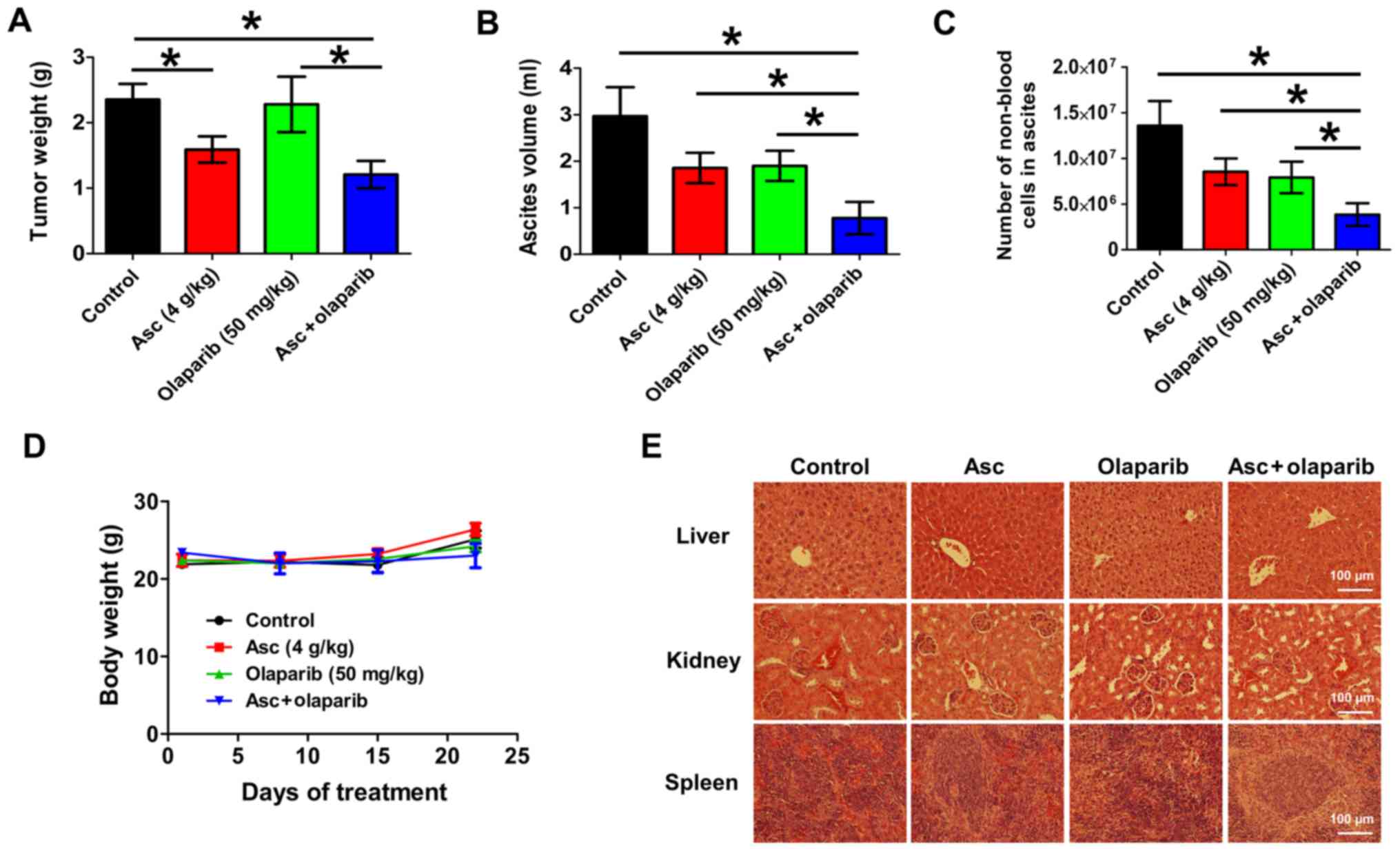
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
https://seer.cancer.gov/statfacts/html/ovary.html
|
|
4
|
Taylor KN and Eskander RN: PARP inhibitors
in epithelial ovarian cancer. Recent Pat Anticancer Drug Discov.
13:145–158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stecklein SR, Kumaraswamy E, Behbod F,
Wang W, Chaguturu V, Harlan-Williams LM and Jensen RA: BRCA1 and
HSP90 cooperate in homologous and non-homologous DNA
double-strand-break repair and G2/M checkpoint activation. Proc
Natl Acad Sci USA. 109:13650–13655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bryant HE and Helleday T: Inhibition of
poly (ADP-ribose) polymerase activates ATM which is required for
subsequent homologous recombination repair. Nucleic Acids Res.
