Introduction
O-linked β-N-acetylglucosaminylation
(O-GlcNAcylation) is a post-translational modification that
occurs at the serine and threonine residues of proteins present in
the cytoplasm or nucleus (1).
O-GlcNAc is attached to target proteins by O-GlcNAc
transferase (OGT) and is removed by O-GlcNAcase (OGA)
(2). Similar to phosphorylation,
O-GlcNAcylation occurs quickly in response to environmental
stimuli, such as stress, hormones, and nutrition, and plays various
roles in the regulation of phosphorylation, protein-protein
interactions, intracellular signaling, nuclear transport,
proteolysis, and epigenetics, according to the histone code
(3–5). Dysregulation of O-GlcNAcylation
is closely associated with various diseases, including type 2
diabetes mellitus (T2DM) (6,7),
Alzheimer's disease (8), heart
failure (9), and cancer (10,11). It
is thought that control of O-GlcNAcylation may be an
effective treatment approach for these diseases.
Under normal conditions, 2–3% of glucose taken up by
the cells is shunted into the hexosamine biosynthesis pathway,
which is a nutrient-sensing pathway that produces uridine diphospho
(UDP)-GlcNAc, an energy substrate of OGT, as an end product
(12). Under hyperglycemic
conditions, more glucose flows toward the hexosamine biosynthesis
pathway, the amount of UDP-GlcNAc increases, and
O-GlcNAcylation of proteins is enhanced (13). In OGT transgenic mice, insulin
resistance and leptin production are enhanced by increased levels
of O-GlcNAc in muscles and adipose tissues (7). It has been shown that the
O-GlcNAcylation of glucose transporter 4 (GLUT-4), is
enhanced in GLUT1-overexpressing muscles (14). Most proteins involved in the
malignant transformation of cells, including β-catenin, p53, the
pRb family, and c-Myc, are known to undergo O-GlcNAcylation
(4). In colorectal and lung cancer
tissues, O-GlcNAcylation and OGT expression are
significantly enhanced, and O-GlcNAcylation promotes the
growth of colorectal and lung cancer cells (15). Noticeably, O-GlcNAcylation is
involved in both T2DM and colorectal cancer.
Recent meta-analyses in epidemiological studies have
demonstrated that T2DM is a risk factor for colorectal
cancer (16) and that the underlying
mechanism may involve hyperglycemia, hyperinsulinemia, and insulin
resistance (17). Thus, we
hypothesized that O-GlcNAcylation may play an important role
in colorectal cancer occurring concurrently with T2DM.
To prove this hypothesis, we aimed to elucidate the relationship
between O-GlcNAcylation levels and colorectal cancer in
patients with T2DM. We report here our results, which
provide important insights into the involvement of
O-GlcNAcylation in cancer progression in patients with
T2DM.
Materials and methods
Patients and samples
Among successive cases histologically diagnosed as
colorectal cancer and subjected to surgical resection at our
facility between 2008 and 2015, those patients whose condition
could be followed up on the basis of clinical information in their
medical records were retrospectively analyzed in this study.
Patients with a history of treatment for T2DM were
classified into the DM group, and patients with no history of
treatment for T2DM were classified into the non-DM (NDM)
group. Tumor and adjacent non-tumor tissue samples were then
examined to determine correlations between the degree of
O-GlcNAcylation and cancer stage, tumor volume, depth of
invasion, lymph node metastasis, and distant metastasis. This study
was approved by the ethics review committee of Osaka Medical
College.
Cell culture
SW480 human colon cancer cells were obtained from
the American Type Culture Collection and maintained in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS containing 100 µg/ml streptomycin and 100 U/ml penicillin.
Another human colon cancer cell line, LoVo cells, were obtained
from Riken Bioresource Center (Riken BRC). Cells were maintained in
DMEM/10% FBS containing 100 µg/ml streptomycin and 100 U/ml
penicillin. Both cells were incubated at 37°C in 5% CO2
and 95% air.
