Introduction
Breast cancer (BC) is one of the most common types
of cancer in women, and occurs in the breast gland epithelium
(1,2). In recent years, BC has accounted for
~22.9% of worldwide cancer diagnoses in women, whilst China
accounted for 12.2% of all new diagnoses of BC and 9.6% of global
BC-associated mortalities (3,4). Despite
significant advances in drug sensitivity and surgical techniques,
metastasis still occurs frequently and represents the leading cause
of advanced BC-associated mortality (2). Recently, research investigating
potential molecular markers associated with the early diagnosis of
relapse and metastasis has gained traction. This may help to
identify possible therapeutic targets for BC.
MicroRNAs (miRNAs/miRs) have been demonstrated to
influence the progression of various cancer types and have
consequently become a research focus. It has been revealed that
miRNAs are associated with tumor development and target mRNAs,
resulting in regulation of cell aggressiveness, migration ability,
invasiveness and apoptosis (5,6).
Additionally, miR-320 has been demonstrated to be expressed
aberrantly in various cancer types. For example, Lieb et al
(7) revealed that miR-320 is
associated with the diagnosis and clinical features of prostate
cancer. miR-320 has also been reported to have an inhibitory effect
on gastric cancer progression and serves as a novel biomarker for
its diagnosis and prognosis (8).
Furthermore, miR-320 suppresses BC cell proliferation and
invasiveness (9); however, the
precise mechanism underpinning the role of miR-320 in BC has not
yet been determined.
E74 Like ETS Transcription Factor 3 (ELF3) is also
called ESE-1, and is typically associated with epithelial carcinoma
(10). It has been reported to serve
important roles in tumor progression and embryonic development
(11). Notably, in various cancer
types, ELF3 expression is dysregulated. For example, it is
upregulated in colorectal (12),
prostate (13) and lung cancer
(14), whereas it is downregulated
in oral squamous cell carcinoma (15) and ovarian cancer (16). Of note, the PI3K/AKT signaling
pathway is considered a canonical regulator of tumorigenesis, the
phosphorylation levels of PI3K and AKT may reflect the change of
PI3K/AKT signaling pathway (17).
Previous studies have revealed that ELF3 is a negative regulator of
epithelial-mesenchymal transition (EMT) in ovarian cancer cells
(16). In addition, miR-320
expression has been revealed to be downregulated in BC, but its
precise molecular mechanism remains to be elucidated.
In the present study, the role of miR-320 and the
exact mechanism underlying its role in modulating BC cell
proliferation, invasiveness and migration ability were
investigated. It was determined that miR-320 exerted an inhibitory
effect on cell progression, and that ELF3 is a specific target of
miR-320. Furthermore, it was revealed that miR-320 suppressed cell
progression by regulating the PI3K/AKT signaling pathway. This
indicated that the miR-320/PI3K/AKT signaling pathway may influence
BC cell progression and may provide a novel therapeutic target for
patients with BC.
Materials and methods
Sample collection and cell
culture
The present study was approved by the Ethics
Committees of Baoding First Central Hospital (approval no.
BD-2015-07-A0034). In total, 52 patients with breast cancer (all
female) were selected as subjects for this study, which was
conducted between January 2015 and December 2018 in Baoding First
Central Hospital (Baoding, China). The patients' tumor tissue and
adjacent normal tissues (≥5 cm from the tumor border) were removed
surgically and collected. Samples were verified as BC by an
experienced pathologist and fresh frozen in liquid N2
prior to use. The ages of the 52 BC patients ranged from 38–69
years, and 59 years was the median age. The entire study was
compliant with the Helsinki Declaration.
RPMI-1640 medium (HyClone; GE Healthcare Life
Sciences) containing 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.), 100 µg/ml streptomycin and 100 U/ml penicillin (Beyotime
Institute of Biotechnology) was used to maintain BC cell lines
(MCF-7, SK-BR-3, MDA-MB-231 and Hs578T) and normal human mammary
gland cells (Hs578Bst), which were purchased from American Type
Culture Collection. The cells were incubated at 37°C with 5%
CO2 in a humidified chamber.
Cell transfection
miR-320 mimic, miR-320 inhibitor, control mimic and
control inhibitor, ELF3 overexpressing pcDNA3.1-ELF3 plasmid (ELF3
vector) and their negative controls (control vector) were produced
by Shanghai GenePharma Co., Ltd. The sequences were: miR-320 mimic,
5′-AAAAGCUGGGUUGAGAGGGCGA-3′; miR-320 inhibitors,
5′-UCGCCCUCUCAACCCAGCUUUU-3′; control mimic,
5′-UCACAACCUCCUAGAAAGAGUAGA-3′; and control inhibitor,
5′-UCUACUCUUUCUAGGAGGUUGUGA-3′ (Shanghai GenePharma Co, Ltd). MCF-7
and SK-BR-3 cells (2.0×105) were inoculated into 6-well
plates and transfected using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) as per the recommended
protocols (100 nM of miRNAs per sample). After transfection for 48
h, the cells were collected for subsequent experiments. The reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) or
western blotting assays were used to evaluate transfection
efficiency.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from BC
cells (MCF-7 and SK-BR-3 cells) or tumor samples, following the
manufacturer's instructions. The first-strand complementary DNA was
synthesized using the ReverTraAce qPCR RT kit (Toyobo Life
Science), according to the manufacturer's protocol. qPCR was
performed using the 7500 FAST RT-PCR system (Thermo Fisher
Scientific, Inc.). The 2−ΔΔCq (18) method was used to calculate and
quantify the relative expression of miR-320 and ELF3 after
standardization to the endogenous controls U6 and GAPDH,
respectively. Reaction conditions are as follows: Pre-denaturation
at 95°C for 10 min, and 35 cycles of denaturation at 95°C for 10
sec, annealing at 60°C for 20 sec and extending at 72°C for 2 min.
The sequences of primers were as follows: miR-320 forward,
5′-ACACTCCAGCTGGGAAAAGCTGGGTTGAG-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′; ELF3 forward,
5′-CATGACCTACGAGAAGCTGAGC-3′ and reverse,
5′-GACTCTGGAGAACCTCTTCCTC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTCGT-3′; and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-ATGGTGGTGAAGACGCCAGT-3′.
