Introduction
According to the global cancer statistics in 2011,
breast cancer is the most commonly diagnosed carcinoma and the
second leading cause of cancer-associated death among women in
China (1). Clinically, all invasive
primary breast cancer cases are analyzed for the expression levels
of estrogen receptor (ER), progesterone receptor (PR) and human
epidermal growth factor receptor 2 (HER2) (2). Correct measurement of these receptors
is essential for accurate therapeutic decision-making (3–5).
Immunohistochemistry (IHC) is recommended for the
detection of ER and PR (6). There
are two commonly used methods for evaluating HER2 status, including
IHC to determine the expression levels of the HER2 protein and
fluorescence in situ hybridization (FISH) for the detection
of HER2 gene amplification (7).
Several studies have considered pre-analytic factors, for example
the effect of cold ischemic time, on the level of biomarkers in
archival breast cancer tissue (2,8–10). However, few studies have considered
the association between the age of the paraffin block and the
levels of ER, PR and HER2.
Given the differences in the testing methods and
reagents, problem can arise between local and centralized HER2
testing in laboratories. It has been reported that HER2 testing is
more accurate when performed at high-volume central laboratories
and results can be quite different between local community-based
laboratories and central laboratories (11). In routine practice, breast cancer
cases are often presented to the Department of Pathology, The First
Affiliated Hospital of Zhejiang University (Hangzhou, China) for
consultation, of which some cases were diagnosed >5 years ago.
Since there is variability among central and local laboratories,
repeated tests are often required to determine the ER, PR and HER2
levels. Importantly, it is not clear whether biomarkers are altered
in cancer tissues that have been stored for long periods of
time.
To address the association between the age of
paraffin blocks and the expression levels of ER, PR and HER2, the
present study compared ER and PR levels between repeated tests and
the original tests. Since the original fluorescence in situ
hybridization (FISH) tests often lacked signal intensity, HER2 and
chromosome enumeration probe 17 (CEP17) were assessed for different
age groups.
Materials and methods
Specimen collection
A total of 100 patients (median age, 56.7 years; age
range, 31–85 years) were recruited between January 2007 and
December 2017. The criteria for the recruitment of samples were as
follows: i) Patients did not receive neoadjuvant chemotherapy; ii)
samples were processed on working days, but not on weekends or
holidays to ensure procedural consistency; and iii) cases had been
tested for the expression levels of ER and PR using IHC, and HER2
gene amplification using FISH. Tissue blocks were collected
according to the following 5 age groups: 1 year ago, defined as new
paraffin blocks (12); 3 years ago;
5 years ago; 7 years ago; and 10 years ago. In each group, 10
mastectomy cases and 10 core needle biopsy cases were selected. Due
to limited tissues, a total of 18 cases were not assessed using IHC
or FISH. The final number of samples included in the study is shown
in (Table I). All the samples were
fixed in 10% neutral-buffered formalin (http://nbtssw.com) at room temperature for 6–24 h. The
present study was approved by The Ethics Committee of the First
Affiliated Hospital, College of Medicine, Zhejiang University
(Hangzhou, China). The Committee waived the need for informed
consent from the patients because the study was completed
anonymously.
 | Table I.Number of breast cancer samples tested
using IHC and FISH in each age group. |
Table I.
Number of breast cancer samples tested
using IHC and FISH in each age group.
|
| M, n | CNB, n |
|---|
|
|
|
|
|---|
| Age of paraffin
blocks, years | IHC | FISH | IHC | FISH |
|---|
| 10 | 7 | 7 | 6 | 4 |
| 7 | 9 | 9 | 8 | 8 |
| 5 | 10 | 7 | 10 | 9 |
| 3 | 10 | 10 | 10 | 9 |
| 1 | 10 | 9 | 10 | 10 |
IHC
Sections were cut at 4 µm, placed on positively
charged slides and dried overnight at 65°C. The slides were
deparaffinized in xylene at room temperature (RT) and dehydrated in
75, 85 and 100% alcohol. Endogenous peroxidase activity was
inhibited by incubating the slides in 3% H2O2
for 10 min at RT. Nonspecific binding sites were blocked with 10%
normal goat serum (Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd.) at 37°C for 10 min. Sections were then incubated with anti-ER
(1:200; cat. no. ab16660; Clone SP1; Abcam) and anti-PR (1:150;
cat. no. M356929; Dako; Agilent Technologies, Inc.) in humid
chambers for 1 h at 37°C. The sections were rinsed three times with
PBS and then incubated with a secondary antibody, Dako Real
Envision /HRP, Rabbit/Mouse (ready-to-use; cat. no. K5007; Dako;
Agilent Technologies, Inc.) for 30 min at RT. DAB (Dako; Agilent
Technologies, Inc) was applied for ~2 min at RT and removed by
rinsing with distilled water. Slides were counterstained with
hematoxylin for 30 sec at RT.
