Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor in children and adolescents globally since
>100 years (1). Although surgery
combined with high-dose drug therapy can improve the survival rate
of patients with OS, a significant number of patients develop fatal
metastatic disease or a serious treatment complication (2). Over the past few decades, despite
increases in the doses of chemotherapy drugs, changes in drug use
times and the use of multidrug combination chemotherapies, the
survival rate of patients with OS has not substantially improved
(3). The use of high dose
chemotherapy drugs has significantly increased side effects in
patients with OS (4). The
development of new and specific treatment options and the discovery
of new biomarkers are therefore necessary to improve treatment
strategies for patients with OS.
2-methoxyestradiol (2-ME; 17β-2-methoxy-est-1, 3,
5(10)-triene-3,17-diol) is a normal metabolite of estradiol in
humans (5). 2-ME is abundant in the
blood and urine of the human body, has certain selective killing
effects on tumor cells and no toxic side effects on normal cells
(6). Numerous studies performed
using cell lines and in animals, demonstrated that 2-ME has a
potent antitumor effect against a number of different types of
tumor (7–10). It has also been reported that 2-ME
has antitumor effects on lung cancer, angiosarcoma, prostate
cancer, colon cancer, melanoma and breast cancer cells (11–16). The
antitumor effect of 2-ME has been widely studied as a promising
anticancer drug. 2-ME exerts a variety of biological effects such
as tumor cell cycle arrest, apoptosis and inhibition of tumor
angiogenesis in cell lines (6). 2-ME
is highly cytotoxic to OS cells, but not cytotoxic to normal
osteoblasts (17). 2-ME may
therefore have potential clinical benefits in the treatment of
tumors, as it inhibits the proliferation of numerous human tumor
cell lines in vitro (18).
Caspase is a protease that promotes apoptosis and is
therefore central to the mechanism of apoptosis (19). Caspase-3 is a frequently activated
death protease that catalyzes the specific cleavage of a number of
key cellular proteins (20).
Caspase-3 is a typical marker of apoptosis and is essential for
apoptosis, chromatin condensation and DNA fragmentation in all cell
types (21). Caspase-3 is also
considered to be the most important scorpion caspase of apoptosis
and can be activated by caspase (22). Cleavage by caspase-3 results in DNA
fragmentation, degradation of the cytoskeleton and nuclear
proteins, cross-linking of proteins, formation of apoptotic bodies,
ligand expression of phagocytic receptors and uptake by phagocytic
cells (23). Caspase-3 is therefore
essential for certain processes associated with the disintegration
of cells and the formation of apoptotic bodies, but it can also
function before the initiation of cell loss (24).
Bcl-2 is an important apoptotic gene and regulatory
protein that can inhibit apoptosis and participate in the
regulation of cell proliferation (25). It plays an important role in
prolonging cell life (26). Bcl-2
activation and abnormal expression is associated with the
occurrence and development of tumors (27). It also plays an important role in the
formation of multidrug resistance in tumors (28). Numerous studies have confirmed that
the Bcl-2 gene is closely associated with the occurrence of a
variety of malignant tumors (29–31). A
study has reported that the Bcl-2 gene is upregulated in the
process of inhibiting apoptosis, greatly increasing the probability
of cell chromosomal mutation, and normal cell infection (32). This could be the key cause of
tumorigenesis in normal cells. Chemotherapy drugs that inhibit
Bcl-2 protein directly or indirectly against the inhibition of
apoptotic proteins increase the sensitivity of chemotherapy drugs
and decrease the occurrence of tumor resistance (19).
Vascularization and nutrition are essential for
tumor growth and progression. VEGF is a member of the angiogenic
factor family, which plays an important role in tumor angiogenesis
by promoting the proliferation and migration of cancer cells
(24). VEGF has been demonstrated to
be one of the most important cytokines regulating angiogenesis
(25). It specifically acts on
vascular endothelial cells, participates in tumor angiogenesis and
is closely associated with biological characteristics such as
invasion and metastasis of tumors (26). Tumor growth is inseparable from
sustained and extensive angiogenesis, and inhibition of
neovascularization of tumors may be an effective method for
treating tumors (27). As the
expression of VEGF is closely associated with angiogenesis and
tumor cell proliferation, inhibiting and blocking the biological
activity of VEGF secreted by tumor cells is important for
suppressing tumor growth and metastasis (28).
