Introduction
Gastrointestinal stromal tumors (GISTs) are the
leading mesenchymal neoplasms of the gastrointestinal system, with
an annual incidence rate of 13.7 per million individuals in Taiwan
(1). Effective systemic treatments
for GISTs were not available globally until 2001 (2). However, identification of the
involvement of constitutively active transmembrane receptor KIT and
platelet-derived growth factor receptor A (PDGFRA) signaling in
GIST oncogenesis justified the use of small-molecule
tyrosine-kinase inhibitors for the treatment of GIST (3). Imatinib mesylate (IM) selectively
inhibits several protein tyrosine kinases, such as the
intracellular ABL kinase, the chimeric BCR-ABL fusion oncoprotein
of chronic myeloid leukemia, KIT and PDGFRs (4–7). The
expression of the cell-surface transmembrane receptor KIT, with
tyrosine kinase activity, is a major diagnostic biomarker of GIST.
The current understanding is that frequent gain-of-function
mutations of KIT occur in GISTs (3),
causing constitutive activation of KIT signaling and resulting in
uncontrolled cell proliferation and resistance to apoptosis
(3). For advanced GIST, IM treatment
also exhibited favorable results in terms of progression-free
survival (PFS) and overall survival (OS) time (8), and several clinical trials have also
reported promising effects of this targeted therapy in increasing
PFS and OS time (6,9–11).
Although IM has been known to result in notable
improvements in the PFS and OS time of patients with GIST, partial
response (PR) and stable disease (SD) was documented in 54% of
cases; however, ~28% of patients will develop advanced or
metastatic GIST (5,6). The majority of patients with GIST will
display drug resistance to imatinib and disease deterioration
(12). A multi-target
tyrosine-kinase inhibitor (TKI) that provides prolonged PFS time
(27 weeks), compared with the placebo in a randomized phase III
trial (13), was approved as the
second-line targeted therapy for GIST after imatinib and sunitinib;
however, resistance to sunitinib also developed (14). Thus, novel TKIs are needed as an
alternative option for patients with GIST, in the event that
resistance to sunitinib resistance develops.
Regorafenib is another multi-kinase inhibitor that
antagonizes various targets, including KIT, PDGFRA, vascular
endothelial growth factor receptor, RAF1, BRAF, RET and fibroblast
growth factor receptor, in in vitro analyses (15). An international, multicenter,
randomized, placebo-controlled, phase III trial (GRID) (16) reported that the median PFS time was
4.8 months for the regorafenib-treated group and this was longer
compared with placebo group by 0.9 months. Based on the GRID study,
regorafenib was then approved by the Food and Drug Administration
in February 2013 for metastatic or unresectable GIST after
resistance to imatinib and sunitinib had developed. Asian patients
enrolled in the phase III GRID trial were from Japan, Korea, China
and Singapore, therefore the present study investigated the
efficacy of regorafenib in a Taiwanese population.
The present prospective, non-randomized,
single-center study aimed to assess the efficacy, prognosis and
safety of regorafenib in inducing an objective response or SD in
population of individuals with advanced inoperable/metastatic GIST,
who either developed resistance to or could not tolerate the
toxicity associated with imatinib or sunitinib. In addition, a
literature review was conducted to elucidate the effect of
regorafenib on GIST globally.
Materials and methods
Patients, study design and efficacy
evaluation
Between April 2014 and December 2017, 40 patients
were diagnosed with advanced inoperable/metastatic GIST
histologically (17) and received
regorafenib therapy. The clinical data was collected prospectively
and reviewed retrospectively. Of note, regorafenib treatment has
been reimbursed by National Health Insurance in Taiwan since August
2016 (16). While 18 patients were
enrolled from the previous trial (18), 22 were enrolled from the health
reimbursement program. In the present study, however, only 28
patients who were refractory or intolerant to imatinib and
sunitinib and with measurable disease based on the Response
Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (19) were eligible for further analysis.
There were 20 males and 8 females with a median age of 61 years
(range, 36–71 years). In addition, these 28 patients had an Eastern
Cooperative Oncology Group (ECOG) performance score of ≤3 and
presented with adequate hepatic, renal and hematological functions.
The dose of regorafenib was 160 mg daily in a 3-week on/1-week off
schedule, every 4 weeks, orally. Regorafenib was not stopped,
unless unmanageable toxicity or disease progression occurred or
consent was withdrawn. Of note, regorafenib could be continued in
spite of documented disease progression if a clinical benefit was
evident to the treating physician. In contrast, postponement of
treatment or lowering of the dose was considered in the event of
adverse drug-associated side effect and dose re-escalation was
allowed after these side effects were resolved. Regular monthly
check-ups of participants included routine physical examinations
and evaluations of their performance status, weight, complete blood
count and serum chemistry, including aspartate aminotransferase,
alanine aminotransferase and total bilirubin to measure hepatic
function, creatinine for renal function, and T3, T4 and thyroid
stimulating hormone for thyroid function. Standard computed
tomography scans for each patient were performed every 3 months.
Tumor size was determined by measuring the diameter of ≥5 target
lesions and the largest dimension was used as a response evaluation
indicator, according to the RECIST 1.1 criteria (19). Time to response (TTR=time point of
the best response-time point of regorafenib administration) was
defined as the interval for the best drug response during the
treatment course. The time to progression (TTP=time point of
disease progression-time point of regorafenib administration) was
defined as the interval for the worse drug response with disease
progression during the clinical course. PFS was defined as no
disease progression after start of regorafenib treatment. OS was
defined as the survival after regorafenib administration, and the
endpoint of the present study was either GIST-associated death or
December 2017. A total of 4 patients were excluded from the
survival analysis due to 2 of them having received regorafenib
<1 month prior to enrolment in the present study, 1 withdrew due
to severe and intolerable adverse events, and 1 was lost to
follow-up. The adverse events of regorafenib were evaluated
according to the National Cancer Institute Common Terminology
Criteria for Adverse Events version 4.0 (20). The protocol of the present study was
approved by The Institutional Review Board of the Chang Gung
Memorial Hospital (approval no. 103-6044A3; Taoyuan, Taiwan), and
written informed consent was provided by all patients for drug
administration and analysis of tumor-associated genetic
alteration.
Analysis of KIT and PDGFRA
mutations
Sections (10-µm-thick) were prepared from
formalin-fixed, paraffin-embedded pretreatment specimens trimmed to
enrich for tumor cells. Tissues were fixed with 10% formalin at
room temperature for ≥24 h. Subsequently, PCR was performed as
previously described (21) on the
DNA isolated from these sections to amplify the genomic DNA
sequences of KIT and PDGFRA by Professor CY Tzen at
Cathay Memorial Hospital (Taipei, Taiwan). Sequences for mutations
of KIT and PDGFRA were analyzed as described
previously (21).