Staining of ER and PR was assessed
semi-quantitatively by using Q-scoring, which incorporates
intensity and distribution of reactivity (8,13).
Intensity was scored as follows: 0, negative (no staining of any
nuclei at high magnification); 1, weak (staining visible only at
high magnification); 2, moderate (staining readily visible at low
magnification); or 3, strong (clear positive staining at low
magnification). The proportion of stained cells was scored as
follows: 0, 0%; +1, 1–25%; +2, 26–50%; +3, 51–75%; or +4, >75%.
Intensity and proportion of stained cells were added for the Q
score, which ranged from 0 to 7 (8).
FISH
Sections (4 µm) were cut and incubated overnight at
65°C. The slides were deparaffinized in xylene at RT, dehydrated in
75, 85 and 100% alcohol for 5 min each at room temperature and
subsequently immersed in distilled water at 90°C for 30 min. The
slides were then incubated for 10 min in 1ug/ml of protease
solution (http://www.gpmedical.com.cn/index.aspx) at 37°C. The
slides were briefly washed in sodium saline citrate (pH 7.2) for 5
min and dehydrated in 70, 85 and 100% ethanol at RT. Subsequently,
the dual color HER2/CEP17 probe (10 µl; ZytoVision GmbH) was
applied onto each slide, a cover slip was placed and sealed with
rubber cement, and then the slides were transferred to a
hybridization oven (S500-24; Abbott Laboratories). The procedure
was as follows: Denaturation at 75°C for 10 min with hybridization
overnight at 37°C. After that, the slides were washed three times
for 5 min in 37°C wash buffer (ZytoVision GmbH) and rinsed in 70%
ethanol. After air-drying, the slides were counterstained with 15
µl DAPI and a cover slip was applied. A total of 30 randomly
selected invasive tumor nuclei in each of two separate distinct
microscopic areas were evaluated under a fluorescent microscope
(magnification, ×100 oil immersion objective; Olympus Corporation).
The interpretation of FISH results was based on the 2018 ASCO/CAP
guidelines (14).
Signal intensity for the FISH assay was scored
utilizing a four-point system: 0, No visible signal; 1, weak signal
barely visible; 2, signal visible but not intense; and 3, intense
signal. This four-point scoring system was applied to HER2 and
CEP17 signals in tumor cells (10).
Statistical analysis