In the present study, different concentrations of
2-ME were used to treat MG63 OS cells in vitro and in
vivo to determine the effects on the proliferation, apoptosis
of OS cells. Side effects were also monitored and the potential
mechanism of action of 2-ME and association with other proteins was
investigated. The antitumor mechanism of 2-ME as a treatment for OS
remains unclear and further studies are required for
elucidation.
Materials and methods
Chemicals and reagents
DMEM was purchased from Invitrogen; Thermo Fisher
Scientific, Inc. 2-ME was obtained from Merck KGaA. The TUNEL
assay, western blotting kits, PCR kits and MTT reagent were
purchased from Boster Biological Technology.
Cell lines and cell culture
The MG63 cell line was obtained from the Department
of Central Laboratory, Renmin Hospital of Wuhan University. The
cells were routinely cultured under 5% (v/v) CO2 at 37°C
and 100% relative humidity; in DMEM medium containing 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and
supplemented with 1% penicillin-streptomycin. The incubator was
periodically sterilized.
MTT assay
OS MG63 cells were seeded into 96-well culture
plates at 5×105 cells/ml. The tested concentrations of
2-ME were 0, 10, 20 and 40 µmol/l. There were 6 replicate wells
evaluated at each concentration. Cells were cultured for 24, 36 or
48 h. A total of 20 µl of MTT (5 mg/ml) solution were added 4 h
before the end of culturing and DNA methylation was evaluated after
the addition of 200 µl DMSO. This was followed by shaking to
dissolve the formazan crystals, with color development (A values)
assessed spectrophotometrically (Sunrise; Tecan Group Ltd.) by
measuring the absorbance at 590 nm using an automated microplate
reader. Cell growth inhibition rates were calculated using the
formula: Inhibition rate (%)=(1-experimental group A value/control
group A value) ×100%. All data are the averages of those from 6
independent experiments.
Flow cytometry for cell cycle
analysis
OS MG63 cells were seeded into 96-well culture
dishes at 106 cells/ml and 2-ME added to 0, 10, 20 or 40
µM (6 wells/group). After 2 days of culture, trypsin was added and
the cell suspensions centrifuged (1,000 × g for 5 min at room
temperature). The cells were collected and incubated with RNase A
for 30 min, propidium iodide (PI) reagent (BD Biosciences) was then
added and the cells incubated in the dark for a further 30 min.
Finally, the cells were analyzed by flow cytometry using a
FACSCanto II (BD Biosciences), and data were analyzed using Cell
Quest Pro software version 5.1 (BD Biosciences).
Apoptosis assay
OS MG63 cells were seeded into 6-well plates at
3×105 cells/well and cultured for 12–24 h with different
concentrations of 2-ME (0, 10, 20 or 40 µM). After 24 h, the cells
were digested with trypsin (without EDTA), and the individual cell
suspensions were transferred to flow tubes and centrifuged at 1,000
× g for 5 min at room temperature. The supernatants were discarded,
the cells washed twice, and 875 µl of apoptosis kit buffer, annexin
V-fluorescein isothiocyanate (FITC), and PI solution (Beijing
TransGen Biotech Co. Ltd.) added according to the manufacturer's
protocol. The extent of apoptosis was measured by flow cytometry
using a FACSCanto II (BD Biosciences), and data were analyzed using
Cell Quest Pro software version 5.1 (BD Biosciences). Dual FITC/PI
staining can accurately distinguish cells in different stages of
apoptosis (33).
Western blotting
Cells in the logarithmic phase of growth were
collected and resuspended in DMEM medium for 24 h at 37°C.
Subsequently, 2-ME was added to the cells at different
concentrations, the cells were incubated. Total proteins were
prepared using the Nuclear and Cytoplasmic Protein Extraction kit
(Beyotime Institute of Biotechnology), and the concentration was
determined by a BCA Protein assay kit (Bio-Rad Laboratories, Inc.).
