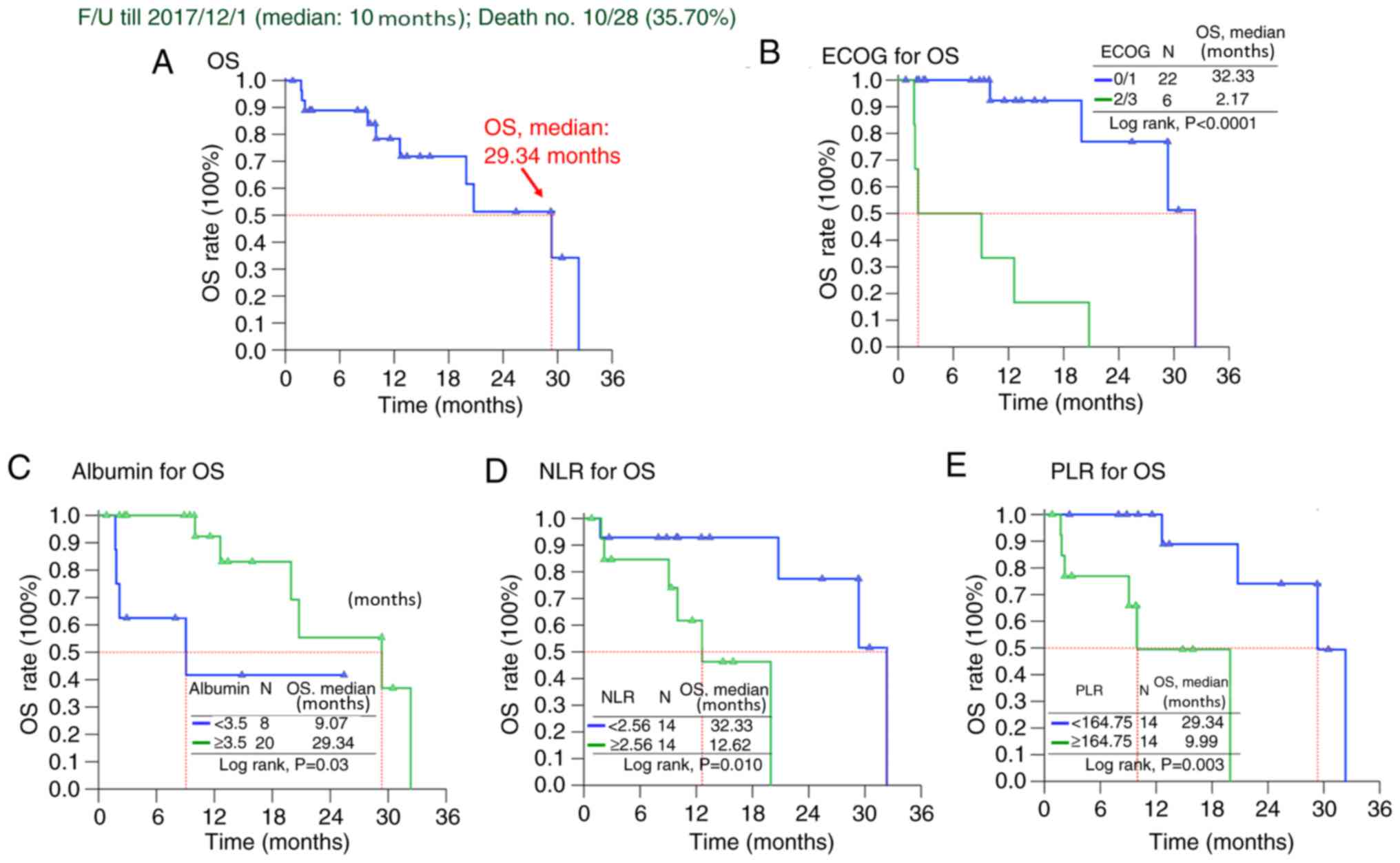
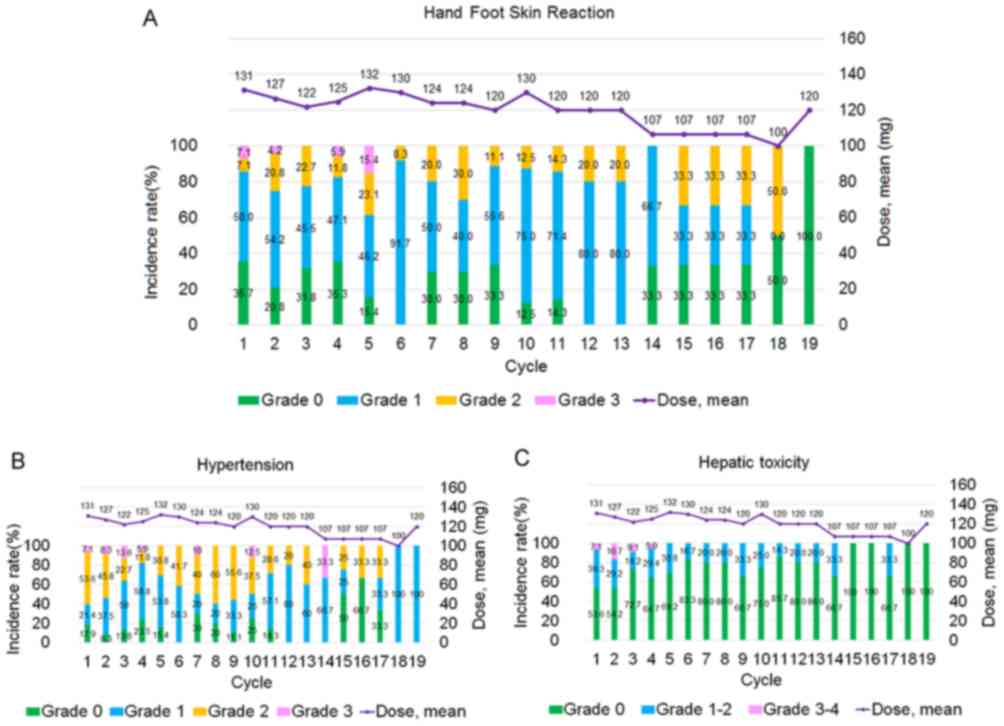
|
1
|
Tzen CY, Wang JH, Huang YJ, Wang MN, Lin
PC, Lai GL, Wu CY and Tzen CY: Incidence of gastrointestinal
stromal tumor: A retrospective study based on immunohistochemical
and mutational analyses. Dig Dis Sci. 52:792–797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blanke CD and Corless CL: State-of-the art
therapy for gastrointestinal stromal tumors. Cancer Invest.
23:274–280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Druker BJ, Tamura S, Buchdunger E, Ohno S,
Segal GM, Fanning S, Zimmermann J and Lydon NB: Effects of a
selective inhibitor of the Abl tyrosine kinase on the growth of
Bcr-Abl positive cells. Nat Med. 2:561–566. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buchdunger E, Cioffi C, Law N, Stover D,
Ohno-Jones S, Druker BJ and Lydon NB: Abl protein-tyrosine kinase
inhibitor STI571 inhibits in vitro signal transduction mediated by
c-kit and platelet-derived growth factor receptors. J Pharmacol Exp
Ther. 295:139–145. 2000.PubMed/NCBI
|
|
6
|
Heinrich MC, Griffith DJ, Druker BJ, Wait
CL, Ott KA and Zigler AJ: Inhibition of c-kit receptor tyrosine
kinase activity by STI 571, a selective tyrosine kinase inhibitor.
Blood. 96:925–932. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang WL, Healy ME, Satter M, Verma S, Lin
J, Maulik G, Stiles CD, Griffin JD, Johnson BE and Salgia R: Growth
inhibition and modulation of kinase pathways of small cell lung
cancer cell lines by the novel tyrosine kinase inhibitor STI 571.
Oncogene. 19:3521–3528. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joensuu H, Roberts PJ, Sarlomo-Rikala M,
Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville
R, Dimitrijevic S, Druker B and Demetri GD: Effect of the tyrosine
kinase inhibitor STI571 in a patient with a metastatic
gastrointestinal stromal tumor. N Engl J Med. 344:1052–1056. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tuveson DA, Willis NA, Jacks T, Griffin
JD, Singer S, Fletcher CD, Fletcher JA and Demetri GD: STI571
inactivation of the gastrointestinal stromal tumor c-KIT
oncoprotein: Biological and clinical implications. Oncogene.