Western blot analysis
RIPA lysis buffer with protease inhibitor (Beyotime
Institute of Biotechnology) was used to extract total protein from
MCF-7 and SK-BR-3 cells. Total protein concentration was calculated
using the bicinchoninic acid (BCA) Protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). A total of 50 µg/lane of protein
was loaded onto 8–15% polyacrylamide gels and then transferred to
nitrocellulose membranes. Non-specific binding was blocked using 5%
non-fat milk for 2 h at room temperature. The primary antibodies
were added to incubate the membrane at 4°C overnight, which was
followed by incubation with horseradish-peroxidase (HRP)-conjugated
secondary antibodies for 2 h at room temperature. Rabbit monoclonal
ELF3 antibody (1:500; cat. no. ab133621), rabbit polyclonal
E-cadherin antibody (1:500; cat. no. ab15148), rabbit monoclonal
N-cadherin antibody (1:500; cat. no. ab92547), rabbit monoclonal
vimentin antibody (1:500; cat. no. ab202030), rabbit monoclonal
PI3K antibody (1:500; cat. no. ab32089), rabbit polyclonal
phosphorylated (p)-PI3K antibody (1:500; cat. no. ab182651), rabbit
polyclonal Akt antibody (1:500; cat. no. ab8805), rabbit monoclonal
p-Akt antibody (1:500; cat. no. ab81283), rabbit polyclonal GAPDH
antibody (1:500; cat. no. ab37168) and secondary goat anti-rabbit
(HRP) IgG antibody (1:2,000; cat. no. ab6721) were all purchased
from Abcam. An enhanced chemiluminescence kit (EMD Millipore) was
used to detect the signals. The target protein:GAPDH expression
level ratio was used to evaluate the relative target protein
expression.
Transwell assay
Cell migration ability and invasiveness were
measured using Transwell assays. In cell migration analysis, MCF-7
and SK-BR-3 cells (1×106) were seeded into the upper
chamber (8-µm pore size inserts). The RPMI-1640 medium in the upper
chamber was serum-free, and the medium in the lower chamber
contained 10% FBS which served as a chemoattractant. After
incubation for 24 h at 37°C, PBS was used to wash the inserts and a
cotton swab was used to remove non-migrated cells. The migrated
cells in the lower chamber were fixed using 100% methanol for 5 min
at 4°C, stained with 0.05% crystal violet for 10 min at 4°C and
then imaged using a light microscope (magnification, ×200; Olympus
Corporation). The process of determining cell invasiveness was
identical to cell migration analysis, except that the Transwell
membranes were pre-coated with 1 mg/ml Matrigel at 4°C.
MTT assay
The MTT assay (Sigma-Aldrich; Merck KGaA) was used
to measure the viability of MCF-7 and SK-BR-3 cells. The cells were
seeded into 96-well plates at a density of 5×103
cells/well and then cultured in an incubator at 37°C with 5%
CO2. MTT (20 µl) was added into each well to measure
cell viability and incubated for another 4 h at 37°C. After
incubation, the medium was removed and 150 µl DMSO was added into
the wells to dissolve the formazan crystals at 37°C. The cell
viability was calculated by measuring the optical density at 490
nm.
Xenograft experiment
A xenograft tumor formation assay was used for the
tumor growth assay in vivo. A total of 16 female nude mice
(3–5 weeks old, 18–22 g) were purchased from the Shanghai SLAC
Laboratory Animal Co., Ltd. All animal experiments were approved by
the Ethics Committee of Baoding First Central Hospital. During the
course of experiments, the mice were kept in pathogen-free animal
facilities with controlled temperature and humidity, under a 12 h
light/dark cycle, and with free access to food and water. The nude
mice were randomly divided into two groups. MCF-7 cells
(1×106) transfected with miR-320 mimic or miR-negative
control were added into the RPMI-1640 medium (100 µl, HyClone; GE
Healthcare Life Sciences). Subsequently, 5×105 MCF-7
cells were injected into the right flank of nude mice
subcutaneously. A vernier caliper was used to measure the xenograft
tumor size every 4 days, and the following formula was applied to
calculate the tumor volume: 0.5 × tumor width × tumor length. At 28
days after inoculation, the mice were sacrificed and the tumors
were used for further analysis.
Immunohistochemistry assay
Firstly, paraffin sections (6-µm thick) were fixed
using 4% paraformaldehyde for 30 min at 37°C and then incubated
with 3% H2O2 in PBS for 15 min at 37°C.
Following blocking with 5% goat serum at temperature for 2 h at
37°C, the primary antibody anti-ELF3 (1:1,000, cat. no. ab133621,
Abcam) was added and incubated for 48 h at 4°C. Subsequently, the
sections were incubated with biotinylated goat anti-rabbit IgG
(1:500; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h
at 37°C. The sections were stained using diaminobenzidine mixture
(Beijing Solarbio Science & Technology Co., Ltd.) for 30 min at
37°C and then dehydrated using a graded alcohol series, cleared
using xylene and coverslipped using neutral balsam. Finally, the
protein density per section was determined using Image Pros Plus
5.0 software (Media Cybernetics). Images were captured with a
fluorescence microscope (IX71, Olympus Corporation).
Dual luciferase reporter assay
Bioinformatics tools (TargetScanHuman 7.1,
http://www.targetscan.org/vert_71/)
were used to predict the miR-320 binding sites of ELF3. The
recombinant pMIR-reporter luciferase vector was applied for
luciferase assays. The wild or mutant type of ELF3-3′ untranslated
region (3′UTR; containing miR-320 binding sites) were synthesized
and then cloned into the pMIR-reporter luciferase vector (Promega
Corporation). MCF-7 cells were co-transfected with miR-320 mimic
and vector using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). MCF-7 cells were seeded into 48-well
plates at a density of 5×104 cells per well. After
transfection for 48 h at 37°C, the Dual Luciferase Reporter assay
system (Promega Corporation) was used to measure the luciferase
activity. The relative luciferase activity was normalized to
Renilla luciferase activity 48 h after transfection.
