Introduction
Additive manufacturing (AM) has become integral to
modern rapid prototyping. Based on computer-aided design (CAD), AM
uses layer-based manufacturing technology to directly produce end
products (1,2). In medicine, by utilizing digital
slicing of CAD, a 3D scan (generated by Mimics or InVesalius
software programs) and microcomputed tomography (µCT) data, AM
enables direct fabrication of customized biological models for
various clinical applications (3,4). These
biological models can be used in disease diagnosis, surgical
planning, clinical teaching and patient communication (5,6). The
process of AM is continuously evolving with advances in the design
and manufacturing of customized implants according to patient needs
(7). AM provides a bridge that
transfers the simulated surgical plan accurately to the surgical
site. Therefore, high accuracy, and efficiency in planning and
execution of surgical procedures are achieved (8–10). There
are >20 individual AM processes, which vary in their method of
layering and manufacturing (1,2). Among
these processes, both selective laser melting and electron-beam
melting (EBM) have been extensively developed and improved upon for
fabrication of surgical metal implants in various clinical settings
(11,12).
Metallic biomaterials, such as 316L stainless steel,
cobalt-chromium-based alloy and titanium, have been used
extensively in manufacturing of surgical implants (13). In contrast to other biocompatible
metals, titanium has improved fatigue resistance and a modulus of
elasticity comparable to bone (14).
Ti6AI4V extra low interstitial (ELI) is an α-β alloy containing 6%
aluminum and 4% vanadium. Controlled interstitial element levels
are designated as an ELI; hence the designation Ti6Al-4V ELI
(15,16). Ti6Al-4V ELI is one of the most widely
used titanium alloys in the production of surgical implants
(17). A previous study has shown
that Ti6AI4V ELI exhibits a number of advantages compared with pure
titanium, including higher tensile strength and improved fatigue
resistance (14). Furthermore,
Ti6AI4V ELI has improved tensile strength, suitable stiffness, a
high degree of resistance to corrosion and an enhanced porous
structure that possesses excellent mechanical properties similar to
those of human bone (18).
Considerable research efforts have been undertaken to evaluate the
biocompatibility of Ti6AI4V ELI using EBM (Ti6AI4V ELI EBM) in the
physiological environment (19,20).
Ti6AI4V ELI EBM exhibits promise for fabrication of hip joints and
knee joints for use clinically (21,22).
The mandible is the largest bone in the human skull,
and it holds the lower teeth in place, assists in mastication and
forms the lower jawline (23). The
mandible is composed of the body and the ramus, and is located
inferior to the maxilla. The body is the horizontally curved
portion that creates the lower jawline (24). Mandibular defects caused by
inflammation, trauma and maxillofacial tumors can cause
dysfunctions in chewing and facial deformities, and may severely
affect a patient's quality of life (25). Due to various sizes of defects and
the effect of radiation therapy (in case of cancer treatment) on
the blood supply of the surrounding tissues, the repair and
reconstruction of mandibular defects has always been a challenging
task for surgeons (26). With the
development of modern medicine, significant progress has been made
in the reconstruction of mandibular defects. At present, there are
a variety of repair methods, including commercial reconstruction
titanium plates (for example the Stryker Leibinger universal
system), non-vascularized autogenous bone grafts, pedicled bone
grafts and vascularized fibula free grafts (27,28);
however, the existing repair methods often do not perfectly restore
the continuity and integrity of the mandible, which may result in a
second trauma (29). Using digital
medical imaging technology and metal 3D printing technology,
according to CT scan data of patients, porous titanium alloy bone
tissue substitute materials that are similar in shape, size and
weight to the bone defect area can be designed and manufactured to
achieve personalized repair and reconstruction of bone defects
(30). At present, this technology
has been applied in the field of hip, knee, spine and skull
reconstruction (31,32); however, to the best of our knowledge,
there are limited studies reporting its use in oral and
maxillofacial defects (33). The
present study included 4 patients with individualized titanium
alloy implants and the outcomes were compared with 16 patients who
received traditional reconstructed titanium plates and vascularized
fibula free flaps through a retrospective controlled study. The
conclusion of the present study may provide clinicians with a
powerful design and manufacturing technique, to improve the
availability of implantable mandibular solutions that overcome the
shortcomings of typical fixation plate-based treatment options,
thereby improving overall patient outcomes.
Materials and methods
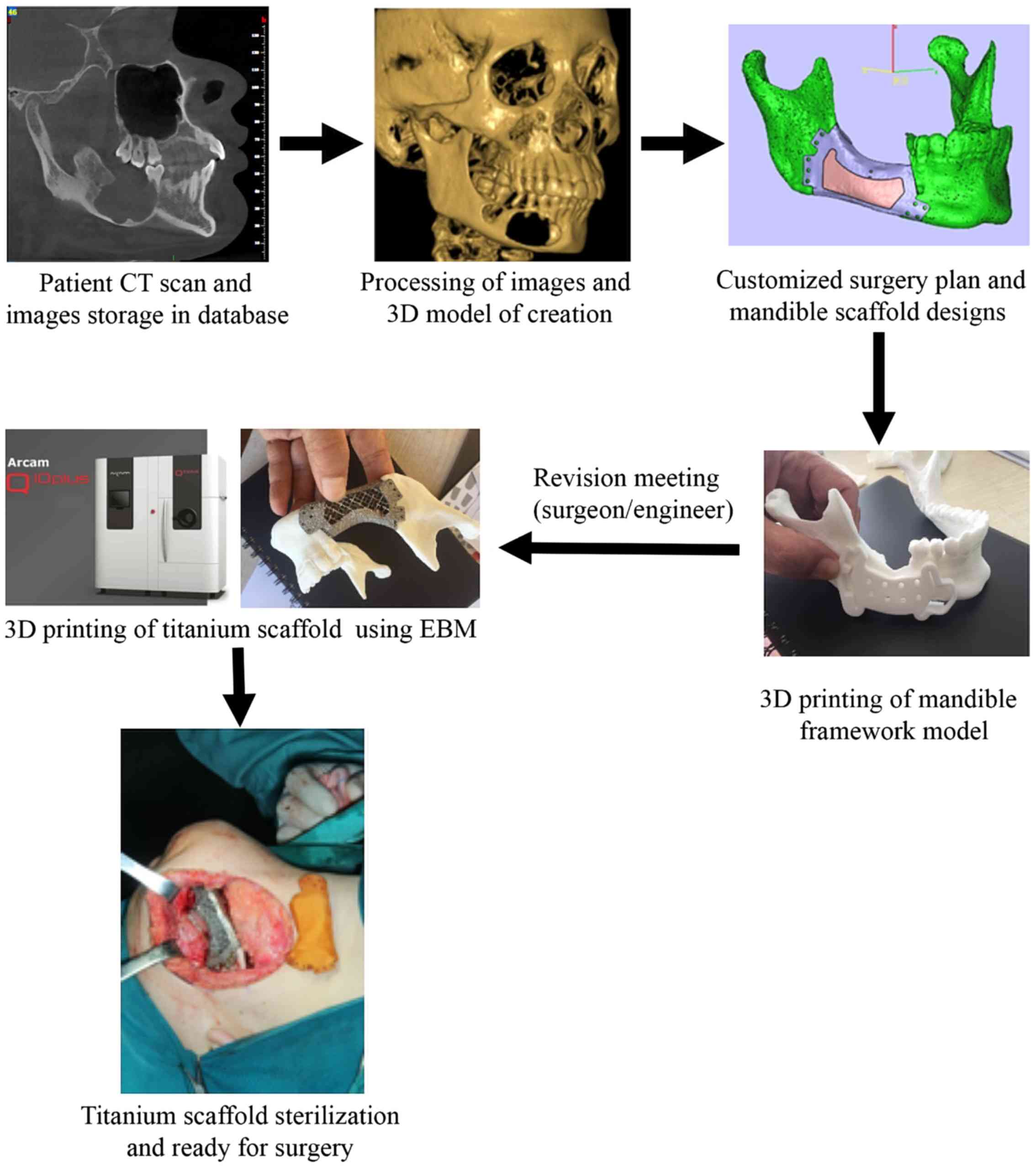
Workflow
The workflow of reconstruction of mandibular
discontinuity using AM technology consisted of several stages: i)
3D digital imaging; ii) data transfer; iii) processing and
segmentation; iv) evaluation of design; and v) AM model production
and evaluation. In the present study, pre-operative preparations,
surgical procedures and post-operative treatment outcomes were
compared among patients who received mandibular reconstruction
using a customized 3D titanium implant, and two other conventional
approaches; titanium reconstruction plates and vascularized
autologous fibular grafting (Fig.