20:5054–5058. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demetri GD, von Mehren M, Blanke CD, Van
den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA,
Singer S, Janicek M, et al: Efficacy and safety of imatinib
mesylate in advanced gastrointestinal stromal tumors. N Engl J Med.
347:472–480. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blanke CD, Demetri GD, von Mehren M,
Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD,
Roberts PJ, Heinz D, et al: Long-term results from a randomized
phase ii trial of standard-versus higher-dose imatinib mesylate for
patients with unresectable or metastatic gastrointestinal stromal
tumors expressing kit. J Clin Oncol. 26:620–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeh CN, Chen TW, Lee HL, Liu YY, Chao TC,
Hwang TL, Jan YY and Chen MF: Kinase mutations and imatinib
mesylate response for 64 Taiwanese with metastatic GIST:
Preliminary experience from Chang Gung Memorial Hospital. Ann Surg
Oncol. 14:1123–1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Demetri GD, von Oosterom AT, Garrett CR,
Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich
MC, Morgan JA, et al: Efficacy and safety of sunitinib in patients
with advanced gastrointestinal stromal tumour after failure of
imatinib: A randomised controlled trial. Lancet. 368:1329–1338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YY, Yeh CN, Cheng CT, Chen TW, Rau
KM, Jan YY and Chen MF: Sunitinib for Taiwanese patients with
gastrointestinal stromal tumor after imatinib treatment failure or
intolerance. World J Gastroenterol. 17:2113–2119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73-4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demetri GD, Reichardt P, Kang YK, Blay JY,
Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M,
Joensuu H, et al: Efficacy and safety of regorafenib for advanced
gastrointestinal stromal tumours after failure of imatinib and
sunitinib (GRID): An international, multicentre, randomised,
placebo-controlled, phase 3 trial. Lancet. 26:295–302. 2013.
View Article : Google Scholar
|
|
17
|
ESMO/European Sarcoma Network Working
Group, . Gastrointestinal stromal tumours: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25
(Suppl 3):iii21–iii26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yeh CN, Chen MH, Chen YY, Yang CY, Yen CC,
Tzen CY, Chen LT and Chen JS: A phase II trial of regorafenib in
patients with metastatic and/or an unresectable gastrointestinal
stromal tumor harboring secondary mutations of exon 17. Oncotarget.
8:44121–44130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 42:228–247.
2009. View Article : Google Scholar
|
|
20
|
"ref-label" rowspan="1" colspan="1">
21
|
Heinrich MC, Corless CL, Demetri GD,
Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den
Abbeele AD, Druker BJ, et al: Kinase mutations and imatinib
response in patients with metastatic gastrointestinal stromal
tumor. J Clin Oncol. 21:4342–4349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kollàr A, Maruzzo M, Messiou C, Cartwright
E, Miah A, Martin-Liberal J, Thway K, McGrath E, Dunlop A, Khabra
K, et al: Regorafenib treatment for advanced, refractory
gastrointestinal stromal tumor: A report of the UK managed access
program. Clin Sarcoma Res. 4:172014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komatsu Y, Doi T, Sawaki A, Kanda T,
Yamada Y, Kuss I, Demetri GD and Nishida T: Regorafenib for
advanced gastrointestinal stromal tumors following imatinib and
sunitinib treatment: A subgroup analysis evaluating Japanese
patients in the phase III GRID trial. Int J Clin Oncol. 20:905–912.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ben-Ami E, Barysauskas CM, von Mehren M,
Heinrich M, Corless CL, Butrynski JE, Morgan JA, Wagner AJ, Choy E,
Yap JT, et al: Long-term follow-up results of the multicenter phase
II trial of regorafenib in patients with metastatic and/or
unresectable GI stromal tumor after failure of standard tyrosine
kinase inhibitor therapy. Ann Oncol. 27:1794–1799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Son MK, Ryu MH, Park JO, Im SA, Kim TY,
Lee SJ, Ryoo BY, Park SR and Kang YK: Efficacy and safety of
regorafenib in korean patients with advanced gastrointestinal
stromal tumor after failure of imatinib and sunitinib: A
multicenter study based on the management access program. Cancer
Res Treat. 49:350–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
George S, Wang Q, Heinrich MC, Corless CL,
Zhu M, Butrynski JE, Morgan JA, Wagner AJ, Choy E, Tap WD, et al:
Efficacy and safety of regorafenib in patients with metastatic
and/or unresectable GI stromal tumor after failure of imatinib and
sunitinib: A multicenter phase II trial. J Clin Oncol.
