively). Tumors were localized in: Stomach, 69%; small
intestine, 16%; esophagus, 4%; enterocoelia, 7%; pelvis, 3%; and
liver, 1%. Detailed clinicopathological characteristics of the
patients are presented in Table
I.
 | Table I.Univariate analysis of disease-free
survival of the 81 patients with gastrointestinal stromal
tumors. |
Table I.
Univariate analysis of disease-free
survival of the 81 patients with gastrointestinal stromal
tumors.
| Characteristic | No. patients (%) | HR | 95% CI | P-value |
|---|
| Sex |
| 2.21 | 1.02–5.07 | 0.060 |
| Male | 38 (47) |
|
|
|
|
Female | 43 (53) |
|
|
|
| Age |
| 0.91 | 0.40–2.01 | 0.800 |
| <60
years | 37 (46) |
|
|
|
| ≥60
years | 44 (54) |
|
|
|
| Location |
|
|
|
|
|
Gastric | 56 (59) | 1.94 | 0.65–5.76 | 0.240a |
| Small
intestinal | 13 (16) |
|
|
|
|
Esophagus | 3 (4) | 4.04 | 1.62–10.09 | 0.003b |
|
Enterocoelia | 6 (7) |
|
|
|
|
Pelvic | 2 (3) |
|
|
|
|
Liver | 1 (1) |
|
|
|
| NCCN risk
classification |
| 0.33 | 0.17–0.75 | 0.009 |
| Very
low | 14 (17) |
|
|
|
| Low | 21 (26) |
|
|
|
|
Moderate | 17 (21.0) |
|
|
|
| High | 29 (36) |
|
|
|
Ski expression levels are correlated
with clinical risk classification
The NCCN risk classification standard was used for
GIST clinical classification. Patients were divided into four risk
groups: High risk, 29 cases; medium risk, 17 cases; low risk, 21
cases; and extremely low risk, 14 cases. Univariate survival
analysis demonstrated that classification as extremely low risk was
significantly correlated with longer DFS (P=0.009; Table I). Patients were also divided into
four groups based on IHC ski expression levels: 3+, 20 cases; 2+,
24 cases; 1+, 18 cases; and 0+, 19 cases; (Fig. 1). The numbers of patients with
different levels of ski expression and in each clinical risk
classification are shown in Table
II. Higher ski expression levels were not significantly
correlated with higher GIST clinical risk and sex, age and tumor
location were not correlated with ski expression levels (Fig. 2)
 | Table II.Ski expression levels in IHC and risk
classification of patients with gastrointestinal stromal
tumors. |
Table II.
Ski expression levels in IHC and risk
classification of patients with gastrointestinal stromal
tumors.
| NCCN risk
classification | 0+ | 1+ | 2+ | 3+ | Total no. of
patients |
|---|
| Very low | 8 | 5 | 0 | 1 | 14 |
| Low | 5 | 3 | 9 | 4 | 21 |
| Moderate | 2 | 5 | 6 | 4 | 17 |
| High | 4 | 5 | 9 | 11 | 29 |
| Total no. of
patients | 19 | 18 | 24 | 20 | 81 |
Higher ski expression levels are
correlated with higher recurrence risk of GISTs
Follow-up data from 71 patients with GISTs were
collected to determine the correlation between recurrence rates and
DFS with ski expression levels. These data were analyzed using the
Kaplan-Meier method. Fewer patients with low ski expression levels
(IHC 0 and IHC +1) had tumor recurrence at 36 months compared with
patients with high ski expression levels (IHC 2+ and 3+; 21 vs.
32%, respectively). At the 80-month follow-up period, a larger
number of patients with high ski expression levels had tumor
recurrence compared with patients with low ski expression levels
(55.3 vs. 24.2% respectively; Table
III). Kaplan-Meier curves showed that the median DFS for the
high ski expression group was shorter compared with the low ski
expression group (72 months vs. N/A, respectively); however, this
difference was not significant (P=0.0586; Fig. 3).
 | Table III.Tumor recurrence rates for patients
with gastrointestinal stromal tumors with low and high ski
expression levels based on IHC. |
Table III.