Data were analyzed using SPSS software (version
25.0; IBM Corp). Values are expressed as mean ± standard deviation.
Comparisons among groups (>2 groups) were carried out with
Kruskal-Wallis and Dunn's post-hoc test. Comparisons between two
groups were carried out with Wilcoxon rank sum tests or
Mann-Whitney tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
ER and PR levels of mastectomy and
core needle biopsy samples for each age group
For mastectomy samples in each age group, the
difference in Q scores for ER expression levels did not change
significantly between original tests and repeated tests (Table II). For core needle biopsies
prepared 10 years ago, Q scores decreased significantly from
6.17±0.75 for original tests to 3.17±1.33 for repeated tests
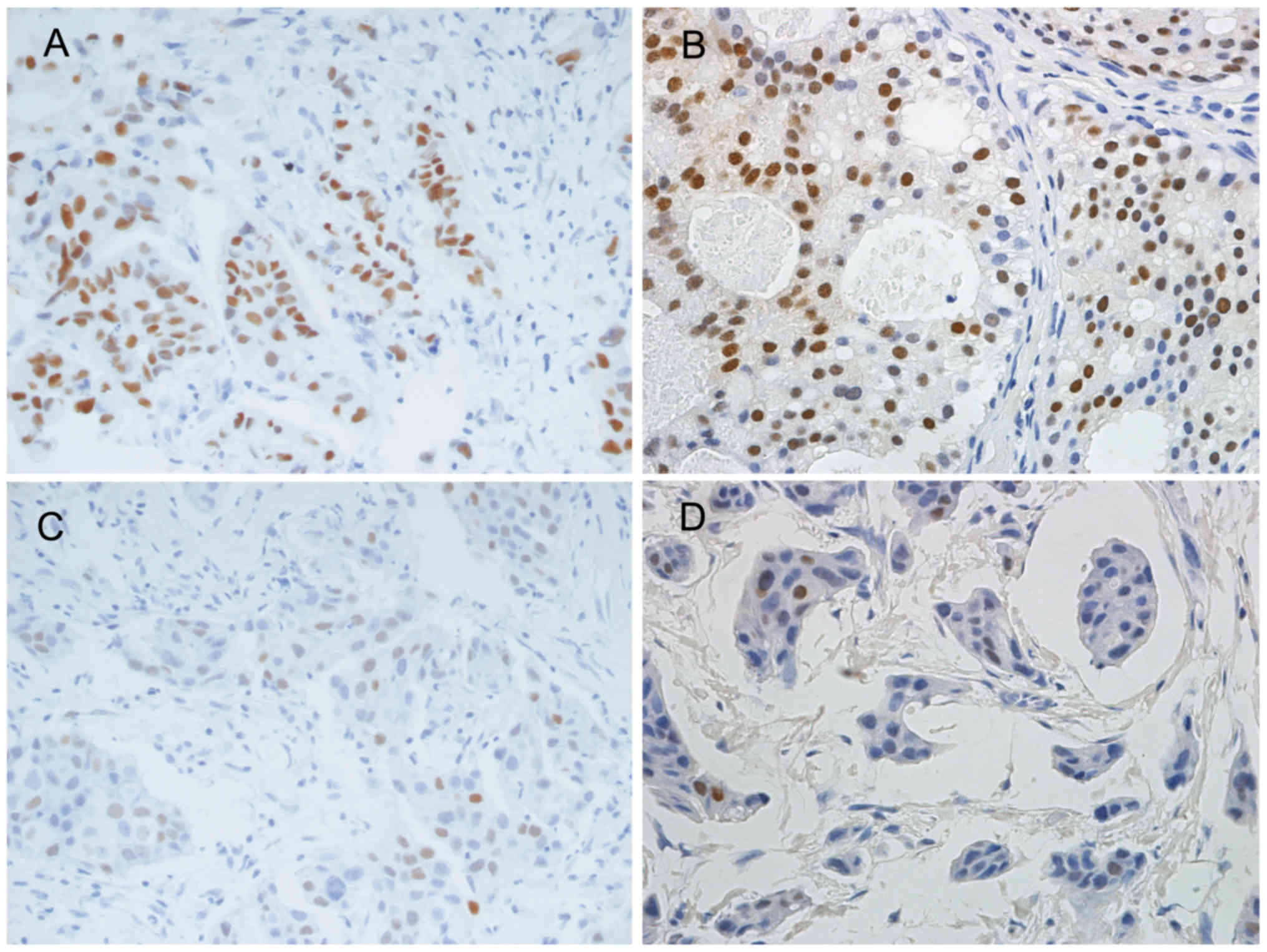
(Table II; Fig. 1A). Regarding all the samples, Q
scores for repeated tests also showed a significant decrease in the
10-year group (Table II). There was
no significant difference for the change in Q scores for other age
groups (Table II). A significant
difference of Q scores for PR expression between original tests and
repeated tests was also observed in the 10 year group, but not the
other groups (Table III; Fig. 1B and D).
 | Table II.Comparison of Q score for estrogen
receptor expression levels in each age group. |
Table II.
Comparison of Q score for estrogen
receptor expression levels in each age group.
| Age of paraffin
block, years | Q score for M
original tests | Q score for M
repeated tests |
P-valuea | Q score for CNB
original tests | Q score for CNB
repeated tests |
P-valuea | Q score for all
original tests | Q score for all
repeated tests |
P-valuea |
|---|
| 10 | 6.71±0.49 | 5.86±1.21 | NS | 6.17±0.75 | 3.17±1.33 | <0.05 | 6.46±0.66 | 4.62±1.85 | <0.05 |
| 7 | 6.33±0.71 | 5.44±1.67 | NS | 5.25±1.98 | 5.13±1.64 | NS | 5.82±1.51 | 5.29±1.61 | NS |
| 5 | 5.70±1.16 | 5.50±0.97 | NS | 5.70±1.06 | 6.40±0.97 | NS | 5.70±1.08 | 5.95±1.05 | NS |
| 3 | 5.70±1.06 | 5.50±0.97 | NS | 5.90±0.99 | 5.90±0.74 | NS | 5.80±1.01 | 5.70±0.86 | NS |
| 1 | 6.20±0.42 | 6.10±0.88 | NS | 5.80±2.04 | 5.20±1.69 | NS | 6.00±1.45 | 5.65±1.39 | NS |
 | Table III.Comparison of Q score for
progesterone receptor expression levels in each age group. |
Table III.