Statistical analysis
Data are presented as the mean ± SD and all
experiments were repeated in triplicate. The difference between two
groups was compared using the unpaired two-tailed Student's t-test,
and one-way ANOVA followed by Bonferroni's post hoc analysis was
used to measure the significance of comparisons among more than two
groups. Pearson's correlation analysis was used to assess the
correlation between miR-320 and ELF3 expression. The overall
survival was calculated using Kaplan-Meier survival analysis and
log-rank tests. SPSS v19.0 software (IBM Corp.) was used to perform
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
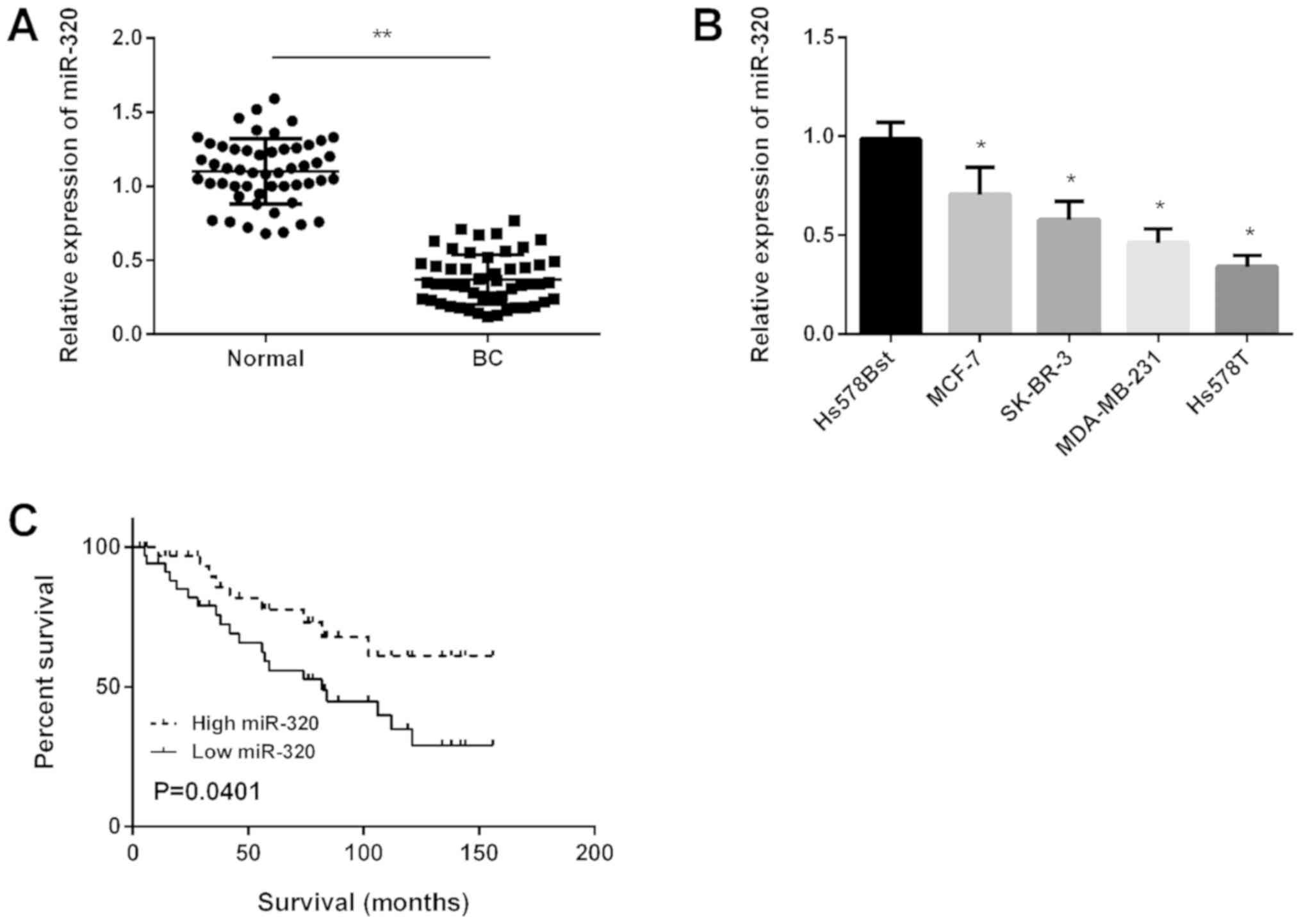
miR-320 is downregulated in BC and
associated with a poor prognosis in patients with BC
Primarily, miR-320 expression in BC tissues was
detected using RT-qPCR and the results revealed that miR-320 was
significantly downregulated in BC tissues compared with in normal
tissues (Fig. 1A). Subsequently,
miR-320 expression in four BC cell lines was determined and it was
demonstrated that miR-320 expression was downregulated
significantly in all BC cell lines compared with in normal cells
(Fig. 1B). Kaplan-Meier analysis
revealed that the survival time of patients with high miR-320
expression was longer compared with patients in the low miR-320
expression group (Fig. 1C).
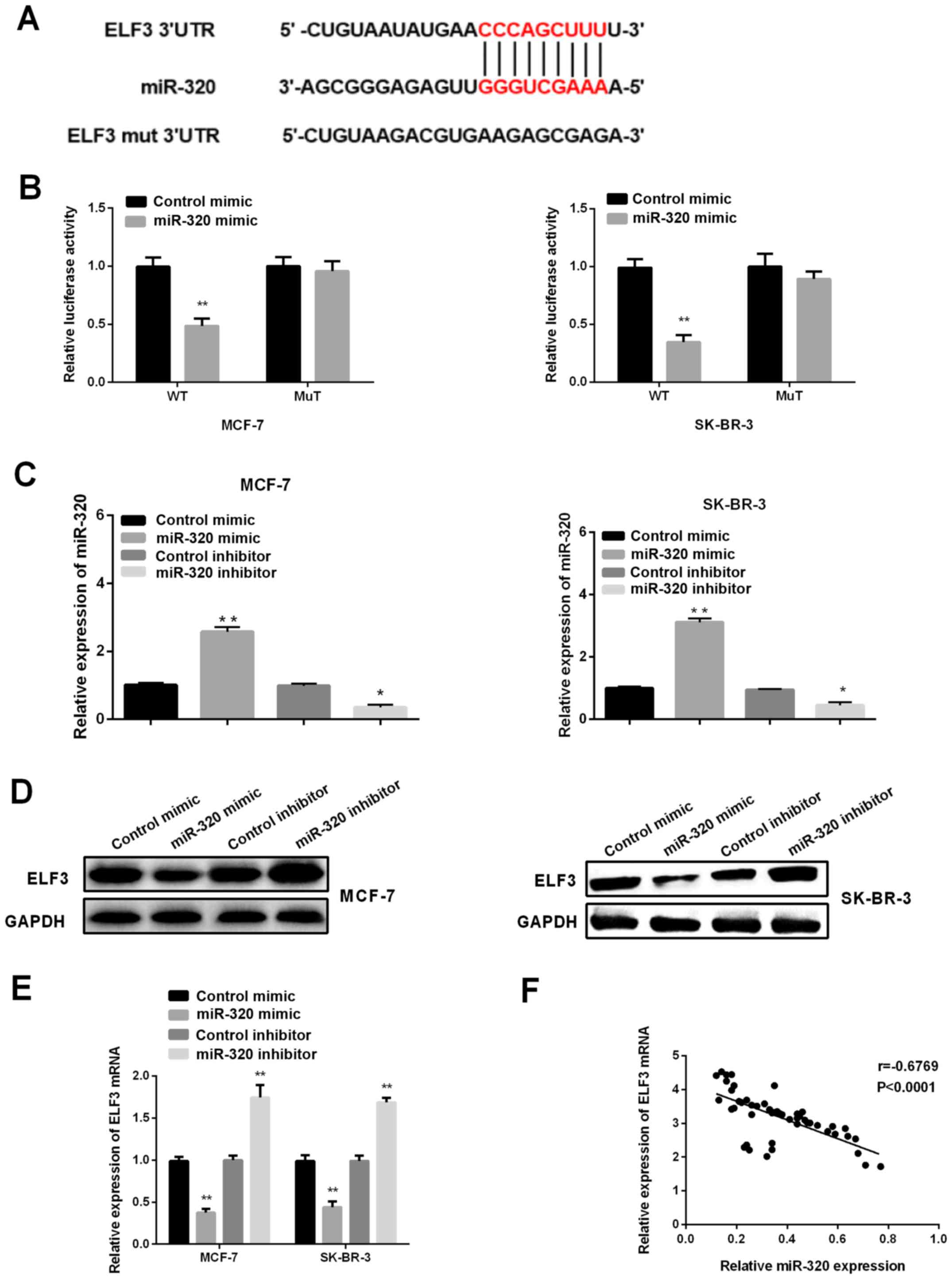
ELF3 is a target of miR-320 in BC
cells
To investigate the mechanism underlying the role of
miR-320 in the progression of BC, TargetScanHuman 7.1 was first
applied to predict the potential targets of miR-320, and ELF3 was
identified as one of the candidate targets (Fig. 2A). Subsequently, a dual-luciferase
reporter assay was performed to further explore whether ELF3
represented a direct target of miR-320 in BC cells. It was revealed