Statistical analysis
For descriptive statistics, all the data are
presented as percentage of patients or mean. Kaplan-Meier and
log-rank tests were performed for time-to-event analysis. Several
potential variables impacting long-term outcomes, including PFS and
OS time, were analyzed for significance, including age (<61 vs.
≥61 years), sex, ECOG performance status (score 0–1 vs. 2–3),
mutational status (presence vs. absence exon 17 mutation), response
[complete response + PR vs. SD vs. progressive disease (PD)],
primary site and metastatic site of GIST, and parameters of the
following: White blood cells with differential counts
[neutrophil:lymphocyte ratio (NLR)], platelet counts,
platelet:lymphocyte ratio (PLR), and hemoglobin and albumin levels.
All aforementioned factors were analyzed using a Cox multivariate
proportional hazard model if statistical significance was
identified using univariate analysis. An ‘enter-selection’
procedure was used to select the most relevant prognostic factors
and only factors that remained significant were included in the
final model. All statistical analyses were performed using SPSS
version 20.0 (IBM Corp). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features
Table I summarizes
the demographic characteristics of 28 patients (20 males and 8
females; median age, 61 years; range, 36–71 years) with advanced
inoperable/metastatic GIST treated with regorafenib. All patients
had received imatinib and sunitinib treatment prior to regorafenib,
and 2/28 (7.14%) had also received nilotinib. The median length of
imatinib treatment was 63.23 months (range, 9.4–155.54 months) and
that of sunitinib treatment was 21.91 months (range, 2.69–67.91
months). Most of the patients had a favorable ECOG score (22/28;
78.6%). While the small bowel was the leading primary site for
GISTs treated with regorafenib (24/28; 85.74%), the liver was the
leading metastatic site (20/28; 71.43%), followed by the peritoneum
(18/28; 64.29%) and lungs (3/28; 10.71%). Out of the 28 patients
with GIST with mutation data, exons 11 and 17 were the most common
(n=10), followed by exons 11, 13 and 17 (n=5), exon 9 (n=5), exon
11 (n=6) and wild-type (n=2).
 | Table I.Clinicopathological characteristics
of patients with advanced GIST treated with regorafenib (n=28). |
Table I.
Clinicopathological characteristics
of patients with advanced GIST treated with regorafenib (n=28).
|
Characteristics | n | Range |
|---|
| Median age at time
of, years |
|
|
|
Diagnosis of GIST | 52 | 28-66 |
|
Diagnosis of metastasis | 52 | 30-67 |
| Start
of imatinib | 52 | 30-68 |
| Start
of sunitinib | 58 | 35-68 |
| Start
of regorafenib | 61 | 36-71 |
| Sex, % |
|
|
|
Male/female | 20/8 | 71.4/28.6 |
| ECOG, % |
|
|
|
0-1/2-3 | 22/6 | 78.6/21.4 |
| Genetic mutation,
% |
|
|
| Exon
9 | 5 | 17.86 |
| Exon
11 | 6 | 21.43 |
| Exons
11 and 17 | 10 | 35.71 |
| Exons
11, 13 and 17 | 5 | 17.86 |
|
Wild-type | 2 | 7.14 |
| Median
length of imatinib treatment, months | 63.23 | 9.4–155.54 |
| Median
length of sunitinib treatment, months | 21.91 | 2.69–67.91 |
| Primary site,
% |
|
|
|
Stomach | 4 | 14.26 |
| Small
bowel | 24 | 85.74 |
| Metastatic site,
% |
|
|
|
Liver | 20 | 71.43 |
|
Peritoneum | 18 | 64.29 |
|
Lung | 3 | 10.71 |
|
Others | 5 | 17.86 |
| Prior failed TKI,
% |
|
|
|
Imatinib | 28 | 100.00 |
|
Sunitinib | 28 | 100.00 |
|
Nilotinib | 2 | 7.14 |
Treatment and outcomes
Regorafenib was administered to patients with
pretreated metastatic GISTs, a starting dose of 160 mg/day was
administered to all 28 patients. All patients were followed up
after regorafenib administration at regular intervals until death
or until December 2017. Table II
summarizes the best antitumor response of regorafenib of all
patients with pretreated metastatic GIST. Overall, 24/28 patients
were available for the efficacy evaluation, four (14.29%)
demonstrated a PR, 10 (35.71%) SD and 10 (35.71%) PD. In addition,
50.00% of patients with GIST exhibited a clinical benefit. Of 24
patients, the median TTR for four patients who presented PR and 10
SD were 6.2 and 2.1 months, respectively. The median OS time for
four patients with PR and 10 with SD were 21.05 and 9.54 months,
respectively. In 10 patients with PD, the median TTP was 2.46
months and the median OS time was 11.69 months (Table II).
 | Table II.Antitumor response of advanced
gastrointestinal stromal tumor treated with regorafenib (n=24). |
Table II.
Antitumor response of advanced
gastrointestinal stromal tumor treated with regorafenib (n=24).