Immunohistochemistry
Paraffin-fixed tumor and adjacent non-tumor tissue
sections cut from surgical blocks were used for
immunohistochemistry. The sections were deparaffinized using
xylene, dehydrated using ethanol, and activated by microwaving
three times in citrate buffer. Next, the sections were blocked
using 3% H2O2 solution and M.O.M blocking
reagent (Vector Laboratories). Next, the sections were stained
using anti-O-GlcNAc antibody (cat. no. MA1-072; Thermo
Fisher Scientific, Inc.) and anti-β-catenin antibody (cat. no.
ab32572; Abcam) as primary antibodies (1:200), followed by staining
with horseradish peroxidase-labeled donkey anti-mouse IgG
(Universal LSAB2 kit, cat. no. K0675; 1:1000; Dako; Agilent
Technologies, Inc.) as the secondary antibody. Color development
was carried out using DAB substrate (Wako Pure Chemical Industries,
Ltd.). Nuclei were counterstained using Mayer's hematoxylin
Solution (Wako Pure Chemical Industries, Ltd.).
Histological analysis
The immunostained tissue sections were observed
under a microscope, and three randomly chosen fields of view were
photographed under the same conditions. All imaging analyses were
performed using ImageJ software [National Institutes of Health
(NIH)]. For evaluation of O-GlcNAcylation, to avoid effects
of variations in glandular duct size, positive cells around all
glandular ducts in each view were counted and divided by the
circumference of the ducts; the resulting value was defined as the
histological score. For evaluation of β-catenin expression,
β-catenin-positive brown areas of the glandular ducts were
extracted, and the integrated density in each view was
measured.
Western blot analysis
Cells were lysed in 1% NP40, 0.25% deoxycholic acid,
0.1 M NaCl, and 25 mM Tris-HCl (pH 7.4). The lysates were subjected
to SDS-PAGE and transferred to a PVDF membrane (EMD Millipore)
using a Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell
(Bio-Rad Laboratories, Inc.). The membrane was probed with
antibodies of interest and exposed with Fusion FX (Vilber-Lourmat).
The results were densitometrically analyzed with ImageJ
software.
Co-immunoprecipitation
Cell lysates were incubated with SureBeads protein G
(Bio-Rad Laboratories, Inc.) pre-incubated with anti-β-catenin
antibody (#8480, Cell Signaling Technologies, Inc.). The
precipitates were eluted according to manufacturer's instructions
and subjected to western blot analysis using anti-O-GlcNAc
antibody (RL-2, Thermo Fisher Scientific, Inc.).
Thiamet G (TMG) treatment
SW480 or LoVo cells were seeded in 6-well plates
(Thermo Fisher Scientific, Inc.) at 1×105 cells/well in
high-glucose (25 mM) growth medium and cultured for 12 h. Next, the
cells were cultured in the presence of 1 µM TMG (Cayman Chemical
Chemical Company), an OGA inhibitor, for another 36 h. Next, cell
lysates were prepared and subjected to western blot analysis.
siRNA-mediated OGA silencing
Three siRNAs against OGA (siOGA 1,
5′-actcatcccacggttaaaa-3′, siOGA 2,
5′-gtgggtttaccctttaaa-3′), and the control siRNA (scrambled) were
purchased from Nippon Gene. siRNA was transfected into SW480 cells
using TransIT-X2 reagent (Takara Bio, Inc.) per the manufacturer's
instructions. At 24 h after transfection, the cells were cultured
in high-glucose (25 mM) or normal-glucose (5 mM) medium for 36 h.
Next, cell lysates were prepared and subjected to western blot
analysis.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 6 software (GraphPad Software, Inc.) as indicated in the
figure legends (Figs. 1B and
3B, Tukey's test; Figs. 2E and S1, Student's t-test; Fig. 2A-D, Pearson's correlation analysis).