that the luciferase activity of ELF3-3′UTR-wild type was
significantly decreased in MCF-7 and SK-BR-3 cells after
transfection with a miR-320 mimic and pMIR-reporter luciferase
vector, whereas there was no significant change in the mutant-type
group (Fig. 2B), suggesting that
ELF3 was a direct target of miR-320. Therefore, to confirm and
further investigate the effects of miR-320 on ELF3 expression, a
miR-320 mimic, miR-320 inhibitor, control mimic and control
inhibitor were transfected to artificially regulate the expression
levels of miR-320 in MCF-7 and SK-BR-3 cell lines. As shown in
Fig. 2C, the introduction of miR-320
mimic could significantly upregulate the expression level of
miR-320, while the miR-320 inhibitor could inhibit the expression
of miR-320. Next, western blotting and RT-qPCR were carried out to
investigate the effects of miR-320 on ELF3 expression. It was
demonstrated that ELF3 expression was suppressed by a miR-320
mimic, and elevated by miR-320 inhibitor in both BC cell lines
(Fig. 2D and E). Pearson correlation
analysis revealed a negative correlation between ELF3 and miR-320
expression in the 52 tumor samples (Fig.
2E; R=−0.6769; P<0.0001), indicating that miR-320 may
regulate ELF3 expression negatively by binding to the 3′UTR of
ELF3.
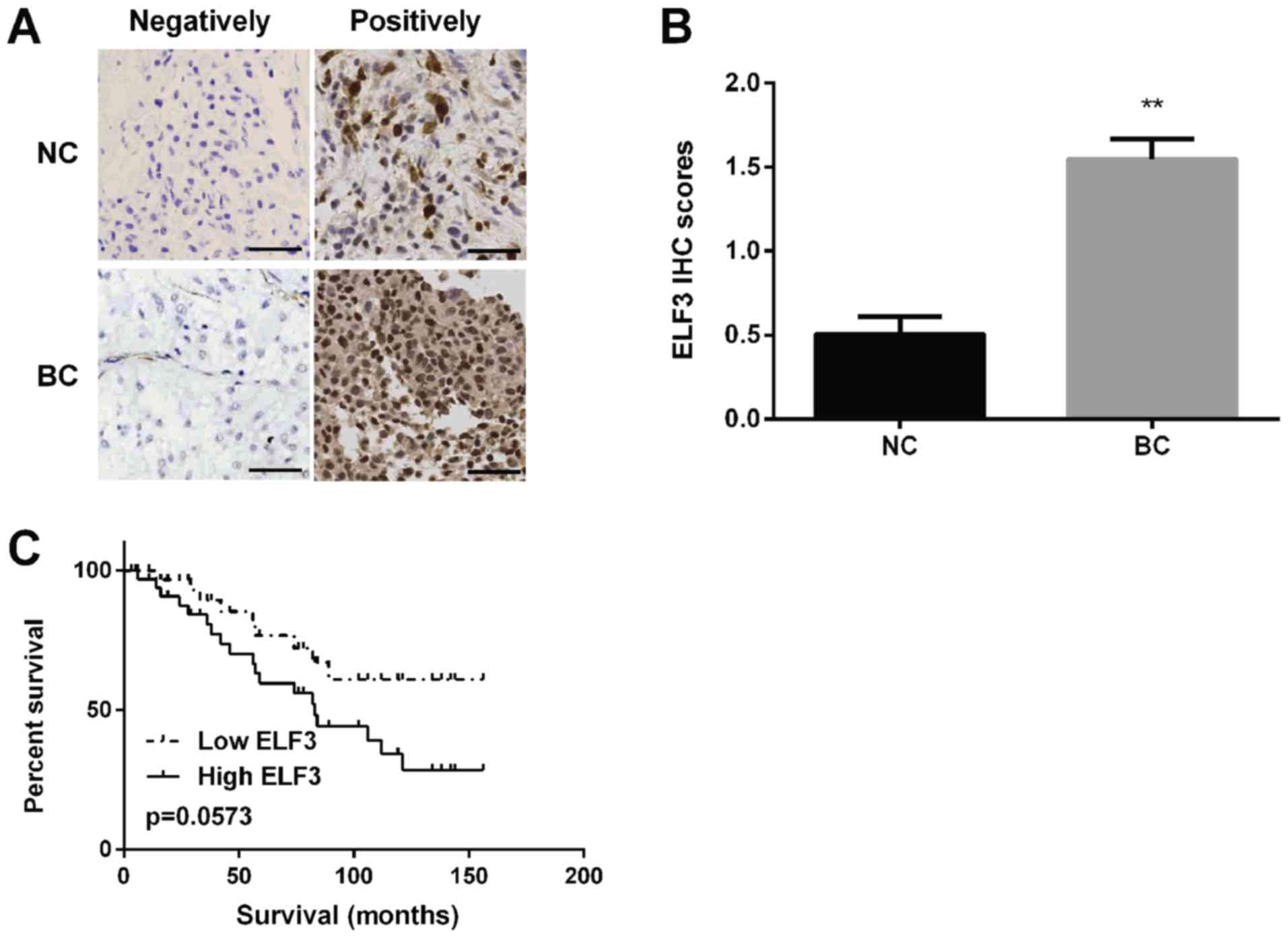
ELF3 is upregulated in BC tissues and
associated with a poor prognosis in patients with BC
ELF3 expression was detected in BC tissues using
immunohistochemistry and it was discovered that ELF3 protein was
localized in the cytoplasm of BC tissues (Fig. 3A) and the staining intensity of ELF3
was higher in BC tissues compared with in normal tissues (Fig. 3B). Kaplan-Meier analysis revealed
that the survival rate of patients with BC in the low-ELF3
expression group was markedly higher compared with that of patients
in the high-ELF3 expression group (Fig.