| Response | n (%) | Sex, male/female,
n | Median regorafenib
duration, months | Median TTR/TTP,
months | Median OS,
months |
|---|
| PR | 4
(14.29) | 3/1 | 15.21 | 6.16 | 21.05 |
| SD | 10 (35.71) | 7/3 |
6.08 | 2.11 | 9.54 |
| PD | 10 (35.71) | 8/2 |
3.09 | 2.46 | 11.69 |
| N/A | 4
(14.29) | 2/2 |
0.46 | N/A | N/A |
Survival analysis of patients with
pretreated metastatic GISTs receiving regorafenib
The median follow-up time after regorafenib
treatment was 14.8 (range, 1.6–110.9) months and GISTs progressed
in 19/28 patients (67.90%) during follow-up. All 28 patients had a
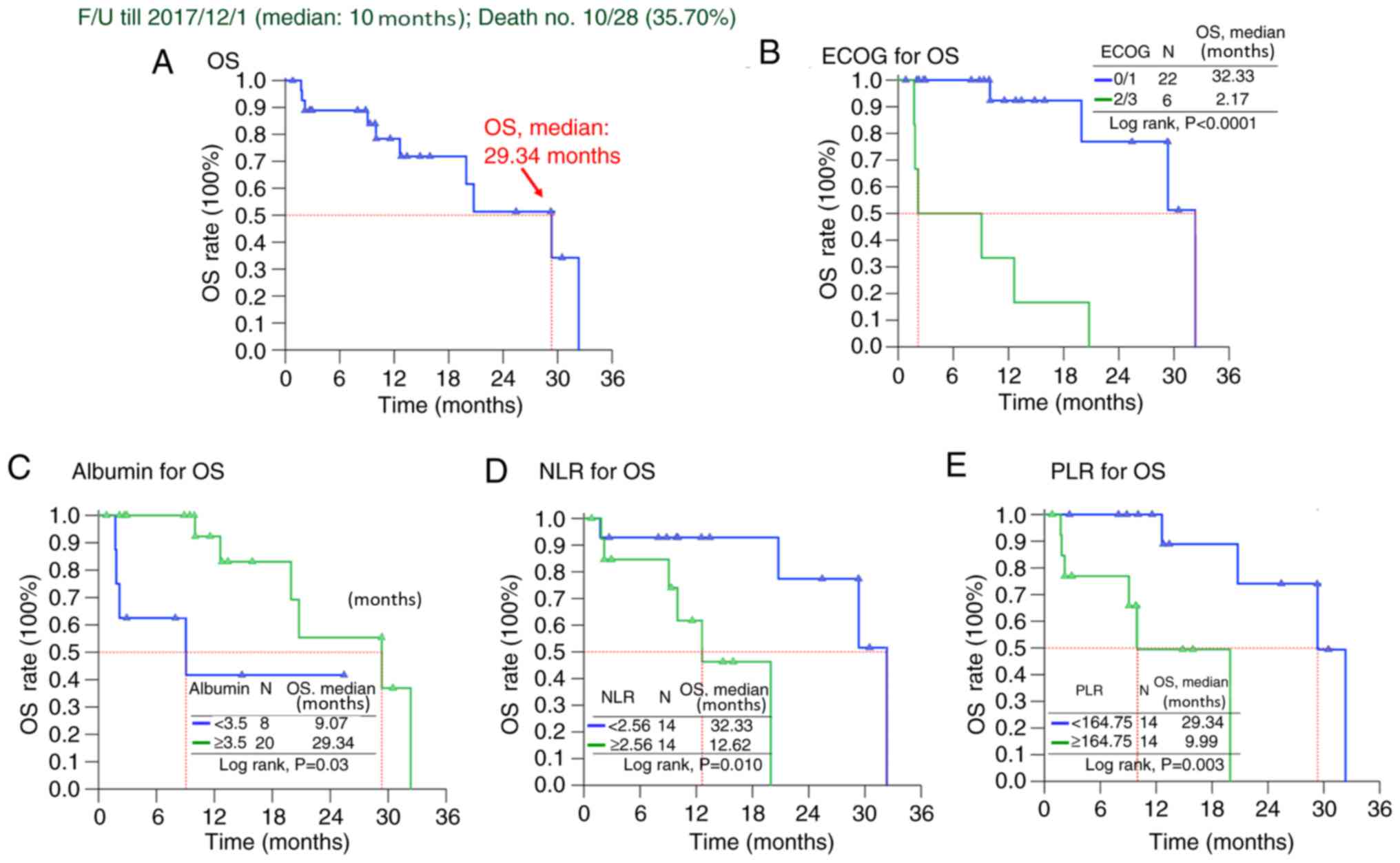
median PFS time of 4.44 months and an OS time of 29.34 months
(Figs. 1 and 2). Tables
III and IV summarize the
survival analysis regarding PFS and OS time, including clinical
features, tumor size, mutational status and laboratory data. Both
univariate and multivariate Cox's proportional hazard analyses
revealed poor performance with ECOG 2 or 3, and primary resistance
was associated with inferior PFS time for patients with GIST
receiving regorafenib treatment (Table
III and Fig. 1). Regarding OS
time, ECOG 2 or 3, absence of exon 17 mutation, NLR ≥2.56, PLR
≥164.7 and albumin <3.5 were associated with a less favorable OS
time in univariate analysis (Table
IV and Fig. 2). However,
multivariate Cox's proportional hazard analysis revealed that good
performance status, lower NLR and PLR (compared with high NLR and
PLR) and good nutritional status with albumin ≥3.5 gm/dl were
independent prognostic factors positively associated the OS time of
patients with advanced inoperable/metastatic GIST after regorafenib
treatment (Table IV and Fig. 2).
 | Table III.Prognostic analysis for the PFS time
for patients with gastrointestinal stromal tumor using the
univariate and multivariate model. |
Table III.
Prognostic analysis for the PFS time
for patients with gastrointestinal stromal tumor using the
univariate and multivariate model.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | Total no. | Events no. | Median PFS
(months) | Log-rank
P-value | P-value | Hazard ratio (95%
CI) |
|---|
| Age, years |
|
|
| 0.378 |
|
|
|
≤61 | 15 | 12 | 4.24 |
|
|
|
|
>61 | 13 | 7 | 9.20 |
|
|
|
| Sex |
|
|
| 0.125 |
|
|
|
Male | 20 | 13 | 5.29 |
|
|
|
|
Female | 8 | 6 | 2.33 |
|
|
|
| ECOG |
|
|
| 0.007 | 0.009 | 4.330 |
|
|
|
|
|
|
| (1.434–13.069) |
|
0-1 | 22 | 14 | 5.03 |
|
|
|
|
2-3 | 6 | 5 | 1.38 |
|
|
|
| Genetic status |
|
|
| 0.422 |
|
|
|
Non-exon 17 | 13 | 9 | 3.19 |
|
|
|
| Exon
17 | 15 | 10 | 5.30 |
|
|
|
| Metastatic
site |
|
|
| 0.136 |
|
|
|
Non-liver | 8 | 4 | 9.20 |
|
|
|
|
Liver | 20 | 15 | 3.75 |
|
|
|
| Total lymphocyte
count |
|
|
| 0.187 |
|
|
|
<1,550 | 14 | 8 | 8.70 |
|
|
|
|
≥1,550 | 14 | 11 | 3.19 |
|
|
|
| NLR |
|
|
| 0.655 |
|
|
|
<2.56 | 14 | 12 | 3.75 |
|
|
|
|
≥2.56 | 14 | 7 | 5.29 |
|
|
|
| PLR |
|
|
| 0.993 |
|
|
|
<164.75 | 14 | 11 | 3.75 |
|
|
|
|
≥164.75 | 14 | 8 | 5.29 |
|
|
|
| Albumin |
|
|
| 0.403 |
|
|
|
<3.5 | 8 | 5 | 4.24 |
|
|
|
|
≥3.5 | 20 | 14 | 4.44 |
|
|
|
| Response |
|
|
| <0.0001 | 0.001 | 8.326 |
|
|
|
|
|
|
| (2.513–27.588) |
| PR +
SD | 14 | 6 | 9.20 |
|
|
|
| PD | 10 | 10 | 2.33 |
|
|
|
 | Table IV.Prognostic analysis for the OS time
of patients with gastrointestinal stromal tumor using the
univariate and multivariate model. |
Table IV.