For western blot data, statistical analyses were performed using
MEPHAS (http://www.gen-info.osaka-u.ac.jp/testdocs/tomocom/)
as indicated in the figure legends (Figs. 4A-C, 5, and S2,
Tukey's test; Fig. 4D, Dunnett's
test). P<0.05 were considered to indicate a statistically
significant difference.
 | Figure 2.O-GlcNAcylation is correlated
with cancer stage, particularly, with distant metastasis. (A)
Correlation between histological score of O-GlcNAcylation
and stage score. Stage score was defined as UICC stage I=0, IIA=1,
IIB=2, IIC=3, IIIA=4, IIIB=5, IIIC=6, IVA=7 and IVB=8. (B)
Correlation between the histological score and tumor volume. Tumor
volume was calculated by multiplying the major tumor axis by the
minor tumor axis. (C) Correlation between the histological score
and depth score. Depth score was defined by the depth of tumor
invasion as M=0, SM=1, MP=2, SS/A=3, SE=4, SI/AI=5 points. (D)
Correlation between the histological score and LN score. LN score
was defined by lymph node metastasis as UICC N0=0, N1=1, N2a=2,
N2b=3 points. (E) Comparison between T2DM (n=6) and
non-T2DM groups (n=5) of the histological score in cases
with metastasis. *P=0.0147. Statistical analyses were performed
using Pearson's correlation analysis for (A-D) and Student t-tests
for (E). T2DM, type 2 diabetes mellitus; UICC, the Union
for International Cancer Control; LN, lymph node. |
 | Figure 5.siRNA-mediated silencing of
OGA in high-glucose condition induces β-catenin and SNAIL.
siOGA 1, siOGA 2, or control siRNA (−) was
transfected into SW480 cells. Twenty-four hours later, the cells
were cultured in normal (5 mM) or high (25 mM) glucose for another
36 h. The cells were lysed and subjected to western blot analysis
using anti-OGA, β-actin (A), O-GlcNAc (B), β-catenin (C),
and SNAIL (D) antibodies. Densitometric was performed using ImageJ
(B-D). β-actin (A) was used as a loading control and for
normalization in densitometry. *P<0.05, Tukey's test. si, small
interfering; OGA, O-GlcNAcase. |
Results
Patient characteristics
Thirty-one and 30 patients were enrolled in the DM
and NDM group, respectively. No significant differences were found
between the two groups in terms of mean age, tumor volume, tumor
localization, macroscopic type, cancer stage, and presence or
absence of metastasis to other organs. Body mass index was