Proteins (40 µg/lane) were separated by 10% SDS-PAGE and
transferred to polyvinylidene fluoride membrane (Bio-Rad
Laboratories, Inc.). Cell debris collected and lysed to extract
proteins. After blocking in 5% skimmed milk for 1 h at room
temperature, the solutions were incubated with primary antibodies
(1:100; cat. no. 1315; Sigma-Aldrich; Merck KGaA) against Bcl-2,
VEGF, caspase-3, or β-actin at 4°C for 16 h, followed by incubation
with an appropriate secondary antibody (1:10,000; cat. no. BA1055;
Boster Biological Technology) conjugated to horseradish peroxidase
at room temperature for 60 min and then the luminescent reagents A
and B from the western blotting kit (Goodbio Technology). The
signal was visualized using enhanced chemiluminescent reagent (cat.
no. KGP1121; Nanjing KeyGen Biotech Co., Ltd.). The membranes were
exposed to X-ray films, which were then developed. β-actin was used
as the reference protein. The expression levels of target proteins
were determined using densitometry (the gray intensities were
derived), and Image J software version 1.0 (National Institutes of
Health) was used for quantitative analysis of the bands.. The
expression levels of target proteins in the control and
experimental groups were statistically compared.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
The PrimeScript RT Reagent kit (Boster
Biological Technology, Ltd.) was used and the samples were
incubated at 37°C for 15 min followed by 85°C for 5 sec and then
maintained at 4°C. Cells in the logarithmic phase of growth were
collected, suspended in fresh medium and different concentrations
of 2-ME added. After 48 h, the cells were collected and 1 ml
TRIzol® reagent (Thermo Fisher Scientific, Inc.) added
to obtain RNA solutions. Each RNA reaction mixture included Taq
enzyme, dNTP mix, loading dye, DNA template, ddH2O, the
reverse transcription reaction product and forward and reverse
primers. The following primers were used: Bcl-2 forward,
5′-CAGGAAACGGCCCGGAT-3′ and reverse, 5′-CTGGGGCCTTTCATCCTCC-3′;
VEGF forward, 5′-GGGAGCAGAAAGCCCATGAA-3′ and reverse,
5′-AGATGTCCACCAGGGTCTCA-3′; caspase-3 forward,
5′-CTCGGTCTGGTACAGATGTCGATG-3′ and reverse,
5′-GGTTAACCCGGGTAAGAATGTGCA-3′; and β-actin forward,
5′-CATTAAGGAGAAGCTGTGCT-3′ and reverse, 5′-GTTGAAGGTAGTTTCGTGGA-3′.
Pre-denaturation was performed for 93°C for 3 min, followed by 36
cycles of 95°C for 5 min, 93°C for 40 sec and 72°C for 60 sec.
β-actin was used as the internal reference gene. Amplification
curves were drawn and the relative mRNA expression levels were
calculated using 2−∆∆Cq (34).
Tumor xenograft growth in nude
mice
BALB/c nude mice (n=32) were purchased from the
Center of Wuhan University; all were male, 4 weeks of age and
weighed 18–24 g, which was the weight at the start of the study.
The animals were raised in specific pathogen free-class sterile
individually ventilated cages. The experimental animal program was
approved by the Experimental Animal Care Committee (approval no.
S01315022I) of Renmin Hospital, Wuhan University (Wuhan, China). OS
MG63 cells (0.1 ml, 1×107) in the logarithmic growth
phase were subcutaneously injected into the right hips of the nude
mice. After 1 week, when all mice had developed subcutaneous
masses, they were randomly divided into 4 groups that received 2-ME
at 0, 10, 20 or 40 mg/kg/day for 30 consecutive days. During drug
administration, the longest diameters (a) and the shortest
diameters (b) of all tumors were measured every 5 days. Tumor
volume was calculated as V=ab2/2 mm3 and
volume curves were drawn. After 30 days of drug administration,
blood was collected from the eyeballs of mice under anesthesia, and
assayed for the serum levels of alanine aminotransferase (ALT),
aspartate aminotransferase (AST), creatinine (Cr) and blood urea
nitrogen (BUN) to evaluate the effects of 2-ME on liver and kidney
function. The mice were then sacrificed by cervical dislocation,
and the subcutaneous tumors isolated and weighed. The main humane
end-point used in the present study was that the tumor burden
should not exceed 10% of the animal's normal body weight.