Tumor recurrence rates for patients
with gastrointestinal stromal tumors with low and high ski
expression levels based on IHC.
| Recurrence status
(%) | Low | High |
|---|
| Before 36
months | 7
(21.2) | 12 (31.6) |
| 37 to 80
months | 1 (3.0) | 9
(23.7) |
| Recurrence
free | 25 (75.8) | 17 (45.7) |
Discussion
Tumor size and mitotic index are the two primary
factors used to assess tumor risk when utilizing the risk
stratification system of the National Institutes of Health
consensus (15). However, tumor
location has subsequently been shown to have independent prognostic
value, therefore, this factor was added into the risk
stratification system and named the Miettinen-Lasota/Armed Forces
Institute of Pathology classification system (16). Tumor size, location and mitotic index
are the primary pathological factors of the risk classification
system recommended by the European Society of Medical Oncology and
NCCN, and are currently used in GIST guidelines (16). Additional prognostic factors have
been reported for GIST, for example, activation of stem cell factor
receptor (KIT) is involved in tumorigenesis of GISTs and specific
KIT mutation types and loci have demonstrated prognostic value
(17). Deletions in exon 11 of the
KIT gene, particularly those involving codons 557 and/or 558, are
associated with higher risk of metastases and have been reported as
independent adverse prognostic factors (18,19). Liu
et al (6) reported that
gastrointestinal bleeding was an independent risk factor for
recurrence. Furthermore, the Japanese Kinki GIST registry group
showed that pre or perioperative ‘GIST rupture’ was associated with
a shorter median overall survival compared with patients with GISTs
without ‘tumor rupture’ (6.4 vs. 11.9 years, respectively)
(20). However, the clinical
definition of ‘tumor rupture’ is impractical and ambiguous,
therefore it is difficult to use ‘tumor rupture’ as an independent
prognostic factor for GIST (21).
High ski expression levels are associated with poor
prognosis in a number of types of cancer, such as esophageal
carcinoma (22,23), leukemia (24), pancreatic cancer (25,26) and
melanoma (27). However, to the best
of our knowledge, the present study is the first to demonstrate the
correlations between ski expression levels and its prognostic value
for GISTs. High ski expression levels were correlated with a high
clinical risk of GISTs, but not correlated with sex, age or tumor
location. Together, these data suggest that ski expression levels
are correlated with the prognosis of patients with GIST. Follow-up
data showed that patients with GISTs in the high ski expression
group had higher recurrence rates compared with patients in the low
ski expression group. These data suggest that patients with GISTs
with high ski expression levels may have a higher risk of
recurrence during the first 3 years after complete surgical
resection and diagnosis of a primary localized GIST. High ski
expression levels were found to be a poor prognostic factor of
median DFS; however, this difference was not significant which may
be due to the small patient cohort size. Therefore, the results of
the present study need to be validated using larger patient cohorts
before ski expression levels can be used clinically as an
independent biomarker of prognosis for patients with GIST.
Acknowledgements
The authors would like to thank Mrs. Li Lin and Mrs.
Ping Fu (Department of Pathology, Daping Hospital and Research
Institute of Surgery, Army Medical University; Chongqing, China)
for their assistance with immunohistochemistry, and Mr. He Xiao
(Cancer center, Daping Hospital and Research Institute of Surgery,
Army Medical University; Chongqing, China) for the assistance with
statistical analysis
Funding
This work was supported by The National Natural
Science Foundation of China (grant no. 81802781).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QSW, YGZ and HLX analyzed and interpreted the data.