3C).
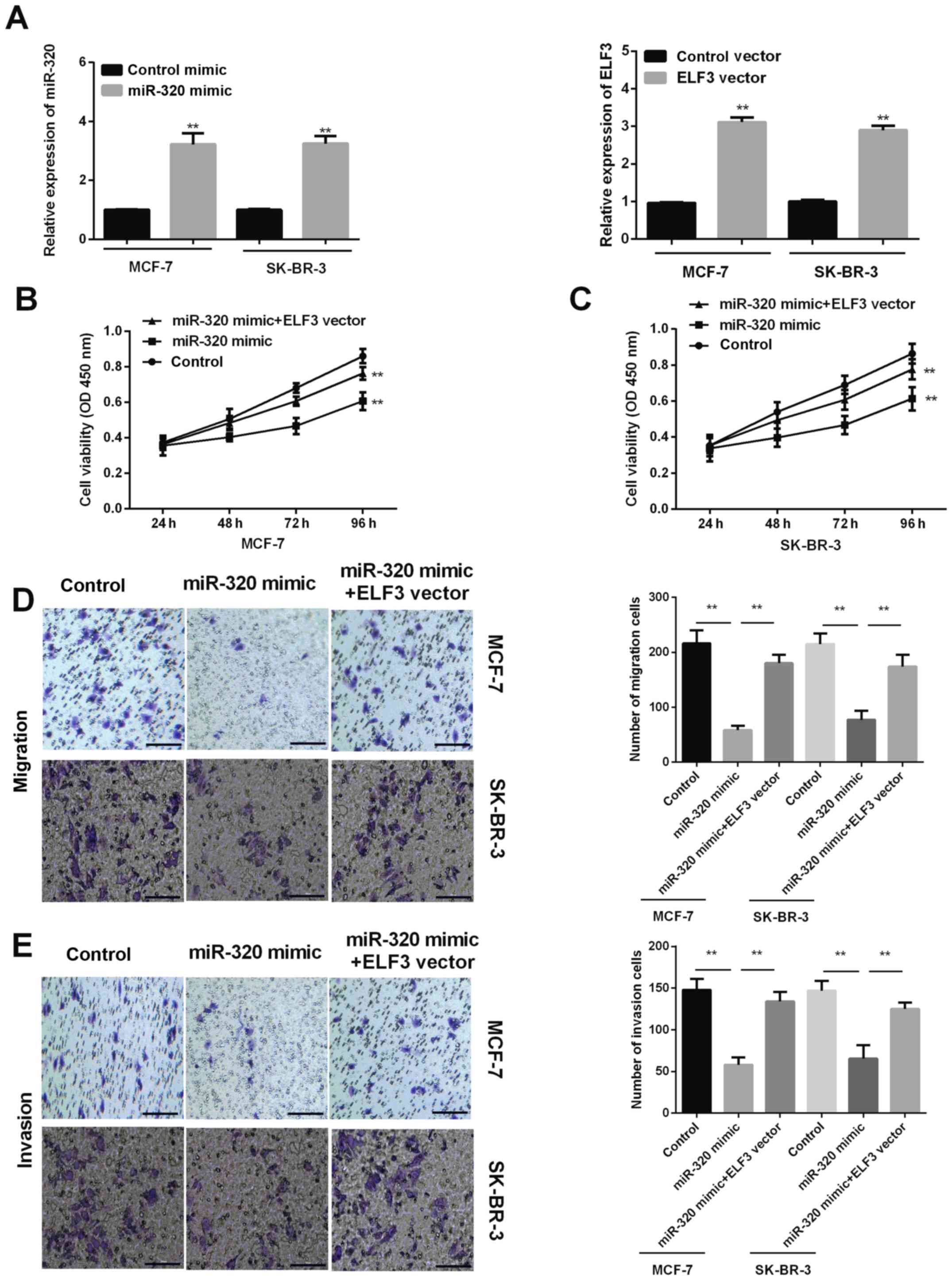
ELF3 rescues the inhibitory effect of
miR-320 on BC cell proliferation, migration ability and
invasiveness
miR-320 mimic was successfully transfected into
MCF-7 and SK-BR-3 cells to increase miR-320 expression levels, and
the ELF3 vector was successfully transfected into MCF-7 and SK-BR-3
cells to increase the ELF3 expression level (Fig. 4A). MTT results determined that
promoting miR-320 expression by transfection with a miR-320 mimic
inhibited the proliferation of MCF-7 and SK-BR-3 cells, and that
ELF3 overexpression partially reversed this effect (Fig. 4B and C). Transwell migration assays
revealed that overexpression of miR-320 suppressed the migration
ability of MCF-7 and SK-BR-3 cells, while ELF3 overexpression
partially rescued this miR-320-mediated suppressive effect
(Fig. 4D). The invasion assay
revealed similar results; ELF3 expression was associated with
promotion of the invasiveness of BC cells reduced by miR-320
(Fig. 4E). Taken together, the
current results indicated that miR-320 inhibits BC cell
proliferation, migration and invasiveness, and that ELF3 expression
partially rescues the aforementioned inhibitory effects.