1).
Selection criteria
The present study included patients with mandibular
defects caused by non-malignant tumors. These tumors have a clear
invasive pattern and margin, and radical therapy is recommended,
which has high immediate cure success rates (34). Furthermore, patients who did not
exhibit tumor recurrence within 1 year of surgery and did not
receive radiation therapy were also included. Cases with any
condition of uncontrolled inflammation were excluded. The surgical
outcome in the prospective cohort of patients was compared with the
retrospective cohort of patients who received mandibular
reconstruction using two other conventional approaches: Titanium
plates or vascularized autologous fibular grafting. The same
inclusion and exclusion criteria were also applied to the
retrospective cohort cases. The surgical pathology database from
Stomatological Hospital of Jiangsu was searched for reported cases
of benign oral tumors between January 2014 and February 2017. A
total of 16 retrospective cases were identified and follow-up time
periods were recorded (Table SI).
Among these retrospective cases, 6 patients received implantation
of titanium plates, and 10 patients received vascularized
autologous fibular grafting. All patients underwent mandibular
reconstruction, and their patient files containing pre- and
post-operative µCT scans were reviewed. All patients were
introduced different types of implants including advantages and
disadvantages for each type. The treatment was based on the
patient's preference after consultation with surgeons.
Written informed consent for participation in the
study was obtained from participants according to the policy of the
Stomatological Hospital of Jiangsu Ethical Review Board, and the
data were anonymized for analysis and research publication. The
present study was approved by the Stomatological Hospital of
Jiangsu Ethical Review Board (approval no. PJ2016-003-01).
Patient information
A total of 4 patients (mean age, 33 years; age
range, 21–50 years) were recruited prospectively at The Affiliated
Hospital of Stomatology of Nanjing Medical University (Nanjing,
China) between March 2016 and March 2017. The patients included 2
men and 1 woman who developed recurring ameloblastoma, and another
woman who was diagnosed with squamous cell carcinoma. According to
the classification scheme proposed by Urken et al (35), the 2 males were classified as having
a ‘RB’ mandibular defect, 1 female was classified as having a ‘BS’
mandibular defect, and the other female was classified as having a
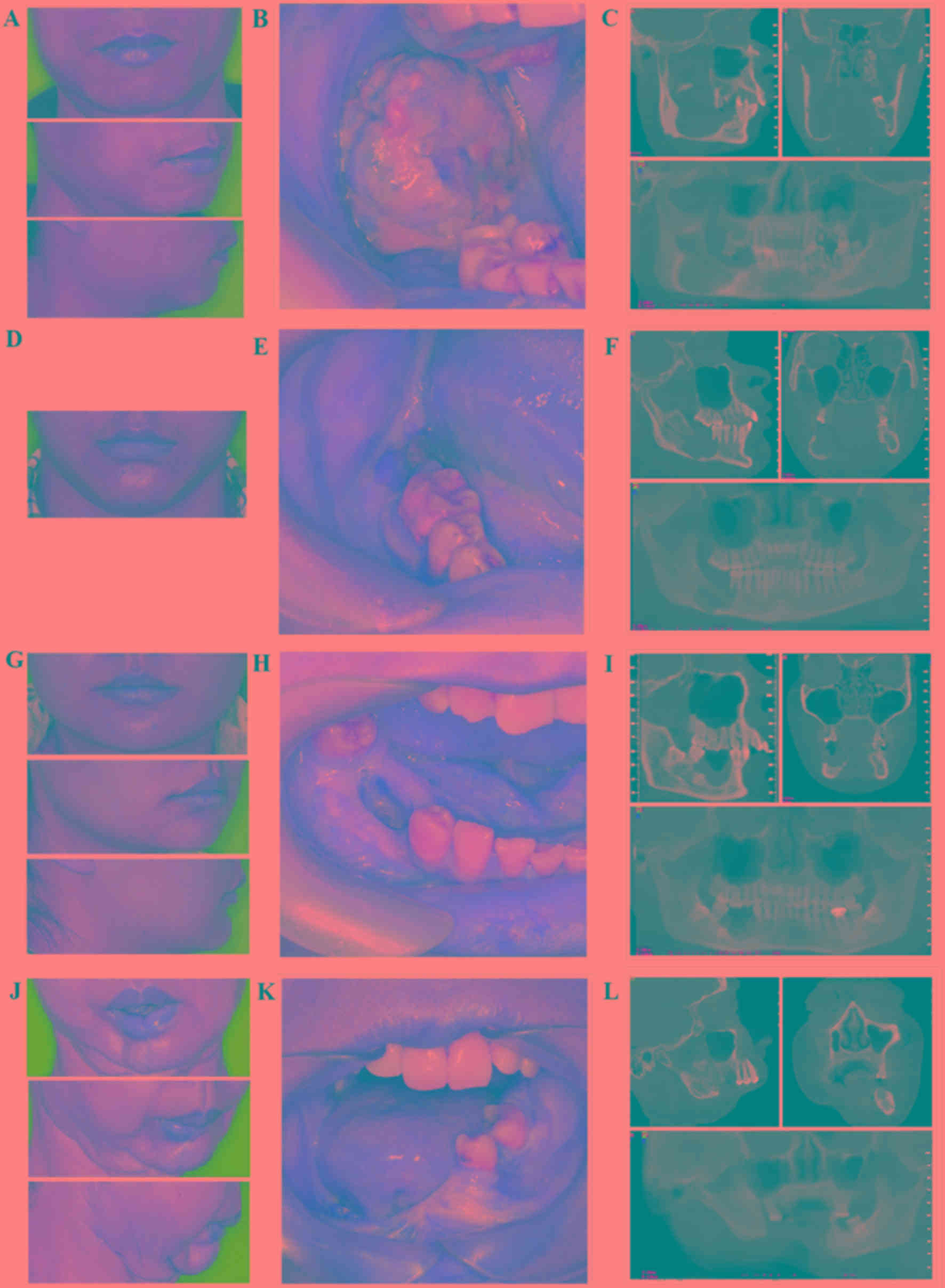
‘BSS’ mandibular defect. Based on CT scans, the 2 male cases
exhibited local invasion in the lower mandibular ridge and the
buccal, and the lingual walls were notably resorbed (Fig. 2A and B). The tumors had spread to the
anterior mandibular ascending branch and the soft tissue of the
posterior molar. The treatment plans included a combination of
mandibular segmental resection and reconstruction following tumor
removal. In the case of the female patient with recurring
ameloblastoma, the tumor had spread to the mandibular canal, and
the treatment plan included a mandibular osteotomy preserving the
lower mandibular ridge (Fig. 2C).