Immunohistochemical staining
Isolated tumor tissues were fixed in 10%
formaldehyde at 4°C for 24 h, soaked in 100% alcohol for 5 min,
rendered transparent with xylene, embedded in paraffin and
sectioned into 4-µm sections. Staining was performed by dropping
hematoxylin and eosin solutions for 15 min at room temperature on
sections placed under an inverted microscope. The sections were
air-dried, soaked in xylene and peroxidase and then 10% FBS (Wuhan
Servicebio Technology Co., Ltd.) was added dropwise at 37°C for 30
min. Rabbit anti-human primary antibody (1:500; cat. no. 250713;
R&D Systems, Inc.) was added and the slides placed in the
refrigerator at 4°C overnight. Following which rabbit anti-human
secondary antibody (1:1,000; cat. no. sc69786; Santa Cruz
Biotechnology, Inc.) VEGF, Bcl-2 and caspase-3 conjugated to
horseradish peroxidase was added at 37°C for 20 min. The sections
were counterstained with hematoxylin, air-dried, and placed in
xylene to render the tissues transparent for 5 min at 37°C.
Immunohistochemical staining was observed under the light
microscope (IX71; Olympus Corporation; magnification, ×200).
TUNEL staining
In order to detect tumor apoptosis, TUNEL staining
was performed using a TUNEL assay kit according to the
manufacturer's instructions. Following treatment with drugs (TMZ,
300 µM; BKM120, 300 nM) for 24 h and washing with PBS 3 times, the
cells were incubated with stationary liquid (4% paraformaldehyde)
for 30 min at room temperature. The sections were air-dried, soaked
in xylene and peroxidase solution added dropwise. The TUNEL
reaction mixture was also added drop-wise at 37°C for 1 h. After
air drying, the converter-POD reaction solution was added
drop-wise, followed by the 0.05% DAB reaction substrate (drop wise)
for 1 h at room temperature. After hematoxylin staining, the slides
were placed in xylene to render the tissues transparent and the
slides were then sealed with a neutral gum and evaluated under a
fluorescence microscope (magnification, ×100). The nuclei of
apoptotic cells stained brown; such cells were counted in 5 random
fields and apoptosis rates calculated.
Statistical analysis
All data are presented as the mean ± SD. All
statistical analyses were performed using SPSS statistical software
for Windows (version 20.0; IBM Corp). All experiments were repeated
at least 3 times. One-way ANOVA followed by the Tukey's test and
unpaired t-tests were used to evaluate the significance of the
differences between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Morphological observations