Prognostic analysis for the OS time
of patients with gastrointestinal stromal tumor using the
univariate and multivariate model.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factors | Total, n | Events,n | Median OS,
months | Log-rank
P-value | P-value | Hazard ratio (95%
CI) |
|---|
| Age, years |
|
|
| 0.321 |
|
|
|
≤61 | 15 | 6 | 32.33 |
|
|
|
|
>61 | 13 | 4 | 29.34 |
|
|
|
| Sex |
|
|
| 0.573 |
|
|
|
Male | 20 | 8 | 20.76 |
|
|
|
|
Female | 8 | 2 | N/A |
|
|
|
| ECOG |
|
|
| <0.0001 | 0.001 | 15.053 |
|
|
|
|
|
|
| (3.024–74.929) |
|
0-1 | 22 | 14 | 32.33 |
|
|
|
|
2-3 | 6 | 6 | 2.17 |
|
|
|
| Genetic status |
|
|
| 0.049 | 0.065 | 3.723 |
|
|
|
|
|
|
| (0.92–15.056) |
|
Non-exon 17 | 13 | 6 | 20.76 |
|
|
|
| Exon
17 | 15 | 4 | 32.33 |
|
|
|
| Metastatic
site |
|
|
| 0.117 |
|
|
|
Non-liver | 8 | 1 | N/A |
|
|
|
|
Liver | 20 | 9 | 19.94 |
|
|
|
| Total lymphocyte
count |
|
|
|
| 0.382 |
|
|
<1,550 | 14 | 5 | 19.94 |
|
|
|
|
≥1,550 | 14 | 5 | 32.33 |
|
|
|
| NLR |
|
|
| 0.010 | 0.033 | 10.876 |
|
|
|
|
|
|
| (1.217–97.211) |
|
<2.56 | 14 | 4 | 32.33 |
|
|
|
|
≥2.56 | 14 | 6 | 12.62 |
|
|
|
| PLR |
|
|
| 0.003 | 0.019 | 13.543 |
|
|
|
|
|
|
|
(1.544–118.822) |
|
<164.75 | 14 | 4 | 29.34 |
|
|
|
|
≥164.75 | 14 | 6 | 9.99 |
|
|
|
| Albumin |
|
|
| 0.03 | 0.045 | 4.221 |
|
|
|
|
|
|
| (1.033–17.246) |
|
<3.5 | 8 | 4 | 9.07 |
|
|
|
|
≥3.5 | 20 | 6 | 29.34 |
|
|
|
| Response |
|
|
| 0.172 |
|
|
| PR +
SD | 14 | 3 | 29.34 |
|
|
|
| PD | 10 | 4 | 19.94 |
|
|
|
Literature review to compare the
effect of regorafenib on GIST globally
For comparison with the present study cohort, a
global literature review of patients with GIST who received
regorafenib treatment was conducted (16,22–25). The
literature review (Table V) revealed
that regorafenib exhibited similar clinical efficacy compared with
the GRID trial (16) comprising of
Asian patients, including Korean and Japanese, with advanced GIST
who experienced treatment failure with imatinib or sunitinib. While
the PFS time ranged between 4.4 and 13.2 months, the OS time ranged
between 12.2 and 29.3 months.
 | Table V.Literature review concerning the
treatment outcomes of patients with metastatic gastrointestinal
stromal tumor treated with regorafenib. |
Table V.
Literature review concerning the
treatment outcomes of patients with metastatic gastrointestinal
stromal tumor treated with regorafenib.
| Author, year | Area | No. of
patients | PFS, months | OS, months | CBR, % | Grade 3 adverse
events | Prognostic factors
for PFS | Prognostic factors
for OS | (Refs.) |
|---|
| Demetri et al,
2013 | Global | 133 | 4.8 | 17.4 | 52.6 | HTN, HFSR,
Diarrhea | N/A | N/A | (16) |
| Kollàr et al,
2014 | UK | 20 | 9.4 | 12.2 | 100 | HTN, HFSR, Skin
rash | N/A | N/A | (22) |
| Komatsu et al,
2015 | Japan | 17 | 7.1 | NA | 52.3 | HTN, HFSR, Skin
rash | N/A | N/A | (23) |
| Ben-Ami et al,
2016 | USA | 33 | 13.2 | 25 | 76 | HTN, HFSR | Exon 11,
SDH-deficient | N/A | (24) |
| Son et al,
2017 | Korea | 57 | 4.5 | 12.9 | 44 | HTN, HFSR, Skin
rash | Liver
metastasis | ECOG Liver
metastasis | (25) |
| Saito et al,
2018 | Japan | 11 | 7.4 | N/A | N/A | HFSR,
HTN | N/A | N/A | (29) |
| Present
study | Taiwan | 28 | 4.4 | 29.3 | 58.3 | HTN, HFSR, Hepatic
toxicity | ECOG, Disease
control | ECOG, Albumin,
NLR, PLR | – |
Safety
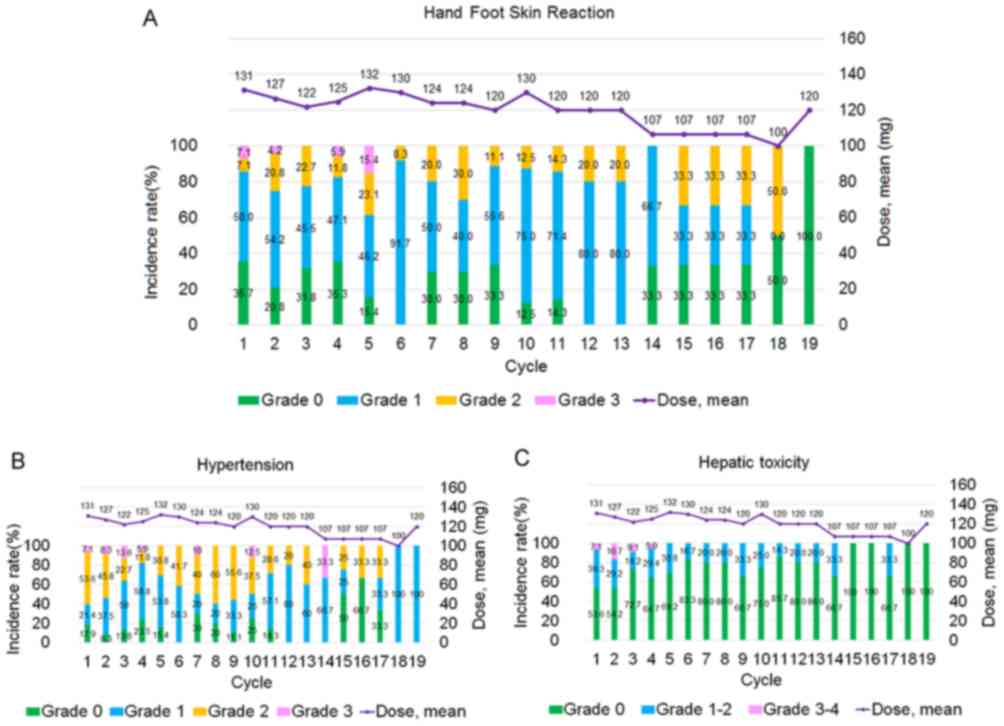
The mean dose of regorafenib per day at 19 weeks was
reduced to 120 mg, and 4/28 patients managed to re-escalate the
dose (14.29%). Safety was assessed in all 28 patients. Despite the
majority of patients requiring ≥1 dose reduction due to toxicity,
some patients (4/28; 14.29%) subsequently had their regorafenib
dose re-escalated without recurrence of unfavorable adverse
effects. Particularly, the mean dose at 18 and 19 cycles of
regorafenib per day was 100 and 120 mg, respectively, since 14.29%
of patients re-escalated their dose. Table VI summarizes the hematological and