YZ and PL performed the experiments. QSW and YGZ wrote the
manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Ethics Committee of Daping Hospital and Research Institute of
Surgery, Army Medical University (Chongqing, China). Patients
provided informed written consent and were informed that their
clinical data may be used in publications.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Du CY, Shi YQ, Zhou Y, Fu H and Zhao G:
The analysis of status and clinical implication of KIT and PDGFRA
mutations in gastrointestinal stromal tumor (GIST). J Surg Oncol.
98:175–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mavroeidis L, Metaxa-Mariatou V,
Papoudou-Bai A, Lampraki AM, Kostadima L, Tsinokou I, Zarkavelis G,
Papadaki A, Petrakis D, Gκoura S, et al: Comprehensive molecular
screening by next generation sequencing reveals a distinctive
mutational profile of KIT/PDGFRA genes and novel genomic
alterations: Results from a 20-year cohort of patients with GIST
from north-western Greece. ESMO Open. 3:e0003352018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopes LF and Bacchi CE: Imatinib treatment
for gastrointestinal stromal tumour (GIST). J Cell Mol Med.
14:42–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanchez Hidalgo JM, Rufian Peña S, Ciria
Bru R, Naranjo Torres A, Muñoz Casares C, Ruiz Rabelo J and Briceño
Delgado J: Gastrointestinal stromal tumors (GIST): A prospective
evaluation of risk factors and prognostic scores. J Gastrointest
Cancer. 41:27–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Qiu H, Wu Z, Zhang P, Feng X, Chen
T, Li Y, Tao K, Li G, Sun X, et al: A Novel pathological prognostic
score (PPS) to identify ‘very high-risk’ patients: A multicenter
retrospective analysis of 506 patients with high risk
gastrointestinal stromal tumor (GIST). J Gastrointest Surg.
22:2150–2157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Q, Li Y, Dong M, Kong F and Dong Q:
Gastrointestinal bleeding is an independent risk factor for poor
prognosis in GIST patients. Biomed Res Int.
2017:71524062017.PubMed/NCBI
|
|
7
|
Stegmüller J, Konishi Y, Huynh MA, Yuan Z,
Dibacco S and Bonni A: Cell-intrinsic regulation of axonal
morphogenesis by the Cdh1-APC target SnoN. Neuron. 50:389–400.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pearson-White S, Deacon D, Crittenden R,
Brady G, Iscove N and Quesenberry PJ: The ski/sno protooncogene
family in hematopoietic development. Blood. 86:2146–2155. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sutrave P, Kelly AM and Hughes SH: Ski can
cause selective growth of skeletal muscle in transgenic mice. Genes
Dev. 4:1462–1472. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Zhang E, Li P, Liu J, Zhou P, Gu
DY, Chen X, Cheng T and Zhou Y: Expression and possible mechanism
of c-ski, a novel tissue repair-related gene during normal and
radiation-impaired wound healing. Wound Repair Regen. 14:162–171.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bravou V, Antonacopoulou A, Papadaki H,
Floratou K, Stavropoulos M, Episkopou V, Petropoulou C and
Kalofonos H: TGF-beta repressors SnoN and Ski are implicated in
human colorectal carcinogenesis. Cell Oncol. 31:41–51.
2009.PubMed/NCBI
|
|
12
|
Kiyono K, Suzuki HI, Morishita Y, Komuro
A, Iwata C, Yashiro M, Hirakawa K, Kano MR and Miyazono K: c-Ski
overexpression promotes tumor growth and angiogenesis through
inhibition of transforming growth factor-beta signaling in
diffuse-type gastric carcinoma. Cancer Sci. 100:1809–1816. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song L, Chen X, Gao S, Zhang C, Qu C, Wang
P and Liu L: Ski modulate the characteristics of pancreatic cancer
stem cells via regulating sonic hedgehog signaling pathway. Tumour
Biol. Oct 12–2016.(Epub ahead of print). View Article : Google Scholar
|
|
14
|
Yang H, Zhan L, Yang T, Wang L, Li C, Zhao
J, Lei Z, Li X and Zhang HT: Ski prevents TGF-β-induced EMT and
cell invasion by repressing SMAD-dependent signaling in non-small
cell lung cancer. Oncol Rep. 34:87–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fletcher CD, Berman JJ, Corless C,
Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti
H, Rubin BP, et al: Diagnosis of gastrointestinal stromal tumors: A
consensus approach. Hum Pathol. 33:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel S: Navigating risk stratification
systems for the management of patients with GIST. Ann Surg Oncol.