significantly higher in the DM group, and although there were no
significant differences in sex between the two groups, there tended
to be more men in the DM group (Table
I). It was very difficult to distinguish DM patients with
normal glucose from those with high glucose levels and thus, the DM
group may contain patients with normal glucose. Therefore, blood
glucose levels were compared between the DM and NDM groups; blood
glucose levels were significantly higher in the DM group (Fig. S1).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | DM | NDM | P-value |
|---|
| Number of
cases | 31 | 30 |
|
| Age, years, mean ±
SD | 68.2±10.7 | 70.0±8.2 | 0.460a |
| Sex |
|
| 0.600b |
|
Female | 7 (22.6%) | 14 (46.7%) |
|
|
Male | 24 (77.4%) | 16 (53.3%) |
|
| BMI, mean ± SD | 23.9±3.4 | 21.4±3.5 | 0.007a |
| Volume,
mm2, mean ± SD | 1,497±1080 | 1,740±1279 | 0.43a |
| Location |
|
| 0.88c |
|
Rectum | 15 (48.4%) | 17 (56.7%) |
|
|
Sigmoid | 10 (32.3%) | 5 (16.6%) |
|
|
Descending | 0 (0%) | 2 (6.7%) |
|
|
Transverse | 3 (9.7%) | 4 (13.3%) |
|
|
Ascending | 2 (6.5%) | 2 (6.7%) |
|
|
Cecum | 1 (3.1%) | 0 (0%) |
|
| Type |
|
| 0.82c |
| 0 | 3 (9.7%) | 3 (10%) |
|
| 1 | 0 (0%) | 0 (0%) |
|
| 2 | 22 (71%) | 24 (80%) |
|
| 3 | 5 (16.1%) | 2 (6.7%) |
|
| 5 | 1 (3.2%) | 1 (3.3%) |
|
| Stage (UICC) |
|
| 0.94c |
| 0 | 0 (0%) | 1 (3.3%) |
|
| I | 5 (16.1%) | 4 (13.3%) |
|
| II | 15 (48.4%) | 7 (23.4%) |
|
|
III | 5 (16.1%) | 13 (43.3%) |
|
| IV | 6 (19.4%) | 5 (16.7%) |
|
| Metastasis |
|
| 1.0b |
| − | 25 (80.6%) | 25 (83.3%) |
|
| + | 6 (19.4%) | 5 (16.7%) |
|
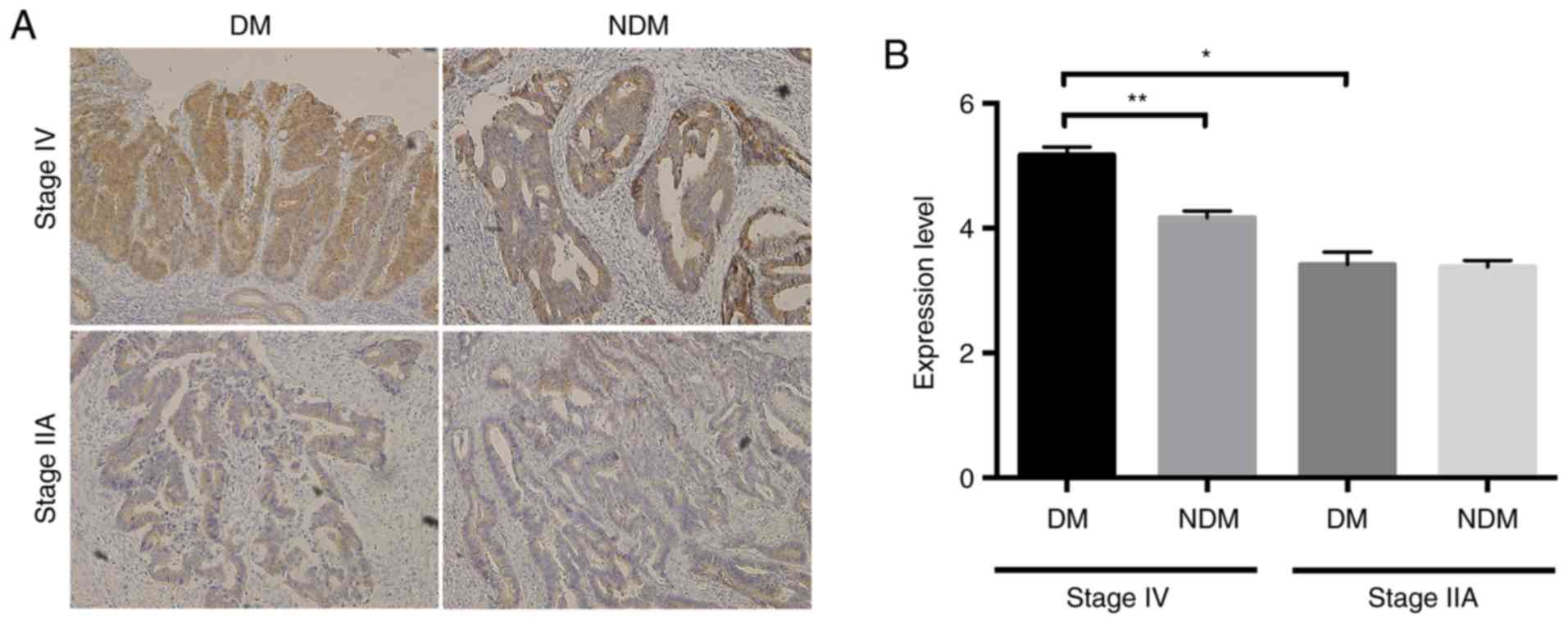
O-GlcNAcylation is enhanced in
colorectal cancer tissues, especially in patients with
T2DM
Next, we examined O-GlcNAc expression in colorectal
cancer and adjacent non-cancerous tissues in patients of the DM and
NDM groups. O-GlcNAc-positivity was detected mainly in the
nucleus and cytoplasm of glandular ductal epithelial cells
(Fig. 1A), suggesting that
glycosylation may occur at these sites. Histological staining
scores were significantly higher in cancer tissues than in
non-cancerous tissues, and histological scores in cancerous tissues
were significantly higher in the DM group than in the NDM group
(Fig. 1B). These results suggested
that O-GlcNAcylation is enhanced in colorectal cancer
tissues, and especially, in patients with T2DM.