The female case with squamous cell carcinoma involved large
segmental defects within the jaw due to invasion by the gingival
squamous cell carcinoma (Fig. 2D).
No tumor recurrences were observed within 1 year after surgery, and
therefore this patient was recommended for customized titanium
mandibular implantation.
In the present study, the four prospective patients
who received customized titanium implants were included in group A
(Table SI). Of the remaining cases
that were analyzed retrospectively, 6 cases underwent titanium
plate implantation and were included in group B, and 10 cases
underwent vascularized autologous fibular grafting and were
included in group C (Fig. S1 and
Table SI). Group B included 3 males
and 3 females, and the age range was 18–77 years, with a mean age
of 41.5 years. Group C consisted of 8 males and 2 females, and the
age range was 19–65 years, with a mean age of 36.8 years. The
breakdown of the benign oral tumor subtypes is presented in
Figure S1 nad Table SI. Among all cases, mandibular
ameloblastoma was the most common subtype (14 cases), followed by
osteofibroma (2 cases), keratocystic odontogenic tumor (2 cases),
mandibular cyst (1 case), and squamous cell carcinoma (1 case). The
diagnosis of benign oral tumor was confirmed in each case.
3D medical imaging acquisition
Full 3D digital dental models were obtained using
integrating cone-beam computed tomography (CBCT; NewTom VG, SRL
Company). These data were handled, stored and transmitted according
to the standards of Digital Imaging and Communications in Medicine
(36). The geometric data obtained
from the CBCT scans were imported into medical image processing
software, including Materialise's interactive medical image control
system (Mimics®; version 15.0; Materialise) and
Analyze® (Mayo Foundation). Using Mimics®, a
3D digital anatomical model comprising the patient's mandible was
constructed by imaging processing and segmentation. This was
followed by construction of a triangular model of bone structure
from the volume data by performing a series of computed steps,
including region growing, mask formation and 3D model calculation.
The anatomical region of mandibular defects was isolated from the
entire data, and the selected bone structure was imported and
processed using Unigraphics NX (version 10.0; Siemens AG) or
Solidworks (version 2014; Dassault Systèmes) for prosthesis design,
including refinement of the internal porous structures and the
patient-specific 3D mesh structure through topology optimization.
The position and model of the personalized implant was designed and
determined according to the patient's mandible and occlusion
defects. Furthermore, the surgical guide was customized and refined
for each reconstruction. After the corresponding stereolithography
(STL) file was generated, the obtained STL file was imported into
Magics (version 20; Materialize) for data error fixing, positing
the part in the build envelop, and for creating the scaffold and
slicing. Finally, the corrected STL file was sent for production of
implants using Ti6AL4V ELI (Arcam AB; model 2016), which meets the
national standards GB/T 13810 of titanium alloy for surgical
implants in China. The implants were produced by Beijing Aikang
Co., Ltd., and were casted in titanium alloy according to the
design of position during printing. (Fig. 3) The entire design and manufacturing
process of personalized porous titanium alloy implant took ~3
weeks.
Surgery
All patients received pre-operative antiseptic oral
rinsing and treatment was maintained with intravenous antibiotics
(Cefazolin Sodium Pentahydrate Inj Pwd F/Sol; 1 g). For patients in
group A (Figs. 4 and 5), customized titanium implants were
designed using computer-aided software and constructed using an EBM
system (37). Prior to surgery, the
titanium implants were cleaned using ultrasonic vibration and then
sterilized using high temperature steam. During the operation,
osteotomy was performed according to the surgical guide, which
controlled the position and direction of resection. Segmental
resection of the mandible including the benign tumor was removed.
After the customized titanium plate was inserted and fixed with 4–8
titanium screws (10-mm; Stryker Leibinger GmbH & Co.), the oral
tissues were stitched back into place using dissolvable stitches.
Patients received antibiotics after surgery [Cefoxitin, 1 g,
intravenously (i.v.), once a day for 5 days]. For patients in group
B, the mandibulectomy area was measured and confirmed based on µCT
images. The sterilized titanium reconstruction plate was pre-bent
to fit the resected mandible. After the benign tumor was removed,
the prebent titanium reconstruction plate was fixed on residual
bone using 3–4 titanium screws on each side. Patients received
antibiotics after surgery as aforementioned. For patients in group
C, the defects were confirmed by µCT scans. A two-team approach was
used for the surgery; one team focused on resection of the benign
tumor; the other team focused on mandibular reconstruction using
vascularized free fibular flap grafting. Harvesting and contouring
of the fibula free flap took ~120 min. The modelled fibula flap was
placed between the mandibular stumps and fixed to the titanium
plate with multiple screws. Finally, the micro-anastomoses were
constructed and the skin was sutured into its definitive position.
Patients received immediate post-operative special care
(Ceftriaxone, 1 g, IV, once a day for 5 days; Ornidazole, 0.5 g,
IV, twice a day for 3 days) with anti-inflammatory (dexamethasone
sodium phosphate injection, 10 mg, i.v., once a day for 3 days) and
anti-coagulant medications (hemocoagulase injection, 1 KU,
intramuscular injection, once a day for 3 days).