30:2401–2407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: (SHARP Investigators Study Group). Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito Y, Takahashi T, Tanaka K, Miyazaki
Y, Makino T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori
M and Doki Y: Treatment of regorafenib in patients with metastatic
or unresectable gastrointestinal stromal tumor after failure of
imatinib and sunitinib. Gan To Kagaku Ryoho. 45:121–123. 2018.(In
Japanese). PubMed/NCBI
|
|
30
|
Italiano A, Cioffi A, Coco P, Maki RG,
Schöffski P, Rutkowski P, Le Cesne A, Duffaud F, Adenis A, Isambert
N, et al: Patterns of care, prognosis, and survival in patients
with metastatic gastrointestinal stromal tumors (GIST) refractory
to first-line imatinib and second-line sunitinib. Ann Surg Oncol.
19:1551–1559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Fletcher CD, Foulis AK, Horgan PG and McMillan DC:
A comparison of inflammation-based prognostic scores in patients
with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer.
47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Glazer ES, Rashid OM, Pimiento JM, Hodul
PJ and Malafa MP: Increased neutrophil-to-lymphocyte ratio after
neoadjuvant therapy is associated with worse survival after
resection of borderline resectable pancreatic ductal
adenocarcinoma. Surgery. 160:1288–1293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goh BK, Kam JH, Lee SY, Chan CY, Allen JC,
Jeyaraj P, Cheow PC, Chow PK, Ooi LL and Chung AY: Significance of
neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and
prognostic nutrition index as preoperative predictors of early
mortality after liver resection for huge (>/=10 cm)
hepatocellular carcinoma. J Surg Oncol. 113:621–627. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dobrzycka B, Mackowiak-Matejczyk B,
Terlikowska KM, Kulesza-Bronczyk B, Kinalski M and Terlikowski SJ:
Serum levels of IL-6, IL-8 and CRP as prognostic factors in
epithelial ovarian cancer. Eur Cytokine Netw. 24:106–113. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perez DR, Baser RE, Cavnar MJ,
Balachandran VP, Antonescu CR, Tap WD, Strong VE, Brennan MF, Coit
DG, Singer S and Dematteo RP: Blood neutrophil-to-lymphocyte ratio
is prognostic in gastrointestinal stromal tumor. Ann Surg Oncol.
20:593–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang C, Hu WM, Liao FX, Yang Q, Chen P,
Rong YM, Guo GF, Yin CX, Zhang B, He WZ and Xia LP: Elevated
preoperative neutrophil-to-lymphocyte ratio is associated with poor
prognosis in gastrointestinal stromal tumor patients. Onco Targets
Ther. 9:877–883. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xue A, Gao X, Fang Y, Shu P, Ling J, Shen
K, Sun Y, Qin J, Qin X and Hou Y: Incorporation of NLR into NIH
stratification system increases predictive accuracy for surgically
resected gastrointestinal stromal tumors. Acta Biochim Biophys Sin
(Shanghai). 49:179–185. 2016.
|
|
39
|
Kumamoto Y, Kaizu T, Tajima H, Nishizawa
N, Ei S, Igarashi K and Watanabe M: Neutrophil-to-lymphocyte ratio
as a predictor of postoperative morbidity in patients with distal
cholangiocarcinoma. Mol Clin Oncol. 9:362–368. 2018.PubMed/NCBI
|
|
40
|
Racz JM, Cleghorn MC, Jimenez MC, Atenafu
EG, Jackson TD, Okrainec A, Venkat Raghavan L and Quereshy FA:
Predictive ability of blood neutrophil-to-lymphocyte and
platelet-to-lymphocyte ratios in gastrointestinal stromal tumors.