34:1685–1691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCabe N, Turner NC, Lord CJ, Kluzek K,
Bialkowska A, Swift S, Giavara S, O'Connor MJ, Tutt AN, Zdzienicka
MZ, et al: Deficiency in the repair of DNA damage by homologous
recombination and sensitivity to poly(ADP-ribose) polymerase
inhibition. Cancer Res. 66:8109–8115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim G, Ison G, McKee AE, Zhang H, Tang S,
Gwise T, Sridhara R, Lee E, Tzou A, Philip R, et al: FDA approval
summary: Olaparib monotherapy in patients with deleterious germline
BRCA-mutated advanced ovarian cancer treated with three or more
lines of chemotherapy. Clin Cancer Res. 21:4257–4261. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pujade-Lauraine E, Ledermann JA, Selle F,
Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A,
Pignata S, et al: Olaparib tablets as maintenance therapy in
patients with platinum-sensitive, relapsed ovarian cancer and a
BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised,
placebo-controlled, phase 3 trial. Lancet Oncol. 18:1274–1284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coleman RL, Oza AM, Lorusso D, Aghajanian
C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G,
et al: Rucaparib maintenance treatment for recurrent ovarian
carcinoma after response to platinum therapy (ARIEL3): A
randomised, double-blind, placebo-controlled, phase 3 trial.
Lancet. 390:1949–1961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mirza MR, Monk BJ, Herrstedt J, Oza AM,
Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I,
et al: Niraparib maintenance therapy in platinum-sensitive,
recurrent ovarian cancer. N Engl J Med. 375:2154–2164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nielsen FC, van Overeem Hansen T and
Sorensen CS: Hereditary breast and ovarian cancer: New genes in
confined pathways. Nat Rev Cancer. 16:599–612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walsh T, Casadei S, Lee MK, Pennil CC,
Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A,
et al: Mutations in 12 genes for inherited ovarian, fallopian tube,
and peritoneal carcinoma identified by massively parallel
sequencing. Proc Natl Acad Sci USA. 108:18032–18037. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Swisher EM, Lin KK, Oza AM, Scott CL,
Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O'Malley DM,
et al: Rucaparib in relapsed, platinum-sensitive high-grade ovarian
carcinoma (ARIEL2 Part 1): An international, multicentre,
open-label, phase 2 trial. Lancet Oncol. 18:75–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loibl S, O'Shaughnessy J, Untch M, Sikov
WM, Rugo HS, McKee MD, Huober J, Golshan M, von Minckwitz G, Maag
D, et al: Addition of the PARP inhibitor veliparib plus carboplatin
or carboplatin alone to standard neoadjuvant chemotherapy in
triple-negative breast cancer (BrighTNess): A randomised, phase 3
trial. Lancet Oncol. 19:497–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bang YJ, Xu RH, Chin K, Lee KW, Park SH,
Rha SY, Shen L, Qin S, Xu N, Im SA, et al: Olaparib in combination
with paclitaxel in patients with advanced gastric cancer who have
progressed following first-line therapy (GOLD): A double-blind,
randomised, placebo-controlled, phase 3 trial. Lancet Oncol.
18:1637–1651. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma Y, Chapman J, Levine M, Polireddy K,
Drisko J and Chen Q: High-dose parenteral ascorbate enhanced
chemosensitivity of ovarian cancer and reduced toxicity of
chemotherapy. Sci Transl Med. 6:222ra182014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Q, Espey MG, Sun AY, Lee JH, Krishna
MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR and Levine
M: Ascorbate in pharmacologic concentrations selectively generates
ascorbate radical and hydrogen peroxide in extracellular fluid in
vivo. Proc Natl Acad Sci USA. 104:8749–8754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Q, Espey MG, Sun AY, Pooput C, Kirk
KL, Krishna MC, Khosh DB, Drisko J and Levine M: Pharmacologic
doses of ascorbate act as a prooxidant and decrease growth of
aggressive tumor xenografts in mice. Proc Natl Acad Sci USA.
105:11105–11109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du J, Martin SM, Levine M, Wagner BA,
Buettner GR, Wang SH, Taghiyev AF, Du C, Knudson CM and Cullen JJ:
Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer.
Clin Cancer Res. 16:509–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schoenfeld JD, Sibenaller ZA, Mapuskar KA,
Wagner BA, Cramer-Morales KL, Furqan M, Sandhu S, Carlisle TL,
Smith MC, Abu Hejleh T, et al: O2− and
H2O2-mediated disruption of fe metabolism
causes the differential susceptibility of NSCLC and GBM cancer
cells to pharmacological ascorbate. Cancer Cell. 32:2682017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoffer LJ, Levine M, Assouline S,
Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L and
Miller WH Jr: Phase I clinical trial of i.v. ascorbic acid in
advanced malignancy. Ann Oncol. 19:1969–1974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Monti DA, Mitchell E, Bazzan AJ, Littman
S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S and Levine
M: Phase I evaluation of intravenous ascorbic acid in combination
with gemcitabine and erlotinib in patients with metastatic
pancreatic cancer. PLoS One. 7:e297942012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Welsh JL, Wagner BA, van't Erve TJ, Zehr
PS, Berg DJ, Halfdanarson TR, Yee NS, Bodeker KL, Du J, Roberts LJ
II, et al: Pharmacological ascorbate with gemcitabine for the
control of metastatic and node-positive pancreatic cancer (PACMAN):
Results from a phase I clinical trial. Cancer Chemother Pharmacol.