non-hematological adverse events in patients. The leading grade 1–2
adverse events were hypertension (20/28; 71.43%), anemia (19/28;
67.86%) and hand-and-foot skin reactions (HFSRs; 18/28; 64.29%;
Fig. 3A). The leading grade 3
adverse events were HFSRs (6/28; 21.43%), hypertension (6/28;
21.43%) and hepatic toxicity (5/28; 17.86%; Fig. 3A).
 | Table VI.Adverse events and laboratory
abnormalities of 28 patients with gastrointestinal stromal tumor
following regorafenib treatment at a starting dose of 160 mg. |
Table VI.
Adverse events and laboratory
abnormalities of 28 patients with gastrointestinal stromal tumor
following regorafenib treatment at a starting dose of 160 mg.
|
| Grade, n (%) |
|---|
|
|
|
|---|
| Adverse effect | Any grade | Grade 1–2 | Grade 3 |
|---|
| Any event | 28 (100.00) | 13 (46.43) | 15 (53.57) |
| Hypertension | 26 (92.86) | 20 (71.43) | 6 (21.43) |
| Hand-and-foot skin
reaction | 24 (85.71) | 18 (64.29) | 6 (21.43) |
| Anemia | 22 (78.57) | 19 (67.86) | 3 (10.71) |
| Hepatic
toxicity | 15 (53.57) | 10 (35.71) | 5 (17.86) |
|
Thrombocytopenia | 9 (32.14) | 8 (28.57) | 1 (3.57) |
| Fatigue | 8 (28.57) | 8 (28.57) | 0 |
| Diarrhea | 7 (25.00) | 7 (25.00) | 0 |
| Hypothyroidism | 6 (21.43) | 6 (21.43) | 0 |
| Hoarseness | 4 (14.29) | 4 (14.29) | 0 |
| Anorexia | 3 (10.71) | 3 (10.71) | 0 |
| Myalgia | 3 (10.71) | 3 (10.71) | 0 |
| Oral mucositis | 2 (7.14) | 2 (7.14) | 0 |
| Palpitation | 2 (7.14) | 2 (7.14) | 0 |
| Alopecia | 1 (3.57) | 1 (3.57) | 0 |
| Leukopenia | 0 | 0 | 0 |
Discussion
The present single-center study investigated
treatment outcomes for patients with pre-treated metastatic GIST
treated with regorafenib. Several points of interest were observed.
Firstly, the median PFS and OS time for all 28 patients were 4.4
and 29.3 months, respectively. Regorafenib exhibited similar
clinical efficacy for Taiwanese patients compared with the GRID
trial comprising of Asian patients, such as Korean and Japanese,
with advanced GIST who experienced treatment failure with imatinib
or sunitinib (23–25). Secondly, regarding
regorafenib-induced adverse events, all patients exhibited similar
treatment-associated toxicity profiles compared with those of the
previous phase II (26) and III GRID
trials (16), but with a lower
incidence of grade III hypertension and diarrhea (23 and 5% in the
phase III GRID trial vs. 21 and 0% in the present study,
respectively). In addition, these adverse events corroborate with
the toxicity profile of other kinase inhibitors with a similar
target spectrum (27,28). Since the dose had to be reduced and
was then re-escalated in some patients, it was not possible to draw
any conclusions regarding the possible dose-response associations
between regorafenib and adverse events in the present study.
HFSR was the most frequently observed adverse event
and the most common reason for dose reduction in the present study.
Although HFSRs are not lethal adverse effects, these conditions are
associated with substantial unfavorable clinical symptoms, such as
intractable pain and dose reduction and treatment may be stopping
(18). Previous studies have
demonstrated that Asian patients are particularly susceptible to
regorafenib-induced HFSRs (18,25,29). The
incidence of HFSR in the present study population (85.71%) was
higher compared with that in the regorafenib group in the GRID
trial (56%) (23) but was similar to
Japanese subgroup (92%) in the GRID trial (29). Genetic polymorphisms of TNF-α, VEGF
and UGT1A9 genes have been reported to be associated with the
increased susceptibility of Asian patients to tyrosine kinase
inhibitor-induced HFSRs, particularly in patients with
hepatocellular carcinoma treated with sorafenib (18). Furthermore, the incidence of HFSRs in
the present study was similar compared with that of Korean patients
(82%) (25). Studies investigating
the underlying molecular mechanisms of this increased
susceptibility to regorafenib-induced HSFRs are required.