Post-operative outcome assessment
The main clinical outcome was evaluated using
assessment scale and measures referring to the EORTC
QLQ-C30/QLQ-H&N35 questionnaires (38,39), and
the University of Washington Quality of Life questionnaire
(40,41). The items related to head and neck
tumors were selected, and modified to the specific situation to
formulate the prognostic evaluation form (Table SII). The questionnaire contained
seven categories: Facial appearance, occlusal force and chewing,
pain [as determined by verbal rating scale (42)], opening degree, speech,
swallowing/eating and mentality. Each category was given a score.
The total score of seven categories ranged between 7–28, and was
subsequently divided into 4 grades. Higher scores corresponded to
worse symptoms and, consequently, a worse quality of life. The
prognosis was classified into one of four grades (grade I–IV).
Briefly, grade I (score 7–12) was characterized as satisfied facial
appearance, balanced mandibular contour symmetry, normal occlusal
force and chewing efficiency, no temporomandibular joint related
pain and normal maximum mouth opening. Grade II (score 13–17) was
characterized as slightly unilateral facial depression or bulging,
restored occlusal function with chewing efficiency of 60–80%, mild
temporomandibular joint related pain, two finger mouth opening, and
slight difficulty to communicate, eat and socialize. Grade III
(score 18–22) was characterized as unilateral facial asymmetry with
obvious depression or bulging, implant fracture, reduced occlusal
force with chewing efficiency of 40–60%, one finger mouth opening
and a high degree of masticatory difficulty, and difficulty to
communicate, eat and socialize. Grade IV (score 23–28) was
characterized as extreme facial asymmetry with significant
depression or bulging, significantly reduced occlusal force
associated with <40% chewing efficiency, severe pain, severely
limited mouth opening, and considerable difficulty communicating,
eating and socializing, implant removal due to post-operative
infection, implant loosening and tumor recurrence. Patients with a
total score between 7 and 12 were classified as grade I (best
outcome); patients with a total score between 13 and 17 were
classified as grade II; patients with a score between 18 and 22
were classified as grade III; and patients with a total score of 23
and 28 were classified as grade IV (the worst outcome).
Statistical analysis
Associations between groups were analyzed using SPSS
version software 19.0 (IBM Corp.). The post-operative outcomes
among group A, B and C were scored according to prognostic
evaluation (Table SII) and analyzed
using Kruskal-Wallis H non-parametric test followed by Dunn's test
for intergroup comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
In group A, mandibular defects were repaired using
customized titanium implants in patients. The mean operative time
of group A was ~130 min (range, 90–160 min), and the mean length of
follow-up was 12 months (range, 3–19 months). In group A, all
patients had post-operative µCT scans verifying the accuracy of
positioning of customized titanium implants and complete tumor
removal (Fig. 4). The continuity of
the mandible of all 4 patients was restored (Fig. 4). Furthermore, they almost fully
recovered the ability to ingest the same foodstuffs they were able
to ingest prior to surgery, along with normal maximum mouth opening
(25–40 mm) (Fig. 5). Among these 4
patients, 2 males and 1 female had good postoperative dental
restoration (Fig. 5). One female
(case 4) had a significantly shorter follow-up time. This female
patient had a smaller range of maximum opening range due to scar
formation from a previous operation, but the continuity of the
mandibula was restored, and the patient was satisfied with their
facial appearance (Fig. 5). Overall,
postoperative dental rehabilitation was completed with satisfactory
results in all patients in group A. No tumor recurrences were
observed.
According to the criteria on facial appearance,
occlusal force and chewing, pain, opening degree, speech,
swallowing/eating and mentality, the prognosis was classified into
four different grades (Table SII).
Group A patients had improved post-operative outcomes compared with
both groups B and C (Table I). In
group A (n=4), 3 patients were grade I and 1 patient was grade II;
however, groups B and C rarely obtained a grade I clinical outcome
score (Table I). In group B (n=6), 3
patients were grade II, 2 patients were grade III and 1 patient was
grade IV. In group C (n=10), 1 patient was grade I, 5 patients were
grade II, 3 patients were grade III and 1 patient was grade IV. The
outcomes among groups A, B and C were analyzed using Kruskal-Wallis
H non-parametric test, which revealed that post-operative treatment
outcomes were significantly different among the three groups
(P=0.033). Subsequently, the outcomes were compared between two
groups by Dunn's test (Table II).
This demonstrated that operative treatment outcomes were
significantly different between groups A and B (P=0.0191), and
between groups A and C (P=0.0378). However, no significant
difference was identified between groups B and C (P=0.873).
 | Table I.Summary and analysis of
post-operative outcomes among group A, B and C. |
Table I.
Summary and analysis of
post-operative outcomes among group A, B and C.
|
| Four-grade clinical
outcome |
|---|
|
|
|
|---|
| Group | I | II | III | IV |
|---|
| A | 3 | 1 | 0 | 0 |
| B | 0 | 3 | 2 | 1 |
| C | 1 | 5 | 3 | 1 |
|
| Total | 4 | 8 | 5 | 2 |
 | Table II.Statistical analysis of the
post-operative outcomes among groups A, B and C. |
Table II.
Statistical analysis of the
post-operative outcomes among groups A, B and C.
| Group | A | B | C |
|---|
| B | 0.0191 | NA | NA |
| C | 0.0378 | 0.873 | NA |
In summary, patients who underwent mandibular
reconstruction using a customized titanium implant exhibited
improved mandibular contour symmetry, restored occlusal function, a
normal range of mouth opening and no temporomandibular joint
related pain (Table SIII). This was
in contrast to the increased risk of unilateral facial asymmetry,
limited mouth opening, decreased chewing function as well as
implant loosening observed in patients who received one of the
other two conventional approaches (Table SIII).
Discussion
The mandible serves a critical role in several
physiological aspects, including craniofacial growth and
development, mastication, swallowing and breathing (23). Accurate mandibular reconstruction has
always been a challenge (43).
Mandibular defects may result from radioactive osteonecrosis,
trauma, inflammation, oral cavity carcinoma and cysts. The
reconstruction of mandibular defects following surgical extirpation
of oral cavity carcinoma accounts for 80% of clinical cases
(40,41). The goals of mandibular reconstruction
are re-establishment of mandibular continuity and completeness,
correction of mandibular deformity and restoration of mandibular
functions (44). Current trends in
mandibular reconstruction with modern medical applications aim to
take into consideration several factors, including accurate
reconstruction of the normal anatomy, the degree of occlusion and
proper biomechanical compatibility with the surrounding bone
tissues (45).
The use of titanium plates in mandibular
reconstruction has been widely implemented (46–48). The
primary advantages of this approach are: i) Lower-complexity
surgical procedures; ii) reduced fatigue of the metal; and iii)
improved adaptation of reconstruction plates (49,50).