Comparison of Q score for
progesterone receptor expression levels in each age group.
| Age of paraffin
block, years | Q score for M
original tests | Q score for M
repeated tests |
P-valuea | Q score for CNB
original tests | Q score for CNB
repeated tests |
P-valuea | Q score for all
original tests | Q score for all
repeated tests |
P-valuea |
|---|
| 10 | 6.29±1.89 | 6.00±1.91 | NS | 4.50±1.38 | 1.50±1.22 | <0.05 | 5.46±1.85 | 3.92±2.81 | <0.05 |
| 7 | 6.67±0.50 | 6.00±0.71 | NS | 3.50±1.85 | 3.25±1.83 | NS | 5.18±2.07 | 4.71±1.93 | NS |
| 5 | 5.20±1.75 | 5.70±1.70 | NS | 5.20±1.62 | 5.30±2.31 | NS | 5.20±1.64 | 5.50±1.99 | NS |
| 3 | 5.50±0.97 | 5.40±1.17 | NS | 4.60±1.65 | 4.80±1.40 | NS | 5.05±1.39 | 5.10±1.29 | NS |
| 1 | 5.00±1.33 | 5.50±1.65 | NS | 5.50±2.07 | 5.10±2.42 | NS | 5.25±1.71 | 5.30±2.03 | NS |
Signal intensity of HER2 and CEP17 in
mastectomy and core needle biopsy samples for each age group
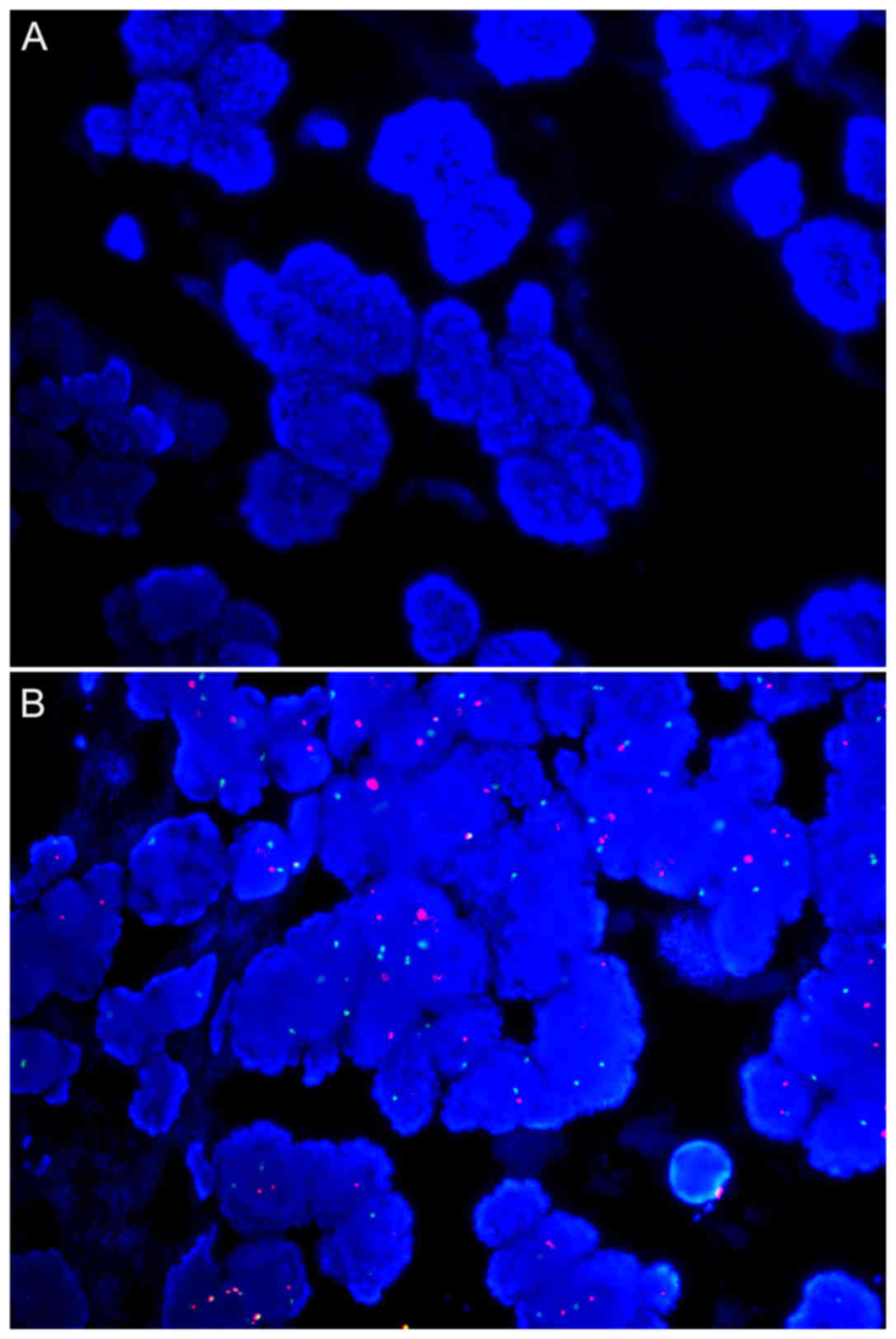
For samples from 10 and 7 years ago, no signal for
HER2 or CEP17 could be detected for core needle biopsy samples
(Tables IV and V; Fig. 2A).