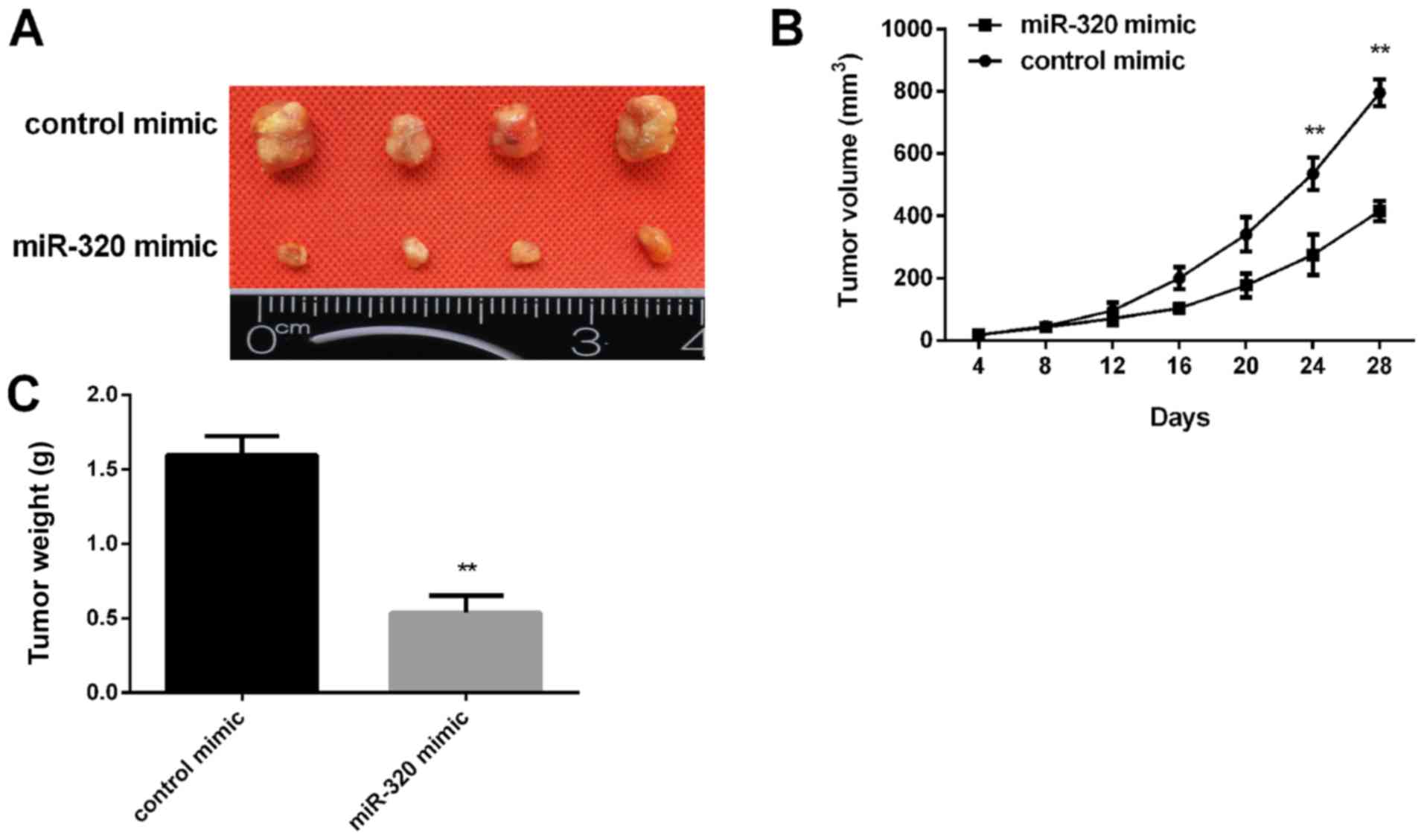
miR-320 inhibits tumor growth in nude
mice
Subsequently, the effect of miR-320 expression on
tumor growth was examined in vivo. MCF-7 cells transfected
with a miR-320 mimic were injected into nude mice subcutaneously.
The tumor volume was measured every 4 days. After 28 days, the mice
were sacrificed, and the xenograft tumors were collected for
further analysis. It was revealed that miR-320 overexpression
inhibited the xenograft tumor growth (Fig. 5A) and the tumor cell xenografts
exhibited a slower growth rate in the miR-320 mimic group compared
with in the control group (Fig. 5B).
In addition, the tumor weight in the miR-320 mimic group was
significantly lower than in the control group (Fig. 5C).
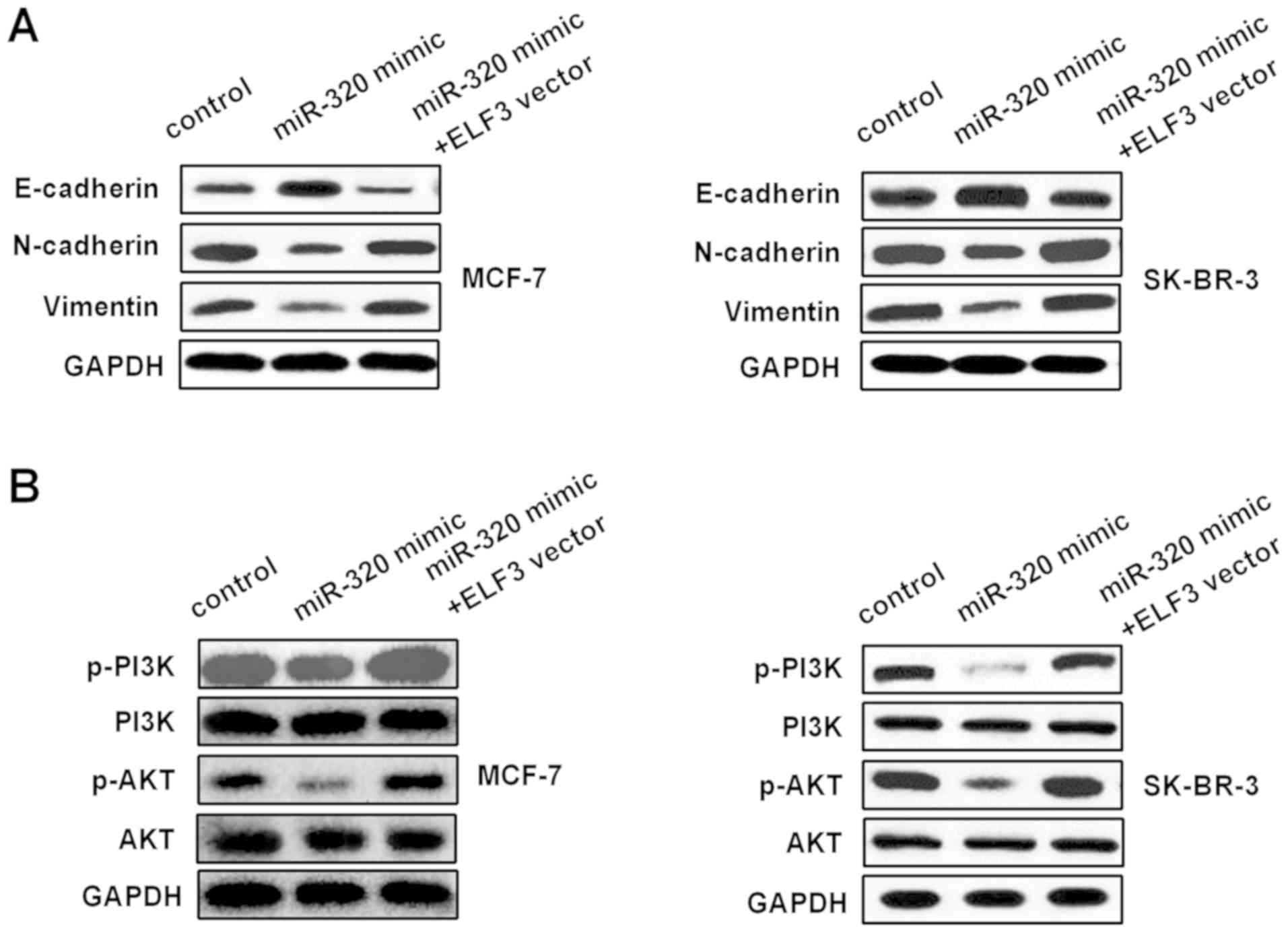
miR-320/ELF3 axis modulates
epithelial-mesenchymal transition (EMT) and PI3K/AKT signaling
pathways in BC cells
The influence of the miR-320/ELF3 axis on the EMT
and PI3K/AKT signaling pathways was examined in BC cells. Western
blotting was used to detect E-cadherin, N-cadherin, vimentin, PI3K,
p-PI3K, AKT and p-AKT protein levels in MCF-7 and SK-BR-3 cells,
following transfection with miR-320 mimic or miR-320 mimic
co-transfected with an ELF3 vector. As Fig. 6A indicates, the miR-320 mimic
increased E-cadherin expression, while it decreased N-cadherin and
vimentin expression. Furthermore, ELF3 partially reversed the
miR-320-mediated effect on EMT-associated markers. In addition,
p-PI3K and p-AKT protein levels in the miR-320 mimic group were
decreased compared with those in the control group, and ELF3
partially rescued the miR-320-mediated inhibitory effect on the
PI3K/AKT signaling pathway (Fig.