However, disadvantages include, decreased rigidity and fracturing
of titanium plates (51,52). Furthermore, if any radiotherapy
patient has inadequate soft tissue coverage over the operative
site, the individual may be at high-risk for plate protrusion and
thus infection, requiring removal of implant and thus surgical
failure (51,53). Although titanium plate systems for
maxillofacial surgery are currently available from several
different manufacturers, the choice of systems from various
manufacturers always results in differences in clinical outcome.
Several of the placed titanium implants later negatively influence
denture repair (54). Autologous
bone grafting can be categorized as non-vascularized and
vascularized. Non-vascularized autologous bone grafting is less
demanding, less invasive and has fewer common complications
(55–57). However, the major limitation of this
approach is that the implant has no blood supply of its own, which
is followed by severe graft resorption. A notable reduction in bony
height further complicates predictable implant rehabilitation
(58). Furthermore, the bone graft
is avascular making it susceptible to delayed union and infection,
and may result in permanent damage to soft tissues thereby
increasing the chances of failure in patients (59,60). By
contrast, vascularized autologous bone grafting overcomes these
limitations by virtue of its inherent own vasculature and can be
harvested with soft tissues (61).
This explains the increased popularity of vascularized autologous
bone grafting as the standard option (62). Multiple reconstructive options have
emerged for mandibular reconstruction, including iliac crest, rib
and fibula (63). The fibula was
first utilized by Hidalgo et al (64) for mandibular reconstruction in 1989.
Compared with other options, the fibula has gained widespread use,
owing to its versatility by offering great length and thickness of
bone, intraosseous and segmental blood supply, and reliable skin
paddle for simultaneous soft tissue reconstruction (65,66).
Therefore, the use of the fibula for mandibular reconstruction is
well accepted among numerous oral maxillofacial surgeons (62). However, despite its merits,
harvesting and contouring of the fibula demands tremendous skill
and expertise. Furthermore, post-operative facial symmetry is an
important aesthetic aspect, which is very difficult to achieve
(67). Previous studies have
reported that harvesting the fibula increases the risk of
donor-site morbidity, and lowers walking endurance in the
short-term and decrease the ability to do strenuous activity in the
long-term (68,69). Certain patients may also experience
mild lameness, and the loss of bony height always results in
notable challenges to post-operative denture restoration (70). Although there have been some
successes, tissue engineering is not yet ready for mandibular
reconstruction in terms of clinical outcomes. Current obstacles
remain to develop an ideal biocompatible bone repair scaffold that
possesses suitable microstructure. Natural biological materials,
such as chitosan-fibrin and hydroxyapatite, possess excellent cell
adhesion and growth support properties (71,72).
However, these polymers lack mechanical strength and physical
stability, which limit their application in reconstruction of
load-bearing areas of the mandible, and are thus likely to result
in the implant failure (70).
Recent advances in digital imaging and CAD have made
it possible to create customized 3D models from medical images
(73). AM technology, also known as
3D printing, allows the use of patient-specific digital models to
manufacture anatomically-specific 3D mesh titanium scaffolds for
the reconstruction of mandibular defects (74). AM technology offers a new level of
control over the architecture of porosity and hardness of titanium
to match the Young's modulus to that of the bone tissue. As a
result, the resulting titanium product has improved
stress-shielding effects, which may stimulate osteogenesis, thereby
significantly enhancing implant-bone interface stability and
reducing the risk of implant loosening (75). Furthermore, titanium with microporous
structure allows better cell adhesion and increased bone in-growth
to achieve biological fixation (76). 3D printing can build complex porous
features inside the implant to enhance biocompatibility (77), and allows the clinician the
capability to design these implants to aesthetically enhance the
cosmetic outcome. Indeed, improved post-operative denture
restoration was observed in patients with customized mandibular
implants in the present study. When compared with other
conventional approaches, the operative time was reduced. Among all
the treatment groups, group A were the most satisfied in regard to
their facial symmetry, and exhibited the most improved maximum
mouth opening and occlusal function. Furthermore, high-precision 3D
printed titanium alloy implants can be quickly positioned and fixed
during surgery, instead of applying traditional finished
reconstructed titanium plates to repair, it takes less time during
the operation to bend to fit the mandible ends and restore
maxillofacial appearance. Overall, in contrast to other treatment
options, patients who received the customized titanium implant
showed improved post-operative life quality.
The strength of AM technology lies in the areas
where traditional implantation approaches are limited, including
personalization. However, customized implants should be designed
with exacting parameters, which require high-resolution imaging
techniques to optimize the bone-to-implant interface. The design
and manufacture of high-quality customized implants for mandibular
reconstruction are complicated, and require close collaboration
between clinicians and engineers. Although the use of AM technology
in mandibular reconstruction is promising, the widespread
application of this technology is limited by several important
factors, including i) Costly materials and equipment maintenance;
ii) sub-optimal porous structure and mechanical performance; iii)
available techniques for implant surface modification; and iv) low
resistance to clinically relevant cumulative radiation doses in
patients with malignant tumors (78).
In conclusion, 3D printing has been applied in the
medical field, including in complex surgical procedure design,
surgical simulation, surgical guide fabrication, implant design and
manufacturing (79). For the
reconstruction of mandibular defects, treatment options include
reconstruction of titanium plates and vascularized autologous bone
grafts, but each method has its advantages and disadvantages
(80). With advances in 3D printing
technology, it has become possible to manufacture custom titanium
implants for mandibular reconstruction. By obtaining digital image
data of patients through CT scans, it is possible to construct 3D
models, optimize surgical schemes, design and manufacture custom
titanium alloy implants, improve the accuracy of the surgical
procedure, reduce the operation time and improve the effect of
repair and reconstruction (81).
Advancements in AM technology within oral and maxillofacial surgery
are positively affecting the health of these patients. The present
study has elucidated a detailed approach of using customized
titanium implants for reconstruction of mandibular defects.
Patients with customized titanium implants exhibited improved
mandibular contour symmetry and functions, with minimum
post-operative complications. Taken together, the present study has
provided a more detailed picture of development of customized
implants. This will aid future studies to take a more efficient and
feasible route to the manufacture of such products in order to
achieve the implant's desired outcomes and complexity. Based on the
results of the present study, surgeons may be able to formulate
adequate strategies and contingency plans to control post-operative
complications. Notably, this work will lead to heightened
confidence in this emerging AM technology for its application in
oral and maxillofacial surgery.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Priority Academic
Program Development of Jiangsu Higher Education Institutions (grant
no. ZCB2012010).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX and HL contributed to the conception, design and
data acquisition. YX, ZCF, CL and HL contributed to data analysis
and interpretation, and drafted and critically revised the
manuscript. HW, CT and LW contributed to data acquisition and
analysis, and critically revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent for participation in the
study was obtained from participants according to the policy of the
Stomatological Hospital of Jiangsu Ethical Review Board, and the
data were anonymized for analysis and research publication. The
present study was approved by the Stomatological Hospital of
Jiangsu Ethical Review Board (approval no. PJ2016-003-01).