HER2 was detected in 5/7 and 7/9 mastectomy samples for these two
age groups, respectively. Except for two samples from 7 years ago,
CEP17 hybridization failed (Table
V). For the remaining age groups, all samples were successfully
hybridized.
 | Table IV.Comparison of the signal intensity of
HER2 between each age group. |
Table IV.
Comparison of the signal intensity of
HER2 between each age group.
| Age of paraffin
block, years | Score of signal
intensity for mastectomies |
P-valuea | Score of signal
intensity for core needle biopsies |
P-valuea |
P-valueb | Score of signal
intensity for all samples |
P-valuea |
|---|
| 10 | 0.86±0.69 | NSc | 0 | NA | NA | 0.55±0.69 | NSc |
| 7 | 1.00±0.71 | NSc | 0 | NA | NA | 0.53±0.72 | NSc |
| 5 | 2.00±0.58 |
<0.05d,e | 1.44±0.73 | NSd | NS | 1.69±0.70 |
<0.05d,e |
| 3 | 2.30±0.48 |
<0.05d,e | 1.78±0.44 | NSd | <0.05 | 2.05±0.52 |
<0.05d,e |
| 1 | 2.33±0.87 |
<0.05d,e | 2.60±0.70 |
<0.05f,g | NS | 2.47±0.77 |
<0.05d–f |
 | Table V.Comparison of the signal intensity
for CEP17 between each age group. |
Table V.
Comparison of the signal intensity
for CEP17 between each age group.
| Age of paraffin
block, years | Score of signal
intensity for mastectomies |
P-valuea | Score of signal
intensity for core needle biopsies |
P-valuea |
P-valueb | Score of signal
intensity for all samples |
P-vlauea |
|---|
| 10 | 0 | NA | 0 | NA | NA | 0 | NA |
| 7 | 0.22±0.44 | NA | 0 | NA | NA | 0.12±0.33 | NA |
| 5 | 1.86±0.38 |
<0.05d,e | 1.22±0.67 | NA | <0.05 | 1.50±0.63 |
<0.05d,e |
| 3 | 2.10±0.32 |
<0.05d,e | 1.78±0.44 |
<0.05f | NS | 1.95±0.40 |
<0.05d,e |
| 1 | 2.00±0.87 |
<0.05d,e | 2.60±0.70 |
<0.05f,g | NS | 2.32±0.82 |
<0.05d,e |
The signal intensity decreased with the age of the
paraffin blocks (Tables IV and
V; Fig.
2A and B). Regarding HER2, the signal intensity demonstrated no
significant difference between mastectomy and core needle biopsy
samples in each age group, except the 3-year group (Table IV). Regarding CEP17, a significant
difference was observed in the signal intensity for mastectomy and
core needle biopsy samples from 5 years ago, 1.86±0.38 and
1.22±0.67, respectively (Table V).
HER2 status did not change for the repeated samples compared with
the original samples.
Signal intensity comparisons for HER2
and CEP17 between each age groups
Core needle biopsy samples from 10 and 7 years ago
were not compared because HER2 and CEP17 signals were not detected.
The signal intensities for HER2 and CEP17 for all samples in the 1
year group were the strongest compared with the samples from other
age groups, with a score of 2.47±0.77 for HER2 and 2.32±0.82 for
CEP17, followed by samples from 3, 5, 7 and 10 years ago (Tables IV and V).
When only mastectomy samples were considered, the
signal intensities of HER2 and CEP17 were stronger in samples from
1, 3 and 5 years ago compared with samples from 10 and 7 years ago
(P<0.05). No difference in signal intensity was observed for any
two groups within the last 5 years. With regard to core needle
biopsy samples, the signal intensities for HER2 and CEP17 decreased
significantly with the age of the paraffin blocks (Tables IV and V).
Discussion
Formalin-fixed paraffin-embedded (FFPE) tissue is a
widely used method to preserve tissue for diagnostic pathology.
Formalin fixation crosslinks amines, amides, aromatic rings,
hydroxyls, guanidine groups, sulfhydryl groups and reactive
hydrogen atoms through a-CH2-linkages (15–17).
Formalin is an ideal fixative, which offers several advantages such
as permanent tissue preservation, easy and long-term affordable
storage, optimal histological quality and efficient preservation of
visual details, including nuclear morphology, cellular morphology
and tissue architecture) (18).