Good performance status and disease control mediated
by regorafenib were independent factors for a favorable PFS time in
the present study, supporting a previous study demonstrating that
good performance status was consistently and independently
associated with favorable PFS and OS time (12). Korean and Japanese patients with
GISTs, who displayed good performance status, also had improved PFS
and OS time with regorafenib treatment (23,25,29).
Regarding OS time, several novel prognostic factors
were found in the present study, including liver metastasis, the
pretreated albumin level, NLR and PLR. Similar to the Korean study
(25), liver metastasis was a
favorable factor for OS time, demonstrated by univariate survival
analysis; however, liver metastasis was not an independent
prognostic factor for OS time. Regarding the pretreated albumin
level, a previous study reported higher pretreated serum albumin
expression levels following two failed lines of TKIs in patients
with pretreated metastatic GIST, and that these increased serum
albumin expression levels were favorable factors associated with an
improved OS time (30); however, in
contrast with the present study, this previous study used
nilotinib, sorafenib and imatinib as third-line TKIs, and therefore
the results cannot be compared with those from the present study.
In a meta-analysis including 29 studies investigating cancer of the
gastrointestinal tract, 26/29 studies found that higher serum
albumin levels were associated with improved survival using
multivariate analysis (31).
Therefore, further studies are required to resolve the molecular
mechanisms underlying the aforementioned association so that
increasing albumin levels may be used as a part of cancer treatment
to improve OS time.
Previously, several studies demonstrated the
association between the inflammatory and immunonutritional status
and the prognosis of patients with cancer, including NLR and PLR
(32–36). A high NLR was associated with poor
prognosis in several malignancies, including pancreatic cancer,
hepatocellular carcinoma, ovarian cancer and GIST (33–36).
Although elevated NLR and PLR have been reported to be associated
with poor treatment outcomes, including PFS and OS time, for
primary GIST (37–41), to the best of our knowledge, the
present study is the first study to demonstrate the association
between lower NLR and PLRs to a more favorable OS time in patients
with pretreated metastatic GIST receiving regorafenib.
The mechanism underlying elevated NLR and poor
prognosis in GISTs is still unknown; however, elevated NLR usually
indicates an imbalance between pro-tumor and the anti-tumor immune
responses (42–44). Lymphocytes inhibit the proliferation
and metastatic ability of cancer cells by inducing cytotoxic
effects and cytokines production (45,46).
Neutrophils have been demonstrated to induce tumor proliferation,
invasion and vascularization by releasing proangiogenic chemokines
(47–49), therefore increased neutrophils can
inhibit the immune system by suppressing the cytolytic activity of
immune cells, such as lymphocytes and nature killer cells (50,51).
Thus, an elevated NLR directs the aforementioned imbalance in favor
of the pro-tumor inflammatory status, which in turn causes an
unfavorable outcome.
PLR has been reported as a poor prognostic factor in
ovarian (52), colorectal (53), esophageal (54), pancreatic (55), endometrial cancer (56) and neuroendocrine tumors (57), as well as in primary GIST (40). A high PLR range between 150 and 300
is associated with less favorable outcomes, in terms of
recurrence-free survival, cancer-specific survival or OS time
(40). Inflammation has been
recognized to be positively associated with PFS and OS outcomes of
malignancy and is a contributor to the shutdown of the anti-tumor
immune response by activating mediating T cells and chemokines
release, facilitating tumor growth and metastasis (58). A non-specific response to
cancer-associated inflammation was represented by the presence of
neutrophilia and thrombocytosis (40). However, both the underlying
mechanism, which links leukocytosis and neutrophilia to the
progression of malignant tumors and explains the increase in
platelets, and the biological pro-inflammatory behavior of cancer
cells, remain unclear (47).
Overall, for Taiwanese patients with pre-treated
GIST treated with regorafenib, poor performance status and poor
disease control predicted an unfavorable PFS time; however, poor
performance status, high NLR, PLR and low serum albumin levels
predicted an unfavorable OS time.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHH collected the data and reviewed the literature.
CNY designed the study, collected the data and wrote the
manuscript. JSC, CYT, SYW, CTC and TSY interpreted the data and
critically revised the manuscript for important intellectual
content. CHH and CNY analyzed the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study protocol was approved by The
Institutional Review Board of the Chang Gung Memorial Hospital
(Taoyuan, Taiwan; approval no. 103-6044A3). Written informed
consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GIST
|
gastrointestinal stromal tumor
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
NLR
|
neutrophil:lymphocyte ratio
|
|
PLR
|
platelet:lymphocyte ratio
|
|
PDGFR
|
platelet-derived growth factor
receptor
|
|
IM
|
Imatinib mesylate
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
TKI
|
tyrosine-kinase inhibitor
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
TTR
|
time to response
|
|
TTP
|
time to progression
|
|
HFSRs
|
hand-and-foot skin reactions
|
References
|
1
|
Tzen CY, Wang JH, Huang YJ, Wang MN, Lin
PC, Lai GL, Wu CY and Tzen CY: Incidence of gastrointestinal
stromal tumor: A retrospective study based on immunohistochemical
and mutational analyses. Dig Dis Sci. 52:792–797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blanke CD and Corless CL: State-of-the art
therapy for gastrointestinal stromal tumors. Cancer Invest.
23:274–280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Druker BJ, Tamura S, Buchdunger E, Ohno S,
Segal GM, Fanning S, Zimmermann J and Lydon NB: Effects of a
selective inhibitor of the Abl tyrosine kinase on the growth of
Bcr-Abl positive cells. Nat Med. 2:561–566. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buchdunger E, Cioffi C, Law N, Stover D,
Ohno-Jones S, Druker BJ and Lydon NB: Abl protein-tyrosine kinase
inhibitor STI571 inhibits in vitro signal transduction mediated by
c-kit and platelet-derived growth factor receptors. J Pharmacol Exp
Ther. 295:139–145. 2000.PubMed/NCBI
|
|
6
|
Heinrich MC, Griffith DJ, Druker BJ, Wait
CL, Ott KA and Zigler AJ: Inhibition of c-kit receptor tyrosine
kinase activity by STI 571, a selective tyrosine kinase inhibitor.
Blood. 96:925–932. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang WL, Healy ME, Satter M, Verma S, Lin
J, Maulik G, Stiles CD, Griffin JD, Johnson BE and Salgia R: Growth
inhibition and modulation of kinase pathways of small cell lung
cancer cell lines by the novel tyrosine kinase inhibitor STI 571.