6B), suggesting that miR-320 regulated the EMT and PI3K/AKT
signaling pathway by targeting ELF3 in BC.
Discussion
miRNA dysregulation is closely associated with a
variety of cancer types, and previous studies have revealed that
miRNAs may be associated with tumor progression and prognosis
(19–21). Comprehending the molecular mechanism
underlying the influence of miRNAs in the progression of cancer may
help identify novel treatment strategies. In the present study, it
was discovered that miR-320 expression was downregulated in BC
tissues and cells. Overexpression of miR-320 inhibited BC cell
progression in vitro and tumor growth in vivo.
Furthermore, ELF3 was identified as the target of miR-320, and
miR-320/ELF3 was demonstrated to regulate the PI3K/AKT signaling
pathway.
It has been demonstrated in large-scale studies that
miRNAs influence BC progression by serving as oncogenes or tumor
suppressors. For example, miR-590 has been revealed to suppress
cell proliferation of BC via targeting of activating transcription
factor 3 (22). Yan et al
(23) reported that miR-125
suppresses BC progression by inhibiting BRCA1 associated protein 1.
Inversely, miR-19b serves as an oncogene in BC and enhances cell
proliferation (24), and
overexpression of miR-181a has been revealed to promote BC cell
migration (25). Regarding studies
of the role of miR-320 in BC, Luo et al (26) reported that increased miR-320
expression suppresses BC cell proliferation, invasiveness and
migration via regulation of aquaporin 1 (AQP1). Furthermore,
miR-320 inhibits BC cell progression via upregulation of SRY-box
transcription factor 4 (9). These
previous studies were consistent with the present study which
reported that miR-320 expression was downregulated in BC tissues
and cells, and miR-320 inhibited BC cell progression. Furthermore,
to the best of our knowledge, it was reported for the first time
that ELF3 represents a direct target of miR-320 in modulating BC
progression.
Previously, it has been revealed that different
miRNAs may regulate a common target gene or one miRNA may regulate
multiple target genes in tumors (27). Numerous genes have been identified as
targets of miR-320 in a wide variety of cancer types. For instance,
AQP1 serves as a target of miR-320 to regulate BC progression
(26). Forkhead box protein M1
influences the progression of glioma and is regulated by miR-320
(28). Notably, several studies have
demonstrated that ELF3 is a target gene of certain miRNAs in other
cancer types, and serves as an oncogene. Zhao et al
(29) reported that ELF3 is
upregulated in lung cancer and serves as the target of miR-320a in
regulating lung cancer progression. Furthermore, ELF3 promotes EMT
in hepatocellular carcinoma and has been identified as a target of
miR-141 (30). In the present study,
the results indicated that ELF3 was a target of miR-320 in BC.
Upregulation of miR-320 inhibited ELF3 expression and there was a
negative correlation between miR-320 and ELF3 expression. ELF3 was
downregulated in BC and associated with the prognosis of patients
with BC.
The PI3K/AKT signaling pathway has been demonstrated
to serve important roles in various pathophysiologic processes,
including tumor growth, apoptosis, migration and invasiveness, as
well as in EMT (31,32). Certainly, the PI3K/AKT signaling
pathway serves a central role in the progression of BC (33). It has previously been reported that
the activation of ELF3 may inhibit the PI3K/AKT signaling pathway
in lung cancer regulated by miR-320 (14,29). The
present study revealed that overexpression of miR-320 in MCF-7 and
SK-BR-3 cells inhibited the PI3K/AKT signaling pathway, and ELF3
may reverse the inhibitory effect of miR-320 on the PI3K/AKT
signaling pathway. The combined use of PI3K/AKT inhibitors may help
to further elucidate the PI3K/AKT signaling pathway. The limitation
of the present study was only exploring whether the PI3K/AKT
signaling pathway influenced miR-320-regulated progression.
In conclusion, it was demonstrated that miR-320
targeted ELF3 to suppress BC cell progression via regulation of
PI3K/AKT signaling. miR-320 may, therefore, represent a novel
cellular therapeutic target for the treatment of patients with
BC.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
2018 Hebei Provincial Key Research and Development Program Health
Care and Biomedicine Special Project (grant no. 18277732D).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZZ and JZ designed the study and performed the
experiments. ZZ, JL and HG analyzed and interpreted the data. JZ
and BZho, BZha and HC analyzed the data. ZZ and JZ prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committees of Baoding First Central Hospital and all patients
provided written informed consent.
Patient consent for publication
Patients or their legal guardians provided written
informed consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hong W and Dong E: The past, present and
future of breast cancer research in China. Cancer Lett. 351:1–5.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ren L, Li Y, Zhao Q, Fan L, Tan B, Zang A
and Yang H: miR-519 regulates the proliferation of breast cancer
cells via targeting human antigen R. Oncol Lett. 19:1567–1576.
2020.PubMed/NCBI
|
|
3
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuo TT, Zheng RS, Zeng HM, Zhang SW and
Chen WQ: Female breast cancer incidence and mortality in China,
2013. Thorac Cancer. 8:214–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dinami R, Buemi V, Sestito R, Zappone A,
Ciani Y, Mano M, Petti E, Sacconi A, Blandino G, Giacca M, et al:
Epigenetic silencing of miR-296 and miR-512 ensures hTERT dependent
apoptosis protection and telomere maintenance in basal-type breast
cancer cells. Oncotarget. 8:95674–95691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao J and Jiang GQ: miR-4282 inhibits
proliferation, invasion and metastasis of human breast cancer by
targeting Myc. Eur Rev Med Pharmacol Sci. 22:8763–8771.