However, DNA and RNA are degraded by formalin-fixation, whereby RNA
extracted from FFPE samples is reported to be of much lower quality
compared with fresh frozen tissues (19). Both IHC and nucleic acid-based assay
results can be compromised over time when stored slides or blocks
are used (20,21) and even after a short time there can
be considerable loss of antigenicity in tissue sections derived
from paraffin blocks (21,22). Compared with stored tissue sections,
degradation within paraffin blocks is relatively slow (21). Reductions in the quantity of nucleic
acids recovered from older tissues can vary from 5 to 50% for each
decade of storage (23).
In the present study, it was observed that ER and PR
expression levels were significantly reduced in 10-year old samples
compared with samples from other age groups. Core needle biopsy
samples from 10 and 7 years ago failed hybridization. Additionally,
signal intensities for HER2 and CEP17 decreased significantly with
the age of the paraffin blocks. The underlying reasons are not
clear, but this may be due to hydration effects and/or oxidation
(24,25). Alternatively, Xie et al
(26) suggested that inadequate
tissue processing allowed for endogenous water retention in tissue
sections and eventual antigen degradation. This endogenous or
exogenous water may explain why some methods of preservation are
ineffective, including cold storage of slides at 4°C (27), paraffin coating (28) or storage of slides in a nitrogen
desiccator (29). Optimal tissue
processing is of particular importance, as if tissues are
efficiently fixed, processed and stored, antigen degradation occurs
at a slower rate in paraffin blocks (26,30).
The Q scores for ER and PR expression levels, and
the signal intensities of HER2 and CEP17 in mastectomy and core
needle biopsy samples from tissue blocks, derived from five
different block age groups, were compared. No HER2 or CEP17 signals
could be detected in core needle biopsy samples >7 years of age;
however, HER2 signals were detected in the majority of mastectomy
samples in the 10 year group. Q scores for ER and PR expression
levels of core needle biopsy samples from 10 years ago were
decreased significantly. However, for the mastectomy samples in
each age group, the difference in Q scores for ER and PR expression
levels were not significantly different between original tests and
repeated tests. Moreover, 6/7 mastectomy samples from 10 years ago
showed no change in Q score for PR expression levels. Based on
these results, mastectomy samples as old as 10 years yielded
excellent PR results. Thus, in addition to variability in tissue
processing, the storage time of paraffin blocks may be a factor
affecting the differences between mastectomy and core needle biopsy
samples; however, the basis for this difference is unknown.
False-negative breast biomarkers are a serious issue
as these biomarkers determine both endocrine and targeted
therapies. False negative results can be due to sampling error,
delay in exposure to formalin, incorrect concentration of the
antibody/probe, incorrect pretreatment, incorrect calibration of
the automated platform, inherent variability in the interpretation
of results and variability of the signal in a given lesion
(8,12,31–34). In
the present study, six core needle biopsy cases were identified as
having a Q score of 0 following repeated tests, of which five had Q
scores of 6, 2, 2, 2 and 2 for PR expression, while one had a Q
score of 2 for ER expression, from the original tests (Table VI; Fig.
3). These cases associated with PR levels for core needle
biopsy samples that scored 2 (Table
VI; Fig. 3). This may be
associated with the occurrence of false-negatives. As such, closer
attention to the interpretation of PR results for repeated tests is
needed for core needle biopsy samples with Q score of 2.
 | Table VI.Cases showing a negative shift of Q
score for PR and ER expression levels. |
Table VI.
Cases showing a negative shift of Q
score for PR and ER expression levels.
| Age of paraffin
blocks, years | Specimen type | Biomarkers | Q score from
original tests | Q score from
repeated tests |
|---|
| 10 | CNB | PR | 6 | 0 |
| 10 | CNB | PR | 2 | 0 |
| 7 | CNB | PR | 2 | 0 |
| 5 | CNB | PR | 2 | 0 |
| 1 | CNB | PR | 2 | 0 |
| 1 | CNB | ER | 2 | 0 |
A significant limitation of the present study was
the small number of patients, limiting the ability to determine the
influence of paraffin block age on biomarker expression levels in
archival breast cancer samples. The major reason for the small
sample size was that very few cases were referred for FISH testing
in the Department of Pathology, The First Affiliated Hospital of
Zhejiang University (Hangzhou, China) for almost 6 years after FISH
testing was introduced in 2007. A larger cohort should be included
in any future study to warrant the reliability of the findings from
the present study.
In conclusion, the age of paraffin blocks has a
significant effect on ER and PR expression levels in core needle
biopsy samples. The expression levels of ER and PR were
considerably reduced in core needle biopsy samples from 10 years
ago. Moreover, samples from >7 years ago were not suitable for
FISH analysis. Furthermore, caution should be exercised for repeat
interpretation of PR expression levels for core needle biopsy
samples with a Q score of 2.