In patients with T2DM and
colorectal cancer, O-GlcNAcylation is correlated with cancer
stage
Next, we examined histological scores of
O-GlcNAcylation in the DM and NDM groups according to colorectal
cancer stage. In the DM group, the histological score increased
with cancer progression, revealing a correlation between cancer
stage and histological score. In contrast, such a tendency was not
found in the NDM group (Fig. 2A).
These findings suggest that in colorectal cancer and comorbid
T2DM, O-GlcNAcylation may play a more important
role in cancer progression than in colorectal cancer alone.
In patients with T2DM and
colorectal cancer, O-GlcNAcylation is correlated with distant
metastasis
In the TNM classification established by the Union
for International Cancer Control (UICC), cancer is staged on the
basis of tumor size and depth of invasion (T factor), lymph node
status (N factor), and presence or absence of distant metastasis (M
factor). To examine which of these factors promoted
O-GlcNAcylation, we examined the correlations among
histological scores, tumor volume, depth of invasion, lymph node
metastasis, and distant metastasis. No correlations were found
between histological score and tumor volume or depth of invasion;
however, there was a weak correlation with lymph node metastasis
(Fig. 2B-D). Moreover, in patients
with distant metastasis, the histological score was significantly
higher in the DM group than in the NDM group (Fig. 2E). These data suggested that in
patients with DM, O-GlcNAcylation may be involved in the
distant metastasis of colorectal cancer.
In patients with T2DM, the
progression of colorectal cancer is accompanied by increased
intracellular accumulation of β-catenin in epithelial cells
Using immunohistochemical staining, we examined the
expression of β-catenin, which is involved in cancer progression,
in patients with colorectal cancer with or without T2DM.
In patients with less advanced cancer stages (e.g., stage IIA), no
differences were found between the two groups in terms of
immunoreactivity; however, in those with advanced cancer stages
(e.g., stage IV), staining for β-catenin was stronger in cases of
colorectal cancer with comorbid T2DM than in those
without T2DM. In addition, β-catenin expression was
higher in patients with advanced cancer than in patients with
earlier cancer (Fig. 3A and B).
β-catenin was localized in the cytoplasm (Fig. 3A). These results suggested that
β-catenin accumulation in the cytoplasm might be involved in the
progression of colorectal cancer, particularly in patients with
T2DM.
Blockage of OGA in high-glucose
condition upregulates β-catenin/SNAIL signaling in human colon
cancer cells
Next, we examined whether the β-catenin/SNAIL
signaling pathway is upregulated by augmented O-GlcNAcylation and
whether the upregulation is amplified in high-glucose condition,
using SW480 cells. After the cells were cultured in the presence or
absence of 1 µM TMG in normal- or high-glucose condition for 36 h,
the expression levels of β-catenin and SNAIL were determined by
western blot analyses. While both TMG treatment and high glucose
significantly increased O-GlcNAcylation, a synergistic
increase was observed under TMG treatment in high-glucose condition
(Fig. 4A). TMG treatment in
normal-glucose condition slightly, albeit insignificantly,
increased the expression of β-catenin and SNAIL (Fig. 4B and C, left 2 lanes). In contrast,
TMG treatment in high-glucose condition significantly increased the
expression of both proteins (Fig. 4B and
C, right 2 lanes). A co-immunoprecipitation assay showed that
TMG treatment did not increase O-GlcNAcylated β-catenin in
normal glucose, whereas O-GlcNAcylation was significantly
increased in high glucose (Fig. 4D).