2018.PubMed/NCBI
|
|
7
|
Lieb V, Weigelt K, Scheinost L, Fischer K,
Greither T, Marcou M, Theil G, Klocker H, Holzhausen HJ, Lai X, et
al: Serum levels of miR-320 family members are associated with
clinical parameters and diagnosis in prostate cancer patients.
Oncotarget. 9:10402–10416. 2017.PubMed/NCBI
|
|
8
|
Zhang H and Lu W: LncRNA SNHG12 regulates
gastric cancer progression by acting as a molecular sponge of
miR-320. Mol Med Rep. 17:2743–2749. 2018.PubMed/NCBI
|
|
9
|
Bai JW, Wang X, Zhang YF, Yao GD and Liu
H: MicroRNA-320 inhibits cell proliferation and invasion in breast
cancer cells by targeting SOX4. Oncol Lett. 14:7145–7152.
2017.PubMed/NCBI
|
|
10
|
Oettgen P, Alani RM, Barcinski MA, Brown
L, Akbarali Y, Boltax J, Kunsch C, Munger K and Libermann TA:
Isolation and characterization of a novel epithelium-specific
transcription factor, ESE-1, a member of the ets family. Mol Cell
Biol. 17:4419–4433. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neve RM, Parmar H, Amend C, Chen C,
Rizzino A and Benz CC: Identification of an epithelial-specific
enhancer regulating ESX expression. Gene. 367:118–125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang JL, Chen ZF, Chen HM, Wang MY, Kong
X, Wang YC, Sun TT, Hong J, Zou W, Xu J and Fang JY: Elf3 drives
β-catenin transactivation and associates with poor prognosis in
colorectal cancer. Cell Death Dis. 5:e12632014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Longoni N, Sarti M, Albino D, Civenni G,
Malek A, Ortelli E, Pinton S, Mello-Grand M, Ostano P, D'Ambrosio
G, et al: ETS transcription factor ESE1/ELF3 orchestrates a
positive feedback loop that constitutively activates NF-κB and
drives prostate cancer progression. Cancer Res. 73:4533–4547. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Yu Z, Huo S, Chen Z, Ou Z, Mai J,
Ding S and Zhang J: Overexpression of ELF3 facilitates cell growth
and metastasis through PI3K/Akt and ERK signaling pathways in
non-small cell lung cancer. Int J Biochem Cell Biol. 94:98–106.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwai S, Amekawa S, Yomogida K, Sumi T,
Nakazawa M, Yura Y, Nishimune Y and Nozaki M: ESE-1 inhibits the
invasion of oral squamous cell carcinoma in conjunction with MMP-9
suppression. Oral Dis. 14:144–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeung TL, Leung CS, Wong KK,
Gutierrez-Hartmann A, Kwong J, Gershenson DM and Mok SC: ELF3 is a
negative regulator of epithelial-mesenchymal transition in ovarian
cancer cells. Oncotarget. 8:16951–16963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ke K and Lou T: MicroRNA-10a suppresses
breast cancer progression via PI3K/Akt/mTOR pathway. Oncol Lett.
14:5994–6000. 2017.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ke SB, Qiu H, Chen JM, Shi W and Chen YS:
MicroRNA-202-5p functions as a tumor suppressor in colorectal
carcinoma by directly targeting SMARCC1. Gene. 676:329–335. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quan H, Li B and Yang J: MicroRNA-504
functions as a tumor suppressor in hepatocellular carcinoma through
inhibiting Frizzled-7-mediated-Wnt/β-catenin signaling. Biomed
Pharmacother. 107:754–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duan S, Dong X, Hai J, Jiang J, Wang W,
Yang J, Zhang W and Chen C: MicroRNA-135a-3p is downregulated and
serves as a tumour suppressor in ovarian cancer by targeting CCR2.
Biomed Pharmacother. 107:712–720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rohini M, Gokulnath M, Miranda PJ and
Selvamurugan N: miR-590-3p inhibits proliferation and promotes
apoptosis by targeting activating transcription factor 3 in human
breast cancer cells. Biochimie. 154:10–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan L, Yu MC, Gao GL, Liang HW, Zhou XY,
Zhu ZT, Zhang CY, Wang YB and Chen X: miR-125a-5p functions as a
tumour suppressor in breast cancer by downregulating BAP1. J Cell
Biochem. 119:8773–8783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Zhang J, Ma Z, Zhang F and Yu W:
miR-19b serves as a prognostic biomarker of breast cancer and
promotes tumor progression through PI3K/AKT signaling pathway. Onco
Targets Ther. 11:4087–4095. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taylor MA, Sossey-Alaoui K, Thompson CL,
Danielpour D and Schiemann WP: TGF-β upregulates miR-181a
expression to promote breast cancer metastasis. J Clin Invest.
123:150–163. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo L, Yang R, Zhao S, Chen Y, Hong S,
Wang K, Wang T, Cheng J, Zhang T and Chen D: Decreased miR-320
expression is associated with breast cancer progression, cell
migration, and invasiveness via targeting Aquaporin 1. Acta Biochim
Biophys Sin (Shanghai). 50:473–480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palumbo T, Faucz FR, Azevedo M, Xekouki P,
Iliopoulos D and Stratakis CA: Functional screen analysis reveals
miR-26b and miR-128 as central regulators of pituitary
somatomammotrophic tumor growth through activation of the PTEN-AKT
pathway. Oncogene. 32:1651–1659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li T, Ma J, Han X, Jia Y, Yuan H, Shui S
and Guo D: MicroRNA-320 enhances radiosensitivity of glioma through
down-regulation of sirtuin type 1 by directly targeting forkhead
box protein M1. Transl Oncol. 11:205–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao W, Sun Q, Yu Z, Mao S, Jin Y, Li J,
Jiang Z, Zhang Y, Chen M, Chen P, et al: miR-320a-3p/ELF3 axis
regulates cell metastasis and invasion in non-small cell lung
cancer via PI3K/Akt pathway. Gene. 670:31–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng L, Xu M, Xu J, Wu K, Fang Q, Liang
Y, Zhou S, Cen D, Ji L, Han W and Cai X: ELF3 promotes
epithelial-mesenchymal transition by protecting ZEB1 from
miR-141-3p-mediated silencing in hepatocellular carcinoma. Cell
Death Dis. 9:3872018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang J, Ding M, Yang L, Liu LZ and Jiang
BH: PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis.
Cell Signal. 19:2487–2497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiu N, He YF, Zhang SM, Zhan YT, Han GD,
Jiang M, He WX, Zhou J, Liang HL, Ao X, et al: Cullin7 enhances
resistance to trastuzumab therapy in Her2 positive breast cancer
via degrading IRS-1 and downregulating IGFBP-3 to activate the
PI3K/AKT pathway. Cancer Lett. 464:25–36. 2019. View Article : Google Scholar : PubMed/NCBI
|