18:1698–1704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wozniak A, Rutkowski P, Piskorz A,
Ciwoniuk M, Osuch C, Bylina E, Sygut J, Chosia M, Rys J, Urbanczyk
K, et al: Prognostic value of KIT/PDGFRA mutations in
gastrointestinal stromal tumours (GIST): Polish Clinical GIST
Registry experience. Ann Oncol. 23:353–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wardelmann E, Losen I, Hans V, Neidt I,
Speidel N, Bierhoff E, Heinicke T, Pietsch T, Büttner R and
Merkelbach-Bruse S: Deletion of Trp-557 and Lys-558 in the
juxtamembrane domain of the c-kit protooncogene is associated with
metastatic behavior of gastrointestinal stromal tumors. Int J
Cancer. 106:887–895. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martín J, Poveda A, Llombart-Bosch A,
Ramos R, López-Guerrero JA, García del Muro J, Maurel J, Calabuig
S, Gutierrez A, González de Sande JL, et al: Deletions affecting
codons 557–558 of the c-KIT gene indicate a poor prognosis in
patients with completely resected gastrointestinal stromal tumors:
A study by the Spanish Group for Sarcoma Research (GEIS). J Clin
Oncol. 23:6190–6198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishida T, Cho H, Hirota S, Masuzawa T,
Chiguchi G and Tsujinaka T; Kinki GIST Study Group, :
Clinicopathological features and prognosis of primary GISTs with
tumor rupture in the real world. Ann Surg Oncol. 25:1961–1969.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asare EA and Feig BW: Raining Frogs,
flying horses, and defining tumor rupture in GIST. Ann Surg Oncol.
26:1601–1603. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fukuchi M, Nakajima M, Fukai Y, Miyazaki
T, Masuda N, Sohda M, Manda R, Tsukada K, Kato H and Kuwano H:
Increased expression of c-Ski as a co-repressor in transforming
growth factor-beta signaling correlates with progression of
esophageal squamous cell carcinoma. Int J Cancer. 108:818–824.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Villanacci V, Bellone G, Battaglia E,
Rossi E, Carbone A, Prati A, Verna C, Niola P, Morelli A, Grassini
M and Bassotti G: Ski/SnoN expression in the sequence
metaplasia-dysplasia-adenocarcinoma of Barrett's esophagus. Hum
Pathol. 39:403–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ritter M, Kattmann D, Teichler S, Hartmann
O, Samuelsson MK, Burchert A, Bach JP, Kim TD, Berwanger B, Thiede
C, et al: Inhibition of retinoic acid receptor signaling by Ski in
acute myeloid leukemia. Leukemia. 20:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heider TR, Lyman S, Schoonhoven R and
Behrns KE: Ski promotes tumor growth through abrogation of
transforming growth factor-beta signaling in pancreatic cancer. Ann
Surg. 246:61–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang P, Chen Z, Meng ZQ, Fan J, Luo JM,
Liang W, Lin JH, Zhou ZH, Chen H, Wang K, et al: Dual role of Ski
in pancreatic cancer cells: Tumor-promoting versus
metastasis-suppressive function. Carcinogenesis. 30:1497–1506.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boone B, Haspeslagh M and Brochez L:
Clinical significance of the expression of c-Ski and SnoN, possible
mediators in TGF-beta resistance, in primary cutaneous melanoma. J
Dermatol Sci. 53:26–33. 2009. View Article : Google Scholar : PubMed/NCBI
|