Oncogene. 19:3521–3528. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joensuu H, Roberts PJ, Sarlomo-Rikala M,
Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville
R, Dimitrijevic S, Druker B and Demetri GD: Effect of the tyrosine
kinase inhibitor STI571 in a patient with a metastatic
gastrointestinal stromal tumor. N Engl J Med. 344:1052–1056. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tuveson DA, Willis NA, Jacks T, Griffin
JD, Singer S, Fletcher CD, Fletcher JA and Demetri GD: STI571
inactivation of the gastrointestinal stromal tumor c-KIT
oncoprotein: Biological and clinical implications. Oncogene.
20:5054–5058. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demetri GD, von Mehren M, Blanke CD, Van
den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA,
Singer S, Janicek M, et al: Efficacy and safety of imatinib
mesylate in advanced gastrointestinal stromal tumors. N Engl J Med.
347:472–480. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blanke CD, Demetri GD, von Mehren M,
Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD,
Roberts PJ, Heinz D, et al: Long-term results from a randomized
phase ii trial of standard-versus higher-dose imatinib mesylate for
patients with unresectable or metastatic gastrointestinal stromal
tumors expressing kit. J Clin Oncol. 26:620–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeh CN, Chen TW, Lee HL, Liu YY, Chao TC,
Hwang TL, Jan YY and Chen MF: Kinase mutations and imatinib
mesylate response for 64 Taiwanese with metastatic GIST:
Preliminary experience from Chang Gung Memorial Hospital. Ann Surg
Oncol. 14:1123–1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Demetri GD, von Oosterom AT, Garrett CR,
Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich
MC, Morgan JA, et al: Efficacy and safety of sunitinib in patients
with advanced gastrointestinal stromal tumour after failure of
imatinib: A randomised controlled trial. Lancet. 368:1329–1338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YY, Yeh CN, Cheng CT, Chen TW, Rau
KM, Jan YY and Chen MF: Sunitinib for Taiwanese patients with
gastrointestinal stromal tumor after imatinib treatment failure or
intolerance. World J Gastroenterol. 17:2113–2119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73-4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demetri GD, Reichardt P, Kang YK, Blay JY,
Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M,
Joensuu H, et al: Efficacy and safety of regorafenib for advanced
gastrointestinal stromal tumours after failure of imatinib and
sunitinib (GRID): An international, multicentre, randomised,
placebo-controlled, phase 3 trial. Lancet. 26:295–302. 2013.
View Article : Google Scholar
|
|
17
|
ESMO/European Sarcoma Network Working
Group, . Gastrointestinal stromal tumours: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25
(Suppl 3):iii21–iii26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yeh CN, Chen MH, Chen YY, Yang CY, Yen CC,
Tzen CY, Chen LT and Chen JS: A phase II trial of regorafenib in
patients with metastatic and/or an unresectable gastrointestinal
stromal tumor harboring secondary mutations of exon 17. Oncotarget.
8:44121–44130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 42:228–247.
2009. View Article : Google Scholar
|
|
20
|
"ref-label" rowspan="1" colspan="1">
21
|
Heinrich MC, Corless CL, Demetri GD,
Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den
Abbeele AD, Druker BJ, et al: Kinase mutations and imatinib
response in patients with metastatic gastrointestinal stromal
tumor. J Clin Oncol. 21:4342–4349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kollàr A, Maruzzo M, Messiou C, Cartwright
E, Miah A, Martin-Liberal J, Thway K, McGrath E, Dunlop A, Khabra
K, et al: Regorafenib treatment for advanced, refractory
gastrointestinal stromal tumor: A report of the UK managed access
program. Clin Sarcoma Res. 4:172014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komatsu Y, Doi T, Sawaki A, Kanda T,
Yamada Y, Kuss I, Demetri GD and Nishida T: Regorafenib for
advanced gastrointestinal stromal tumors following imatinib and
sunitinib treatment: A subgroup analysis evaluating Japanese
patients in the phase III GRID trial. Int J Clin Oncol. 20:905–912.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ben-Ami E, Barysauskas CM, von Mehren M,
Heinrich M, Corless CL, Butrynski JE, Morgan JA, Wagner AJ, Choy E,
Yap JT, et al: Long-term follow-up results of the multicenter phase
II trial of regorafenib in patients with metastatic and/or
unresectable GI stromal tumor after failure of standard tyrosine
kinase inhibitor therapy. Ann Oncol. 27:1794–1799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Son MK, Ryu MH, Park JO, Im SA, Kim TY,
Lee SJ, Ryoo BY, Park SR and Kang YK: Efficacy and safety of
regorafenib in korean patients with advanced gastrointestinal
stromal tumor after failure of imatinib and sunitinib: A
multicenter study based on the management access program. Cancer
Res Treat. 49:350–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
George S, Wang Q, Heinrich MC, Corless CL,
Zhu M, Butrynski JE, Morgan JA, Wagner AJ, Choy E, Tap WD, et al:
Efficacy and safety of regorafenib in patients with metastatic
and/or unresectable GI stromal tumor after failure of imatinib and
sunitinib: A multicenter phase II trial. J Clin Oncol.
30:2401–2407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: (SHARP Investigators Study Group). Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito Y, Takahashi T, Tanaka K, Miyazaki
Y, Makino T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori
M and Doki Y: Treatment of regorafenib in patients with metastatic
or unresectable gastrointestinal stromal tumor after failure of
imatinib and sunitinib. Gan To Kagaku Ryoho. 45:121–123. 2018.(In
Japanese). PubMed/NCBI
|
|
30
|
Italiano A, Cioffi A, Coco P, Maki RG,
Schöffski P, Rutkowski P, Le Cesne A, Duffaud F, Adenis A, Isambert
N, et al: Patterns of care, prognosis, and survival in patients
with metastatic gastrointestinal stromal tumors (GIST) refractory
to first-line imatinib and second-line sunitinib. Ann Surg Oncol.
19:1551–1559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Fletcher CD, Foulis AK, Horgan PG and McMillan DC:
A comparison of inflammation-based prognostic scores in patients
with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer.