To confirm that the elevation of protein O-GlcNAcylation
affected the metastatic phenotype of colon cancer cells as
indicated by the high β-catenin and SNAIL expression levels, we
used siOGA to increase protein O-GlcNAcylation genetically.
SW480 cells were treated with siOGA1 or 2 and then subjected to
western blot analysis. OGA expression in SW480 cells was
effectively decreased by siOGA transfection (Fig. 5A), and, subsequently, protein
O-GlcNAcylation was increased (Fig. 5B). The expression levels of β-catenin
and SNAIL were increased in high-glucose condition after silencing
of OGA (Fig. 5C and D), which was
consistent with the findings in TMG-treated cells. To evaluate
whether the effect of augmented O-GlcNAcylation observed in
SW480 cells can be generalized to other colon cancer cells, we used
another human colon cancer cell line, LoVo. The β-catenin/SNAIL
signaling pathway was upregulated in high-glucose, but not in
normal-glucose condition in LoVo cells after TMG treatment,
consistent with the findings in SW480 cells (Fig. S2). These data suggested that high
glucose has a synergistic effect on the upregulation of the
β-catenin/SNAIL signaling pathway, probably through
O-GlcNAcylation of β-catenin, in colorectal cancer tissues
where O-GlcNAcylation is augmented.
Discussion
It is well known that patients with T2DM
are at significantly higher risk for many types of cancer, but
potential biological links between the two diseases are not fully
elucidated. Here, we showed one of the mechanisms through which
T2DM influences the prognosis of colorectal cancer
patients. There was a significant correlation between
O-GlcNAcylation and cancer stage only in the DM group.
However, a comparison based on factors regulating the stage of
cancer progression revealed no association between
O-GlcNAcylation and horizontal or vertical progression,
namely infiltration (Fig. 2B and C),
whereas a weak association was found with lymph node metastasis
(Fig. 2D). Additionally, in patients
with metastasis to other organs, O-GlcNAcylation was more
strongly enhanced in the DM group (Fig.
2E), suggesting that O-GlcNAcylation may play a more
important role in the distant metastasis of colorectal cancer with
comorbid T2DM than in that of colorectal cancer without
T2DM.
Epithelial-mesenchymal transition (EMT) is believed
to be required for cancers to undergo metastasis (18,19). In
healthy living organisms, EMT is involved in embryogenesis, organ
formation, wound healing, and fibrosis, and contributes to the
maintenance of homeostasis (20).
However, in cancer cells, the transition of epithelial cells toward
highly mobile mesenchymal cells causes a loosening of intercellular
adhesion; as a result, cancer cells are more likely to infiltrate
by passing through the basal membrane (21). Various molecules, including
β-catenin, are involved in EMT.
Abnormal activation of the β-catenin/SNAIL signaling
pathway has been found in various types of malignant tumors,
including colorectal cancer (22,23).
Studies in breast cancer and oral squamous cell carcinoma have
shown that enhancement of the β-catenin/SNAIL signaling pathway is
associated with EMT and cancer cell invasiveness (24,25).
According to our immunohistochemical analysis, β-catenin expression
was strongly enhanced in patients with stage IV colorectal cancers
with comorbid T2DM (Fig. 3A
and B). Thus, EMT mediated by β-catenin/SNAIL signaling may be
more strongly involved in the metastasis of colorectal cancer with
comorbid T2DM.