71:765–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma E, Chen P, Wilkins HM, Wang T, Swerdlow
RH and Chen Q: Pharmacologic ascorbate induces neuroblastoma cell
death by hydrogen peroxide mediated DNA damage and reduction in
cancer cell glycolysis. Free Radic Biol Med. 113:36–47. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stordal B, Timms K, Farrelly A, Gallagher
D, Busschots S, Renaud M, Thery J, Williams D, Potter J, Tran T, et
al: BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only
one functionally deleterious BRCA1 mutation. Mol Oncol. 7:567–579.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van de Wetering M, Barker N, Harkes IC,
van der Heyden M, Dijk NJ, Hollestelle A, Klijn JG, Clevers H and
Schutte M: Mutant E-cadherin breast cancer cells do not display
constitutive Wnt signaling. Cancer Res. 61:278–284. 2001.PubMed/NCBI
|
|
32
|
Hall TA: BioEdit: A user-friendly
biological sequence alignment editor and analysis program for
windows 95/98/NT. Nucleic Acids Symp Ser. 41:95–98. 1999.
|
|
33
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Imai S, Kiyozuka Y, Maeda H, Noda T and
Hosick HL: Establishment and characterization of a human ovarian
serous cystadenocarcinoma cell line that produces the tumor markers
CA-125 and tissue polypeptide antigen. Oncology. 47:177–184. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaidyanathan A, Sawers L, Gannon AL,
Chakravarty P, Scott AL, Bray SE, Ferguson MJ and Smith G: ABCB1
(MDR1) induction defines a common resistance mechanism in
paclitaxel- and olaparib-resistant ovarian cancer cells. Br J
Cancer. 115:431–441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Food and Drug Administration, . FDA
approves olaparib tablets for maintenance treatment in ovarian
cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-tablets-maintenance-treatment-ovarian-cancerMay
15–2018
|
|
37
|
Food and Drug Administration, . FDA
approves rucaparib for maintenance treatment of recurrent ovarian,
fallopian tube, or primary peritoneal cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-rucaparib-maintenance-treatment-recurrent-ovarian-fallopian-tube-or-primary-peritonealMay
15–2018
|
|
38
|
Ison G, Howie LJ, Amiri-Kordestani L,
Zhang L, Tang S, Sridhara R, Pierre V, Charlab R, Ramamoorthy A,
Song P, et al: FDA approval summary: Niraparib for the maintenance
treatment of patients with recurrent ovarian cancer in response to
platinum-based chemotherapy. Clin Cancer Res. 24:4066–4071. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kamel D, Gray C, Walia JS and Kumar V:
PARP inhibitor drugs in the treatment of breast, ovarian, prostate
and pancreatic cancers: An update of clinical trials. Curr Drug
Targets. 19:21–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Polireddy K, Dong R, Reed G, Yu J, Chen P,
Williamson S, Violet PC, Pessetto Z, Godwin AK, Fan F, et al: High
dose parenteral ascorbate inhibited pancreatic cancer growth and
metastasis: Mechanisms and a phase I/IIa study. Sci Rep.
7:171882017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Panieri E and Santoro MM: ROS homeostasis
and metabolism: A dangerous liason in cancer cells. Cell Death Dis.
7:e22532016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Doskey CM, Buranasudja V, Wagner BA,
Wilkes JG, Du J, Cullen JJ and Buettner GR: Tumor cells have
decreased ability to metabolize H2O2:
Implications for pharmacological ascorbate in cancer therapy. Redox
Biol. 10:274–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shibata A: Regulation of repair pathway
choice at two-ended DNA double-strand breaks. Mutat Res.
803-805:51–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patel AG, Sarkaria JN and Kaufmann SH:
Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP)
inhibitor lethality in homologous recombination-deficient cells.
Proc Natl Acad Sci USA. 108:3406–3411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Do TV, Hirst J, Hyter S, Roby KF and
Godwin AK: Aurora A kinase regulates non-homologous end-joining and
poly(ADP-ribose) polymerase function in ovarian carcinoma cells.
Oncotarget. 8:50376–50392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott CL, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
patients with platinum-sensitive relapsed serous ovarian cancer: A
preplanned retrospective analysis of outcomes by BRCA status in a
randomised phase 2 trial. Lancet Oncol. 15:852–861. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Padayatty SJ, Sun AY, Chen Q, Espey MG,
Drisko J and Levine M: Vitamin C: Intravenous use by complementary
and alternative medicine practitioners and adverse effects. PLoS
One. 5:e114142010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ngo B, Van Riper JM, Cantley LC and Yun J:
Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev
Cancer. 19:271–282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
BRCA1 BRCA1 DNA repair associated [Homo
sapiens (human)]. Gene ID: 672. https://www.ncbi.nlm.nih.gov/gene/?term=NM_007294Updated.
November 25–2019.
|
|
50
|
BRCA2 BRCA2 DNA repair associated [Homo
sapiens (human)]. Gene ID: 675. https://www.ncbi.nlm.nih.gov/gene/?term=NM_000059Updated.
November 18–2019.
|