Patient consent for publication
Participants provided informed consent for
publication of identifying information/images in an online
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiménez M, Romero L, Domínguez I, Espinosa
M and Domínguez M: Additive Manufacturing Technologies: An Overview
about 3D Printing Methods and Future Prospects. Complexity.
2019:302019. View Article : Google Scholar
|
|
2
|
Berman B: 3-D printing: The new industrial
revolution. Bussiness Horizons. 55:155–162. 2012. View Article : Google Scholar
|
|
3
|
Truscott M, Beer D, Vicatos G, Hosking K,
Barnard L, Booysen G and Ian Campbell R: Using RP to promote
collaborative design of customised medical implants. Rapid
Prototyping J. 13:82007. View Article : Google Scholar
|
|
4
|
Khan SF and Dalgarno KW: Design of
customised bioceramic medical implants by layered manufacturing.
2009. View Article : Google Scholar
|
|
5
|
Rodby KA, Turin S, Jacobs RJ, Cruz JF,
Hassid VJ, Kolokythas A and Antony AK: Advances in oncologic head
and neck reconstruction: Systematic review and future
considerations of virtual surgical planning and computer aided
design/computer aided modeling. J Plast Reconstr Aesthet Surg.
67:1171–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rengier F, Mehndiratta A, von
Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU and
Giesel FL: 3D printing based on imaging data: Review of medical
applications. Int J Comput Assist Radiol Surg. 5:335–341. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tack P, Victor J, Gemmel P and Annemans L:
3D-printing techniques in a medical setting: A systematic
literature review. Biomed Eng Online. 15:1152016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salmi M, Tuomi J, Paloheimo KS,
Björkstrand R, Paloheimo M, Salo J, Kontio R, Mesimäki K and
Mäkitie A: Patient-specific reconstruction with 3D modeling and
DMLS additive manufacturing. Rapid Prototyp J. 18:209–214. 2012.
View Article : Google Scholar
|
|
9
|
Philippe B: Custom-made prefabricated
titanium miniplates in Le Fort I osteotomies: Principles, procedure
and clinical insights. Int J Oral Maxillofac Surg. 42:1001–1006.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng C, Xing W, Wu Z, Huang H and Huang W:
A combination of three-dimensional printing and computer-assisted
virtual surgical procedure for preoperative planning of acetabular
fracture reduction. Injury. 47:2223–2227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prochor P and Mierzejewska ZA: Influence
of the surface roughness of PEEK GRF30 and Ti6Al4V SLM on the
viability of primary human osteoblasts determined by the MTT Test.
Materials (Basel). 12:41892019. View Article : Google Scholar
|
|
12
|
Vaezi M, Drescher P and Seitz H: Beamless
metal additive manufacturing. Materials (Basel). 13:9222020.
View Article : Google Scholar
|
|
13
|
Chen Q and Thouas GA: Metallic implant
biomaterials. Materials Science Engineering. 87:1–57. 2015.
View Article : Google Scholar
|
|
14
|
Vandenbroucke B and Kruth JP: Selective
laser melting of biocompatible metals for rapid manufacturing of
medical parts. Rapid Prototyp J. 13:196–203. 2007. View Article : Google Scholar
|
|
15
|
Zhou C, Lei F, Chodosh J and Paschalis EI:
The role of titanium surface microtopography on adhesion,
proliferation, transformation, and matrix deposition of corneal
cells. Invest Ophthalmol Vis Sci. 57:1927–1938. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Traini T, Mangano C, Sammons RL, Mangano
F, Macchi A and Piattelli A: Direct laser metal sintering as a new
approach to fabrication of an isoelastic functionally graded
material for manufacture of porous titanium dental implants. Dent
Mater. 24:1525–1533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdelrhman Y, Gepreel MA, Kobayashi S,
Okano S and Okamoto T: Biocompatibility of new low-cost (α+β)-type
Ti-Mo-Fe alloys for long-term implantation. Mater Sci Eng C Mater
Biol Appl. 99:552–562. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ponader S, von Wilmowsky C, Widenmayer M,
Lutz R, Heinl P, Körner C, Singer RF, Nkenke E, Neukam FW and
Schlegel KA: In vivo performance of selective electron beam-melted
Ti-6Al-4V structures. J Biomed Mater Res A. 92:56–62. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parthasarathy J, Starly B, Raman S and
Christensen A: Mechanical evaluation of porous titanium (Ti6Al4V)
structures with electron beam melting (EBM). J Mech Behav Biomed
Mater. 3:249–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murr LE, Amato KN, Li SJ, Tian YX, Cheng
XY, Gaytan SM, Martinez E, Shindo PW, Medina F and Wicker RB:
Microstructure and mechanical properties of open-cellular
biomaterials prototypes for total knee replacement implants
fabricated by electron beam melting. J Mech Behav Biomed Mater.
4:1396–1411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cronskär M, Rännar LE and Bäckström M:
Production of Customized Hip Stem Prostheses: A Comparison Between
Machining and Additive Manufacturing. Rapid Prototyp J. 19:365–372.
2011. View Article : Google Scholar
|
|
22
|
Jardini AL, Larosa MA, Maciel Filho R,
Zavaglia CA, Bernardes LF, Lambert CS, Calderoni DR and
Kharmandayan P: Cranial reconstruction: 3D biomodel and
custom-built implant created using additive manufacturing. J
Craniomaxillofac Surg. 42:1877–1884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Breeland G, Aktar A and Patel BC: Anatomy,
Head and Neck, Mandible. In: StatPearls. (Treasure Island (FL)).
2020.
|
|
24
|
Zhang J, Liu M, Wang L, Chen S, Yuan P, Li
J, Shen SG, Tang Z, Chen KC, Xia JJ and Shen D: Context-guided
fully convolutional networks for joint craniomaxillofacial bone
segmentation and landmark digitization. Med Image Anal.
60:1016212020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee S, Choi D, Shim JH and Nam W: Efficacy
of three-dimensionally printed polycaprolactone/beta tricalcium
phosphate scaffold on mandibular reconstruction. Sci Rep.