47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Glazer ES, Rashid OM, Pimiento JM, Hodul
PJ and Malafa MP: Increased neutrophil-to-lymphocyte ratio after
neoadjuvant therapy is associated with worse survival after
resection of borderline resectable pancreatic ductal
adenocarcinoma. Surgery. 160:1288–1293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goh BK, Kam JH, Lee SY, Chan CY, Allen JC,
Jeyaraj P, Cheow PC, Chow PK, Ooi LL and Chung AY: Significance of
neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and
prognostic nutrition index as preoperative predictors of early
mortality after liver resection for huge (>/=10 cm)
hepatocellular carcinoma. J Surg Oncol. 113:621–627. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dobrzycka B, Mackowiak-Matejczyk B,
Terlikowska KM, Kulesza-Bronczyk B, Kinalski M and Terlikowski SJ:
Serum levels of IL-6, IL-8 and CRP as prognostic factors in
epithelial ovarian cancer. Eur Cytokine Netw. 24:106–113. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perez DR, Baser RE, Cavnar MJ,
Balachandran VP, Antonescu CR, Tap WD, Strong VE, Brennan MF, Coit
DG, Singer S and Dematteo RP: Blood neutrophil-to-lymphocyte ratio
is prognostic in gastrointestinal stromal tumor. Ann Surg Oncol.
20:593–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang C, Hu WM, Liao FX, Yang Q, Chen P,
Rong YM, Guo GF, Yin CX, Zhang B, He WZ and Xia LP: Elevated
preoperative neutrophil-to-lymphocyte ratio is associated with poor
prognosis in gastrointestinal stromal tumor patients. Onco Targets
Ther. 9:877–883. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xue A, Gao X, Fang Y, Shu P, Ling J, Shen
K, Sun Y, Qin J, Qin X and Hou Y: Incorporation of NLR into NIH
stratification system increases predictive accuracy for surgically
resected gastrointestinal stromal tumors. Acta Biochim Biophys Sin
(Shanghai). 49:179–185. 2016.
|
|
39
|
Kumamoto Y, Kaizu T, Tajima H, Nishizawa
N, Ei S, Igarashi K and Watanabe M: Neutrophil-to-lymphocyte ratio
as a predictor of postoperative morbidity in patients with distal
cholangiocarcinoma. Mol Clin Oncol. 9:362–368. 2018.PubMed/NCBI
|
|
40
|
Racz JM, Cleghorn MC, Jimenez MC, Atenafu
EG, Jackson TD, Okrainec A, Venkat Raghavan L and Quereshy FA:
Predictive ability of blood neutrophil-to-lymphocyte and
platelet-to-lymphocyte ratios in gastrointestinal stromal tumors.
Ann Surg Oncol. 22:2343–2350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goh BK, Chok AY, Allen JC Jr, Quek R, Teo
MC, Chow PK, Chung AY, Ong HS and Wong WK: Blood
neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are
independent prognostic factors for surgically resected
gastrointestinal stromal tumors. Surgery. 159:1146–1156. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
An X, Ding PR, Li YH, Wang FH, Shi YX,
Wang ZQ, He YJ, Xu RH and Jiang WQ: Elevated neutrophil to
lymphocyte ratio predicts survival in advanced pancreatic cancer.
Biomarkers. 15:516–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shibutani M, Maeda K, Nagahara H, Noda E,
Ohtani H, Nishiguchi Y and Hirakawa K: A high preoperative
neutrophil-to-lymphocyte ratio is associated with poor survival in
patients with colorectal cancer. Anticancer Res. 33:3291–3294.
2013.PubMed/NCBI
|
|
44
|
Li X, Chen ZH, Ma XK, Chen J, Wu DH, Lin
Q, Dong M, Wei L, Wang TT, Ruan DY, et al: Neutrophil-to-lymphocyte
ratio acts as a prognostic factor for patients with advanced
hepatocellular carcinoma. Tumor Biol. 35:11057–11063. 2014.
View Article : Google Scholar
|
|
45
|
Ownby HE, Roi LD, Isenberg RR and Brennan
MJ: Peripheral lymphocyte and eosinophil counts as indicators of
prognosis in primary breast cancer. Cancer. 52:126–130. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology.
138:2101–2114.e5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shamamian P, Schwartz JD, Pocock BJ, Monea
S, Whiting D, Marcus SG and Mignatti P: Activation of progelatinase
A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: A
role for inflammatory cells in tumor invasion and angiogenesis. J
Cell Physiol. 189:197–206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gong Y and Koh DR: Neutrophils promote
inflammatory angiogenesis via release of preformed VEGF in an in
vivo corneal model. Cell Tissue Res. 339:437–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Neagoe PE, Brkovic A, Hajjar F and Sirois
MG: Expression and release of angiopoietin-1 from human
neutrophils: Intracellular mechanisms. Growth Factors. 27:335–344.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Müller I, Munder M, Kropf P and Hansch GM:
Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows
or brothers in arms? Trends Immunol. 30:522–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
el-Hag A and Clark RA: Immunosuppression
by activated human neutrophils. Dependence on the myeloperoxidase
system. J Immunol. 139:2406–2413. 1987.PubMed/NCBI
|
|
52
|
Asher V, Lee J, Innamaa A and Bali A:
Preoperative platelet-lymphocyte ratio as an independent prognostic
marker in ovarian cancer. Clin Transl Oncol. 13:499–503. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kwon HC, Kim SH, Oh SY, Lee S and Lee JH,
Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ and Lee JH: Clinical
significance of preoperative neutrophil-lymphocyte versus
platelet-lymphocyte ratio in patients with operable colorectal
cancer. Biomarkers. 17:216–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feng JF, Huang Y, Zhao Q and Chen QX:
Clinical significance of preoperative neutrophil-lymphocyte ratio
versus platelet-lymphocyte ratio in patients with small cell
carcinoma of the esophagus. ScientificWorldJournal.
2013:5043652013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Smith RA, Bosonnet L, Raraty M, Sutton R,
Neoptolemos JP, Campbell F and Ghaneh P: Preoperative
platelet-lymphocyte ratio is an independent significant prognostic
marker in resected pancreatic ductal adenocarcinoma. Am J Surg.
197:466–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang D, Yang JX, Cao DY, Wan XR, Feng FZ,
Huang HF, Shen K and Xiang Y: Preoperative neutrophil-lymphocyte
and platelet-lymphocyte ratios as independent predictors of
cervical stromal involvement in surgically treated endometrioid
adenocarcinoma. Onco Targets Ther. 6:211–216. 2013.PubMed/NCBI
|
|
57
|
Sakka N, Smith RA, Whelan P, Ghaneh P,
Sutton R, Raraty M, Campbell F and Neoptolemos JP: A preoperative
prognostic score for resected pancreatic and periampullary
neuroendocrine tumours. Pancreatology. 9:670–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bhatti I, Peacock O, Lloyd G, Larvin M and
Hall RI: Preoperative hematologic markers as independent predictors
of prognosis in resected pancreatic ductal adenocarcinoma:
Neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg.
200:197–203. 2010. View Article : Google Scholar : PubMed/NCBI
|