Ann Surg Oncol. 22:2343–2350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goh BK, Chok AY, Allen JC Jr, Quek R, Teo
MC, Chow PK, Chung AY, Ong HS and Wong WK: Blood
neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are
independent prognostic factors for surgically resected
gastrointestinal stromal tumors. Surgery. 159:1146–1156. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
An X, Ding PR, Li YH, Wang FH, Shi YX,
Wang ZQ, He YJ, Xu RH and Jiang WQ: Elevated neutrophil to
lymphocyte ratio predicts survival in advanced pancreatic cancer.
Biomarkers. 15:516–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shibutani M, Maeda K, Nagahara H, Noda E,
Ohtani H, Nishiguchi Y and Hirakawa K: A high preoperative
neutrophil-to-lymphocyte ratio is associated with poor survival in
patients with colorectal cancer. Anticancer Res. 33:3291–3294.
2013.PubMed/NCBI
|
|
44
|
Li X, Chen ZH, Ma XK, Chen J, Wu DH, Lin
Q, Dong M, Wei L, Wang TT, Ruan DY, et al: Neutrophil-to-lymphocyte
ratio acts as a prognostic factor for patients with advanced
hepatocellular carcinoma. Tumor Biol. 35:11057–11063. 2014.
View Article : Google Scholar
|
|
45
|
Ownby HE, Roi LD, Isenberg RR and Brennan
MJ: Peripheral lymphocyte and eosinophil counts as indicators of
prognosis in primary breast cancer. Cancer. 52:126–130. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology.
138:2101–2114.e5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shamamian P, Schwartz JD, Pocock BJ, Monea
S, Whiting D, Marcus SG and Mignatti P: Activation of progelatinase
A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: A
role for inflammatory cells in tumor invasion and angiogenesis. J
Cell Physiol. 189:197–206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gong Y and Koh DR: Neutrophils promote
inflammatory angiogenesis via release of preformed VEGF in an in
vivo corneal model. Cell Tissue Res. 339:437–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Neagoe PE, Brkovic A, Hajjar F and Sirois
MG: Expression and release of angiopoietin-1 from human
neutrophils: Intracellular mechanisms. Growth Factors. 27:335–344.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Müller I, Munder M, Kropf P and Hansch GM:
Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows
or brothers in arms? Trends Immunol. 30:522–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
el-Hag A and Clark RA: Immunosuppression
by activated human neutrophils. Dependence on the myeloperoxidase
system. J Immunol. 139:2406–2413. 1987.PubMed/NCBI
|
|
52
|
Asher V, Lee J, Innamaa A and Bali A:
Preoperative platelet-lymphocyte ratio as an independent prognostic
marker in ovarian cancer. Clin Transl Oncol. 13:499–503. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kwon HC, Kim SH, Oh SY, Lee S and Lee JH,
Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ and Lee JH: Clinical
significance of preoperative neutrophil-lymphocyte versus
platelet-lymphocyte ratio in patients with operable colorectal
cancer. Biomarkers. 17:216–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feng JF, Huang Y, Zhao Q and Chen QX:
Clinical significance of preoperative neutrophil-lymphocyte ratio
versus platelet-lymphocyte ratio in patients with small cell
carcinoma of the esophagus. ScientificWorldJournal.
2013:5043652013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Smith RA, Bosonnet L, Raraty M, Sutton R,
Neoptolemos JP, Campbell F and Ghaneh P: Preoperative
platelet-lymphocyte ratio is an independent significant prognostic
marker in resected pancreatic ductal adenocarcinoma. Am J Surg.
197:466–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang D, Yang JX, Cao DY, Wan XR, Feng FZ,
Huang HF, Shen K and Xiang Y: Preoperative neutrophil-lymphocyte
and platelet-lymphocyte ratios as independent predictors of
cervical stromal involvement in surgically treated endometrioid
adenocarcinoma. Onco Targets Ther. 6:211–216. 2013.PubMed/NCBI
|
|
57
|
Sakka N, Smith RA, Whelan P, Ghaneh P,
Sutton R, Raraty M, Campbell F and Neoptolemos JP: A preoperative
prognostic score for resected pancreatic and periampullary
neuroendocrine tumours. Pancreatology. 9:670–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bhatti I, Peacock O, Lloyd G, Larvin M and
Hall RI: Preoperative hematologic markers as independent predictors
of prognosis in resected pancreatic ductal adenocarcinoma:
Neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg.
200:197–203. 2010. View Article : Google Scholar : PubMed/NCBI
|