10:49792020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alfotawi R and Ayoub A: Reconstruction of
maxillofacial bone defects: Contemporary methods and future
techniques. Am J Adv Med Sci. 2:18–27. 2014.
|
|
27
|
Ansari E, Chargi N, van Gemert JTM, van Es
RJJ, Dieleman FJ, Rosenberg AJWP, Van Cann EM and de Bree R: Low
skeletal muscle mass is a strong predictive factor for surgical
complications and a prognostic factor in oral cancer patients
undergoing mandibular reconstruction with a free fibula flap. Oral
Oncol. 101:1045302020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mesgarzadeh AH, Abadi A and Keshani F:
Seven-year follow-up of spontaneous bone regeneration following
segmental mandibulectomy: Alternative option for mandibular
reconstruction. Dent Res J (Isfahan). 16:435–440. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tatara AM, Koons GL, Watson E,
Piepergerdes TC, Shah SR, Smith BT, Shum J, Melville JC, Hanna IA,
Demian N, et al: Biomaterials-aided mandibular reconstruction using
in vivo bioreactors. Proc Natl Acad Sci USA. 116:6954–6963. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang M, Lin R, Wang X, Xue J, Deng C,
Feng C, Zhuang H, Ma J, Qin C, Wan L, et al: 3D printing of
Haversian bone-mimicking scaffolds for multicellular delivery in
bone regeneration. Sci Adv. 6:eaaz67252020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsukamoto Y, Akagi T and Akashi M:
Vascularized cardiac tissue construction with orientation by
layer-by-layer method and 3D printer. Sci Rep. 10:54842020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Belaid H, Nagarajan S, Teyssier C, Barou
C, Barés J, Balme S, Garay H, Huon V, Cornu D, Cavaillès V and
Bechelany M: Development of new biocompatible 3D printed graphene
oxide-based scaffolds. Mater Sci Eng C Mater Biol Appl.
110:1105952020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pacifici A, Polimeni A and Pacifici L:
Additive manufacturing and biomimetic materials in oral and
maxillofacial surgery: A topical overview. J Biol Regul Homeost
Agents. 32:1579–1582. 2018.PubMed/NCBI
|
|
34
|
Rink B: Mandibular resection in cancer of
the tongue and/or mouth floor. HNO. 39:224–226. 1991.(In German).
PubMed/NCBI
|
|
35
|
Urken ML, Weinberg H, Vickery C,
Buchbinder D, Lawson W and Biller HF: Oromandibular reconstruction
using microvascular composite free flaps. Report of 71 cases and a
new classification scheme for bony, soft-tissue, and neurologic
defects. Arch Otolaryngol Head Neck Surg. 117:733–744. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burgess J: Digital DICOM in dentistry.
Open Dent J. 9:330–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parthasarathy J: 3D modeling, custom
implants and its future perspectives in craniofacial surgery. Ann
Maxillofac Surg. 4:9–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wan Leung S, Lee TF, Chien CY, Chao PJ,
Tsai WL and Fang FM: Health-related quality of life in 640 head and
neck cancer survivors after radiotherapy using EORTC QLQ-C30 and
QLQ-H&N35 questionnaires. BMC Cancer. 11:1282011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tschudi D, Stoeckli S and Schmid S:
Quality of life after different treatment modalities for carcinoma
of the oropharynx. Laryngoscope. 113:1949–1954. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boyapati RP, Shah KC, Flood V and Stassen
LF: Quality of life outcome measures using UW-QOL questionnaire v4
in early oral cancer/squamous cell cancer resections of the tongue
and floor of mouth with reconstruction solely using local methods.
Br J Oral Maxillofac Surg. 51:502–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Viana TSA, Silva PGB, Pereira KMA, Mota
MRL, Alves APNN, de Souza EF and Sousa FB: Prospective evaluation
of quality of life in patients undergoing primary surgery for oral
cancer: Preoperative and postoperative analysis. Asian Pac J Cancer
Prev. 18:2093–2100. 2017.PubMed/NCBI
|
|
42
|
Williamson A and Hoggart B: Pain: A review
of three commonly used pain rating scales. J Clin Nurs. 14:798–804.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dimitroulis G: Mandibular reconstruction
following ablative tumour surgery: An overview of treatment
planning. Aust N Z J Surg. 70:120–126. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bak M, Jacobson AS, Buchbinder D and Urken
ML: Contemporary reconstruction of the mandible. Oral Oncol.
46:71–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang M, Rao P, Xia D, Sun L, Cai X and
Xiao J: Functional reconstruction of mandibular segment defects
with individual preformed reconstruction plate and computed
tomographic angiography-aided iliac crest flap. J Oral Maxillofac
Surg. 77:1293–1304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kumar BP, Venkatesh V, Kumar KA, Yadav BY
and Mohan SR: Mandibular Reconstruction: Overview. J Maxillofac
Oral Surg. 15:425–441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bowerman JE: A review of reconstruction of
the mandible. Proc R Soc Med. 67:610–614. 1974.PubMed/NCBI
|
|
48
|
Gullane PJ and Holmes H: Mandibular
reconstruction. New concepts. Arch Otolaryngol Head Neck Surg.
112:714–719. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schusterman MA, Reece GP, Kroll SS and
Weldon ME: Use of the AO plate for immediate mandibular
reconstruction in cancer patients. Plast Reconstr Surg. 88:588–593.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vuillemin T, Raveh J and Sutter F:
Mandibular reconstruction with the titanium hollow screw
reconstruction plate (THORP) system: evaluation of 62 cases. Plast
Reconstr Surg. 82:804–814. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu YF, Xu LW, Zhu HY and Liu SS:
Technical procedures for template-guided surgery for mandibular
reconstruction based on digital design and manufacturing. Biomed
Eng Online. 13:632014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wikesjo UM, Susin C, Qahash M, Polimeni G,
Leknes KN, Shanaman RH, Prasad HS, Rohrer MD and Hall J: The
critical-size supraalveolar peri-implant defect model:
Characteristics and use. J Clin Periodontol. 33:846–854. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sadr-Eshkevari P, Rashad A, Vahdati SA,
Garajei A, Bohluli B and Maurer P: Alloplastic mandibular
reconstruction: A systematic review and meta-analysis of the
current century case series. Plast Reconstr Surg. 132:413e–427e.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shibahara T, Noma H, Furuya Y and Takaki
R: Fracture of mandibular reconstruction plates used after tumor
resection. J Oral Maxillofac Surg. 60:182–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gadre PK, Ramanojam S, Patankar A and
Gadre KS: Nonvascularized bone grafting for mandibular
reconstruction: Myth or reality? J Craniofac Surg. 22:1727–1735.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Handschel J, Hassanyar H, Depprich RA,
Ommerborn MA, Sproll KC, Hofer M, Kübler NR and Naujoks C:
Nonvascularized iliac bone grafts for mandibular
reconstruction-requirements and limitations. In Vivo. 25:795–799.
2011.PubMed/NCBI
|
|
57
|
Warnke PH, Wiltfang J, Springer I, Acil Y,
Bolte H, Kosmahl M, Russo PA, Sherry E, Lützen U, Wolfart S and
Terheyden H: Man as living bioreactor: Fate of an exogenously
prepared customized tissue-engineered mandible. Biomaterials.