Acknowledgements
The authors would like to thank Mr. Liming Xu, Mr.
Jian Dong and Mr. Jinlong Cui for their assistance with
immunohistochemistry staining, and Miss Yanfeng Bai for her
assistance in histological analysis [all from the Department of
Pathology, The First Affiliated Hospital of the College of
Medicine, Zhejiang University (Hangzhou, China)].
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
BW designed the study, performed the experiments and
analyzed the data. HC and QQF collected and analyzed the data and
wrote the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of the First Affiliated Hospital, College of Medicine,
Zhejiang University (Hangzhou, China; approval no. 20181096). The
Committee waived the need for informed consent from the patients,
since the study was completed anonymously.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yildiz-Aktas IZ, Dabbs DJ and Bhargava R:
The effect of cold ischemic time on the immunohistochemical
evaluation of estrogen receptor, progesterone receptor, and HER2
expression in invasive breast carcinoma. Mod Pathol. 25:1098–1105.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barnes DM, Millis RR, Beex LV, Thorpe SM
and Leake RE: Increased use of immunohistochemistry for oestrogen
receptor measurement in mammary carcinoma: The need for quality
assurance. Eur J Cancer. 34:1677–1682. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr; American
Society of Clinical Oncology, : American Society of Clinical
Oncology 2007 update of recommendations for the use of tumor
markers in breast cancer. J Clin Oncol. 25:5287–5312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dowsett M, Allred C, Knox J, Quinn E,
Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, et
al: Relationship between quantitative estrogen and progesterone
receptor expression and human epidermal growth factor receptor 2
(HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or
in Combination trial. J Clin Oncol. 26:1059–1065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rhodes A, Jasani B, Balaton AJ, Barnes DM
and Miller KD: Frequency of oestrogen and progesterone receptor
positivity by immunohistochemical analysis in 7016 breast
carcinomas: Correlation with patient age, assay sensitivity,
threshold value, and mammographic screening. J Clin Pathol.
53:688–696. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al American Society of Clinical Oncology; College of American
Pathologists, : American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for human epidermal
growth factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khoury T, Sait S, Hwang H, Chandrasekhar
R, Wilding G, Tan D and Kulkarni S: Delay to formalin fixation
effect on breast biomarkers. Mod Pathol. 22:1457–1467. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moatamed NA, Nanjangud G, Pucci R, Lowe A,
Shintaku IP, Shapourifar-Tehrani S, Rao N, Lu DY and Apple SK:
Effect of ischemic time, fixation time, and fixative type on
HER2/neu immunohistochemical and fluorescence in situ hybridization
results in breast cancer. Am J Clin Pathol. 136:754–761. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Portier BP, Wang Z, Downs-Kelly E, Rowe
JJ, Patil D, Lanigan C, Budd GT, Hicks DG, Rimm DL and Tubbs RR:
Delay to formalin fixation ‘cold ischemia time’: Effect on ERBB2
detection by in-situ hybridization and immunohistochemistry. Mod
Pathol. 26:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reddy JC, Reimann JD, Anderson SM and
Klein PM: Concordance between central and local laboratory HER2
testing from a community-based clinical study. Clin Breast Cancer.
7:153–157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nuovo AJ, Garofalo M, Mikhail A, Nicol AF,
Vianna-Andrade C and Nuovo GJ: The effect of aging of
formalin-fixed paraffin-embedded tissues on the in situ
hybridization and immunohistochemistry signals in cervical lesions.
Diagn Mol Pathol. 22:164–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldstein NS, Ferkowicz M, Odish E, Mani A
and Hastah F: Minimum formalin fixation time for consistent
estrogen receptor immunohistochemical staining of invasive breast
carcinoma. Am J Clin Pathol. 120:86–92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human Epidermal Growth Factor Receptor 2 Testing in
Breast Cancer: American Society of Clinical Oncology/College of
American Pathologists Clinical Practice Guideline Focused Update. J
Clin Oncol. 36:2105–2122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moelans CB, Oostenrijk D, Moons MJ and van
Diest PJ: Formaldehyde substitute fixatives: Effects on nucleic
acid preservation. J Clin Pathol. 64:960–967. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dapson RW: Glyoxal fixation: How it works
and why it only occasionally needs antigen retrieval. Biotech
Histochem. 82:161–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dapson RW: Macromolecular changes caused
by formalin fixation and antigen retrieval. Biotech Histochem.