Our findings suggest that O-GlcNAcylation,
which was enhanced in colorectal cancer tissues from patients with
T2DM, may be involved in the development of distant
metastases. Given that this was a retrospective study and that
there were no cases of early-stage cancer with markedly enhanced
O-GlcNAcylation, the possibility of enhancement of
O-GlcNAcylation as a result of the development of distant
metastases cannot be ruled out. However, based on the findings from
recent molecular biology studies of EMT (26,27), we
hypothesize that enhanced O-GlcNAcylation of β-catenin in
colorectal cancer tissues of patients with T2DM may have
promoted β-catenin/SNAIL-mediated EMT, which may have promoted the
development of distant metastases. For example, Jiang et al
reported that O-GlcNAcylation, which is negatively regulated
by miR-101, likely promotes colorectal cancer metastasis by
enhancing enhancer of zeste homolog 2 (EZH2) protein stability and
function, which plays important roles in EMT (28). In turn, O-GlcNAcylation in
cancer cells is promoted, stimulating EMT-mediated metastasis in
colorectal cancer (28). In
addition, according to Ling et al, EMT in podocytes is
controlled by demethylation of the promoter region of MMP-9,
which occurs under hyperglycemic conditions in diabetic nephropathy
(29). These findings strongly
support our hypothesis that EMT may be involved in the development
of distant metastasis in the presence of colorectal cancer and
hyperglycemia.
In conclusion, we found that O-GlcNAcylation
was more strongly enhanced in colorectal cancer tissues from
patients with T2DM than in those from patients without
T2DM. Moreover, it was shown that this modification
promoted cancer progression, particularly the development of
distant metastasis, through upregulation of the β-catenin/SNAIL
pathway, probably through O-GlcNAcylation of β-catenin.
O-GlcNAcylation has been shown to be enhanced in cancer
tissues and in patients with diabetes, although no previous studies
have examined O-GlcNAcylation in cancer tissues from
patients with T2DMtional studies with more participants
are needed to elucidate the underlying mechanisms; however, our
findings provided important insights into the development and
etiopathology of colorectal cancers with comorbid T2DM,
and may facilitate the establishment of therapeutic strategies
targeting O-GlcNAcylation for the treatment and prevention
of colorectal cancer with comorbid T2DM.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Nozomi Tokuhara
(Osaka Medical College, Japan) for technical assistance.
Funding
This study was supported by the KAKEN grant (grant
no. 16K09296) from the Japan Society for the Promotion of Science,
and the OMC Internal Research Grant.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
TO, MA and KH designed the current study. YN, TO,
TN, EK, YK, YT, HT, YHira, KKaw, and KKak conducted the current
study. TI, TT, SF, YHiro and KU analyzed the data. TO, TN, and MA
wrote the manuscript. KH revised the manuscript. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics review
committee of Osaka Medical College. All patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hart GW, Housley MP and Slawson C: Cycling
of O-linked β-N-acetylglucosamine on nucleocytoplasmic
proteins. Nature. 446:1017–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iyer SP and Hart GW: Dynamic nuclear and
cytoplasmic glycosylation: Enzymes of O-GlcNAc cycling.
Biochemistry. 42:2493–2499. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zachara NE, O'Donnell N, Cheung WD, Mercer
JJ, Marth JD and Hart GW: Dynamic O-GlcNAc modification of
nucleocytoplasmic proteins in response to stress. A survival
response of mammalian cells. J Biol Chem. 279:30133–30142. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hart GW, Slawson C, Ramirez-Correa G and
Lagerlof O: Cross talk between O-GlcNAcylation and
phosphorylation: Roles in signaling, transcription, and chronic
disease. Annu Rev Biochem. 80:825–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Griffith LS and Schmitz B: O-linked
N-acetylglucosamine levels in cerebellar neurons respond
reciprocally to pertubations of phosphorylation. Eur J Biochem.