27:3163–3167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Moura LB, Carvalho PH, Xavier CB, Post LK,
Torriani MA, Santagata M and Chagas Júnior OL: Autogenous
non-vascularized bone graft in segmental mandibular reconstruction:
A systematic review. Int J Oral Maxillofac Surg. 45:1388–1394.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mericli AF, Boukovalas S, Rhines LD,
Adelman DM, Hanasono MM and Chang EI: Free Fibula Flap for
Restoration of Spinal Stability after Oncologic Vertebrectomy Is
Predictive of Bony Union. Plast Reconstr Surg. 145:219–229. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rao Janardhan S, Kotrashetti SM, Lingaraj
JB, Pinto PX, Keluskar KM, Jain S, Sone P and Rao S: Anterior
segmental distraction osteogenesis in the hypoplastic cleft
maxilla: Report of five cases. Sultan Qaboos Univ Med J.
13:454–459. 2013.PubMed/NCBI
|
|
61
|
Wu V, Helder MN, Bravenboer N, Ten
Bruggenkate CM, Jin J, Klein-Nulend J and Schulten EAJM: Bone
tissue regeneration in the oral and maxillofacial region: A review
on the application of stem cells and new strategies to improve
vascularization. Stem Cells Int. 2019:62797212019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vidal L, Kampleitner C, Brennan MA,
Hoornaert A and Layrolle P: Reconstruction of large skeletal
defects: Current clinical therapeutic strategies and future
directions using 3D Printing. Front Bioeng Biotechnol. 8:612020.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang MM, Flores RL, Witek L, Torroni A,
Ibrahim A, Wang Z, Liss HA, Cronstein BN, Lopez CD, Maliha SG and
Coelho PG: Dipyridamole-loaded 3D-printed bioceramic scaffolds
stimulate pediatric bone regeneration in vivo without disruption of
craniofacial growth through facial maturity. Sci Rep. 9:184392019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hidalgo DA: Fibula free flap: A new method
of mandible reconstruction. Plast Reconstr Surg. 84:71–79. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Byun SH, Lim HK, Yang BE, Kim SM and Lee
JH: Delayed reconstruction of palatomaxillary defect using fibula
free flap. J Clin Med. 9:8842020. View Article : Google Scholar
|
|
66
|
Hashemi S, Oda M, Onoue K, Basa K, Rubin
SJ, Sakai O, Salama A and Ezzat WH: Determining the optimal
osteotomy distance with the fibula free flap in mandibular
reconstruction. Am J Otolaryngol:. 41:1024362020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lonie S, Herle P, Paddle A, Pradhan N,
Birch T and Shayan R: Mandibular reconstruction: Meta-analysis of
iliac-versus fibula-free flaps. ANZ J Surg. 86:337–342. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Iamaguchi RB, de Moraes MA, Silva GB, Cho
AB, Iwase FDC, Wei TH, de Rezende MR and Mattar R Jr: Is obesity a
risk factor for free vascularized fibular flap complications? Acta
Ortop Bras. 27:192–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Prevost R, Kimakhe J, Diep D, Drouet J,
Benateau H and Veyssiere A: The significance of computer-assisted
surgery in avoiding double-barrel fibula grafts in reconstruction
of the horizontal mandibular ramus. J Stomatol Oral Maxillofac
Surg. 120:167–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bartaire E, Mouawad F, Mallet Y, Milet P,
El Bedoui S, Ton Van J, Chevalier D and Lefebvre JL: Morphologic
assessment of mandibular reconstruction by free fibula flap and
donor-site functional impairment in a series of 23 patients. Eur
Ann Otorhinolaryngol Head Neck Dis. 129:230–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Vedakumari WS, Ayaz N, Karthick AS,
Senthil R and Sastry TP: Quercetin impregnated chitosan-fibrin
composite scaffolds as potential wound dressing
materials-Fabrication, characterization and in vivo analysis. Eur J
Pharm Sci. 97:106–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhao W, Li Y, Zhou A, Chen X, Li K, Chen
S, Qiao B and Jiang D: Controlled release of basic fibroblast
growth factor from a peptide biomaterial for bone regeneration. R
Soc Open Sci. 7:1918302020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yuan X, Xuan M, Tian W and Long J:
Application of digital surgical guides in mandibular resection and
reconstruction with fibula flaps. Int J Oral Maxillofac Surg.
45:1406–1409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Foley BD, Thayer WP, Honeybrook A, McKenna
S and Press S: Mandibular reconstruction using computer-aided
design and computer-aided manufacturing: An analysis of surgical
results. J Oral Maxillofac Surg. 71:e111–e119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pattanayak DK, Fukuda A, Matsushita T,
Takemoto M, Fujibayashi S, Sasaki K, Nishida N, Nakamura T and
Kokubo T: Bioactive Ti metal analogous to human cancellous bone:
Fabrication by selective laser melting and chemical treatments.
Acta Biomater. 7:1398–1406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jia ZJ, Li M, Xiu P, Xu X, Cheng Y, Zheng
Y, Xi T, Wei S and Liu Z: A novel cytocompatible, hierarchical
porous Ti6Al4V scaffold with immobilized silver nanoparticles.
Mater Lett. 157:143–146. 2015. View Article : Google Scholar
|
|
77
|
Bertollo N, Da Assuncao R, Hancock NJ, Lau
A and Walsh WR: Influence of electron beam melting manufactured
implants on ingrowth and shear strength in an ovine model. J
Arthroplasty. 27:1429–1436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Azuma M, Yanagawa T, Ishibashi-Kanno N,
Uchida F, Ito T, Yamagata K, Hasegawa S, Sasaki K, Adachi K,
Tabuchi K, Sekido M and Bukawa H: Mandibular reconstruction using
plates prebent to fit rapid prototyping 3-dimensional printing
models ameliorates contour deformity. Head Face Med. 10:452014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Crafts TD, Ellsperman SE, Wannemuehler TJ,
Bellicchi TD, Shipchandler TZ and Mantravadi AV: Three-dimensional
printing and its applications in otorhinolaryngology-head and neck
surgery. Otolaryngol Head Neck Surg. 156:999–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sweed AH, Bolzoni AR, Kadubiec A,
Beltramini GA, Cherchi A and Baj A: Factors influencing CAD/CAM
accuracy in fibula free flap mandibular reconstruction. Acta
Otorhinolaryngol Ital. 40:138–143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Friedli L, Kloukos D, Kanavakis G,
Halazonetis D and Gkantidis N: The effect of threshold level on
bone segmentation of cranial base structures from CT and CBCT
images. Sci Rep. 10:73612020. View Article : Google Scholar : PubMed/NCBI
|