82:133–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perlmutter MA, Best CJM, Gillespie JW,
Gathright Y, González S, Velasco A, Linehan WM, Emmert-Buck MR and
Chuaqui RF: Comparison of snap freezing versus ethanol fixation for
gene expression profiling of tissue specimens. J Mol Diagn.
6:371–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scicchitano MS, Dalmas DA, Bertiaux MA,
Anderson SM, Turner LR, Thomas RA, Mirable R and Boyce RW:
Preliminary comparison of quantity, quality, and microarray
performance of RNA extracted from formalin-fixed,
paraffin-embedded, and unfixed frozen tissue samples. J Histochem
Cytochem. 54:1229–1237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nirmalan NJ, Harnden P, Selby PJ and Banks
RE: Development and validation of a novel protein extraction
methodology for quantitation of protein expression in
formalin-fixed paraffin-embedded tissues using western blotting. J
Pathol. 217:497–506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hewitt SM, Lewis FA, Cao Y, Conrad RC,
Cronin M, Danenberg KD, Goralski TJ, Langmore JP, Raja RG, Williams
PM, et al: Tissue handling and specimen preparation in surgical
pathology: Issues concerning the recovery of nucleic acids from
formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med.
132:1929–1935. 2008.PubMed/NCBI
|
|
22
|
Chung JY, Braunschweig T, Williams R,
Guerrero N, Hoffmann KM, Kwon M, Song YK, Libutti SK and Hewitt SM:
Factors in tissue handling and processing that impact RNA obtained
from formalin-fixed, paraffin-embedded tissue. J Histochem
Cytochem. 56:1033–1042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cronin M, Pho M, Dutta D, Stephans JC,
Shak S, Kiefer MC, Esteban JM and Baker JB: Measurement of gene
expression in archival paraffin-embedded tissues: Development and
performance of a 92-gene reverse transcriptase-polymerase chain
reaction assay. Am J Pathol. 164:35–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fergenbaum JH, Garcia-Closas M, Hewitt SM,
Lissowska J, Sakoda LC and Sherman ME: Loss of antigenicity in
stored sections of breast cancer tissue microarrays. Cancer
Epidemiol Biomarkers Prev. 13:667–672. 2004.PubMed/NCBI
|
|
25
|
Wester K, Wahlund E, Sundström C, Ranefall
P, Bengtsson E, Russell PJ, Ow KT, Malmström PU and Busch C:
Paraffin section storage and immunohistochemistry. Effects of time,
temperature, fixation, and retrieval protocol with emphasis on p53
protein and MIB1 antigen. Appl Immunohistochem Mol Morphol.
8:61–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie R, Chung JY, Ylaya K, Williams RL,
Guerrero N, Nakatsuka N, Badie C and Hewitt SM: Factors influencing
the degradation of archival formalin-fixed paraffin-embedded tissue
sections. J Histochem Cytochem. 59:356–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van den Broek LJ and van de Vijver MJ:
Assessment of problems in diagnostic and research
immunohistochemistry associated with epitope instability in stored
paraffin sections. Appl Immunohistochem Mol Morphol. 8:316–321.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jacobs TW, Prioleau JE, Stillman IE and
Schnitt SJ: Loss of tumor marker-immunostaining intensity on stored
paraffin slides of breast cancer. J Natl Cancer Inst. 88:1054–1059.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
DiVito KA, Charette LA, Rimm DL and Camp
RL: Long-term preservation of antigenicity on tissue microarrays.
Lab Invest. 84:1071–1078. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mirlacher M, Kasper M, Storz M, Knecht Y,
Dürmüller U, Simon R, Mihatsch MJ and Sauter G: Influence of slide
aging on results of translational research studies using
immunohistochemistry. Mod Pathol. 17:1414–1420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stoler MH: Toward objective cervical
cancer screening: Maybe the eyes do have it. Am J Clin Pathol.
134:5–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Copete M, Garratt J, Gilks B, Pilavdzic D,
Berendt R, Bigras G, Mitchell S, Lining LA, Cheung C and Torlakovic
EE: Inappropriate calibration and optimisation of pan-keratin
(pan-CK) and low molecular weight keratin (LMWCK)
immunohistochemistry tests: Canadian Immunohistochemistry Quality
Control (CIQC) experience. J Clin Pathol. 64:220–225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Denton KJ, Bergeron C, Klement P, Trunk
MJ, Keller T and Ridder R; European CINtec Cytology Study Group, :
The sensitivity and specificity of p16(INK4a) cytology vs HPV
testing for detecting high-grade cervical disease in the triage of
ASC-US and LSIL pap cytology results. Am J Clin Pathol. 134:12–21.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer (Unabridged Version). Arch Pathol Lab Med.
134:e48–e72. 2010.PubMed/NCBI
|