262:824–831. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vosseller K, Wells L, Lane MD and Hart GW:
Elevated nucleocytoplasmic glycosylation by O-GlcNAc results
in insulin resistance associated with defects in Akt activation in
3T3-L1 adipocytes. Proc Natl Acad Sci USA. 99:5313–5318. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McClain DA, Lubas WA, Cooksey RC, Hazel M,
Parker GJ, Love DC and Hanover JA: Altered glycan-dependent
signaling induces insulin resistance and hyperleptinemia. Proc Natl
Acad Sci USA. 99:10695–10699. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lefebvre T, Ferreira S, Dupont-Wallois L,
Bussière T, Dupire MJ, Delacourte A, Michalski JC and
Caillet-Boudin ML: Evidence of a balance between phosphorylation
and O-GlcNAc glycosylation of Tau proteins--a role in
nuclear localization. Biochim Biophys Acta. 1619:167–176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yokoe S, Asahi M, Takeda T, Otsu K,
Taniguchi N, Miyoshi E and Suzuki K: Inhibition of phospholamban
phosphorylation by O-GlcNAcylation: Implications for
diabetic cardiomyopathy. Glycobiology. 20:1217–1226. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slawson C, Pidala J and Potter R:
Increased N-acetyl-beta-glucosaminidase activity in primary breast
carcinomas corresponds to a decrease in N-acetylglucosamine
containing proteins. Biochim Biophys Acta. 1537:147–157. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moriwaki K and Asahi M: Augmented TME
O-GlcNAcylation Promotes Tumor Proliferation through the
Inhibition of p38 MAPK. Mol Cancer Res. 15:1287–1298. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McClain DA and Crook ED: Hexosamines and
insulin resistance. Diabetes. 45:1003–1009. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buse MG, Robinson KA, Marshall BA, Hresko
RC and Mueckler MM: Enhanced O-GlcNAc protein modification
is associated with insulin resistance in GLUT1-overexpressing
muscles. Am J Physiol Endocrinol Metab. 283:E241–E250. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X,
Cong Q and Yu W: O-GlcNAcylation is a novel regulator of
lung and colon cancer malignancy. Biochim Biophys Acta.
1812:514–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng L, Gui Z, Zhao L, Wang J and Shen L:
Diabetes mellitus and the incidence of colorectal cancer: An
updated systematic review and meta-analysis. Dig Dis Sci.
57:1576–1585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: Epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue C, Plieth D, Venkov C, Xu C and
Neilson EG: The gatekeeper effect of epithelial-mesenchymal
transition regulates the frequency of breast cancer metastasis.
Cancer Res. 63:3386–3394. 2003.PubMed/NCBI
|
|
20
|
Greenburg G and Hay ED: Epithelia
suspended in collagen gels can lose polarity and express
characteristics of migrating mesenchymal cells. J Cell Biol.
95:333–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prasad CP, Mirza S, Sharma G, Prashad R,
DattaGupta S, Rath G and Ralhan R: Epigenetic alterations of CDH1
and APC genes: Relationship with activation of Wnt/beta-catenin
pathway in invasive ductal carcinoma of breast. Life Sci.
83:318–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iwai S, Yonekawa A, Harada C, Hamada M,
Katagiri W, Nakazawa M and Yura Y: Involvement of the Wnt-β-catenin
pathway in invasion and migration of oral squamous carcinoma cells.
Int J Oncol. 37:1095–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vu T and Datta PK: Regulation of EMT in
Colorectal Cancer: A Culprit in Metastasis. Cancers (Basel).
9:171–192. 2017. View Article : Google Scholar
|
|
28
|
Jiang M, Xu B, Li X, Shang Y, Chu Y, Wang
W, Chen D, Wu N, Hu S, Zhang S, et al: O-GlcNAcylation
promotes colorectal cancer metastasis via the
miR-101-O-GlcNAc/EZH2 regulatory feedback circuit. Oncogene.
38:301–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ling L, Chen L, Zhang C, Gui S, Zhao H and
Li Z: High glucose induces podocyte epithelial-to-mesenchymal
transition by demethylation-mediated enhancement of MMP9
expression. Mol Med Rep. 17:5642–5651. 2018.PubMed/NCBI
|