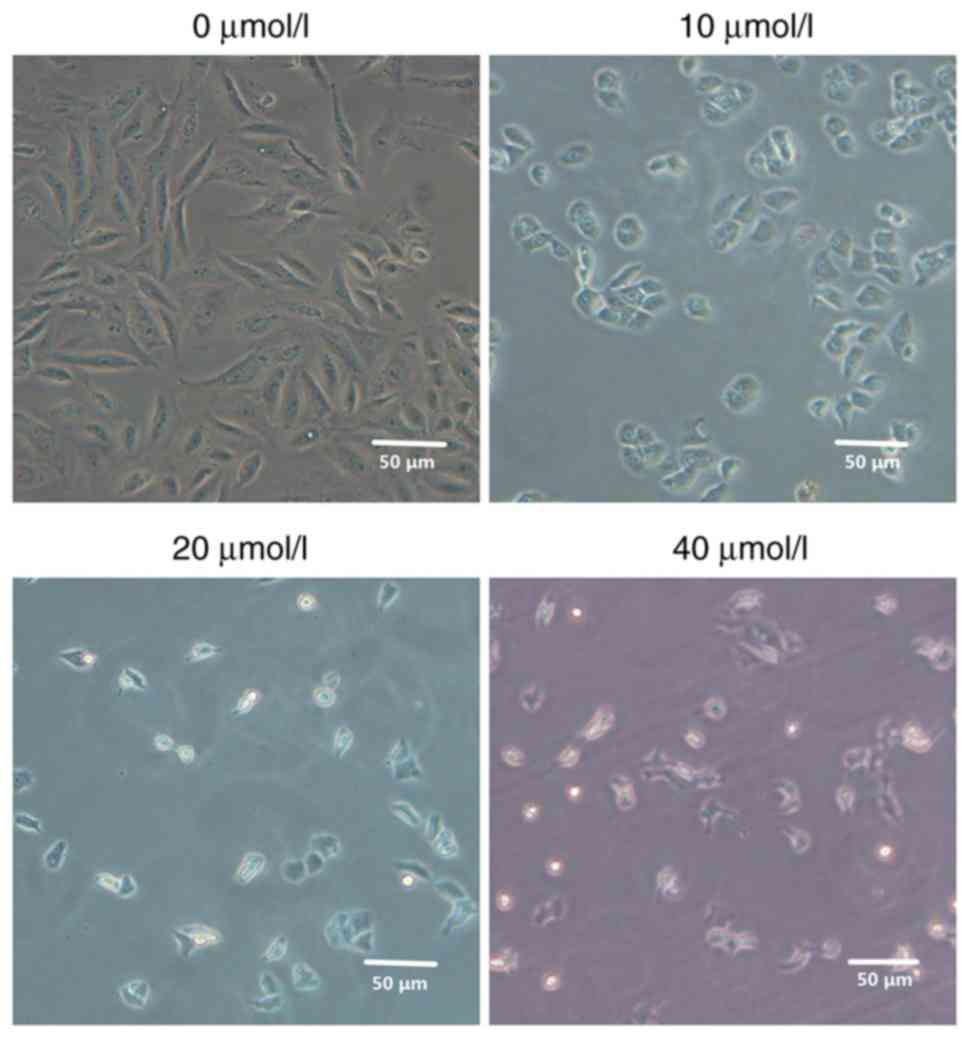
Following treatment of MG63 cells with different
concentrations of 2-ME, the effects on cell morphology were
evaluated. Compared with the control group, MG63 cells exhibited
morphological changes that increased significantly as the 2-ME
concentration increased. Also, the cell density was significantly
decreased and the cells became gradually rounded and shrunken in a
2-ME dose-dependent manner (Fig.
1).
2-ME inhibits OS cell proliferation in
a dose- and time-dependent manner
The effects of 2-ME on OS cell proliferation were
investigated using the MTT assay. Following 24 h of different doses
of 2-ME exposure, cell proliferation was inhibited in a
dose-dependent manner (Fig. 2A).
After 24, 36 or 48 h of drug exposure, 2-ME was observed to inhibit
cell proliferation in a time-dependent manner (Fig. 2B).
2-ME exposure causes cell cycle arrest
and increased apoptosis of MG63 cells
In order to further evaluate the effect of 2-ME
treatment on cell proliferation, flow cytometry was performed. The
results demonstrated that 2-ME affected the MG63 cell cycle. The
majority of the cells were arrested in the
G0/G1 phase of the cell cycle, proportions of
cells in S and G2/M phases of the cell cycle were
decreased (Fig. 3A). Taken together,
these results demonstrated that 2-ME inhibits cell
proliferation.
The effect of 2-ME on apoptosis in MG63 cells was
investigated using Annexin V-FITC/PI double staining followed by
flow cytometry. MG63 cells were exposed to 0, 10, 20 or 40 µM 2-ME
for 24 h and the apoptosis rate was measured. The early and late
apoptosis rates compared to the control gradually increased as
concentration of 2-ME increased from 10 to 40 µM and were
significant (P<0.05; Fig. 3B).
The flow cytometry results demonstrated that 2-ME induced apoptosis
in MG63 OS cells in a dose-dependent manner (Fig. 3B).
2-ME has a dose-dependent effect on
the expression levels of VEGF, Bcl-2 and caspase-3
In order to elucidate the biological mechanisms
involved in the proliferation and apoptotic effects of 2-ME, the
expression levels of VEGF, Bcl-2 and caspase-3 were measured. MG63
cells were exposed to different doses of 2-ME for 24 h, and VEGF,
Bcl-2 and caspase-3 expression levels were assessed via RT-qPCR and
western blotting. The mRNA expression levels of Bcl-2 (Fig. 4B) and VEGF (Fig. 4C) in MG63 osteosarcoma cells
decreased gradually with increasing 2-ME doses, whereas the
expression of caspase-3 (Fig. 4D)
increased gradually; the protein expression levels of Bcl-2 and
VEGF in MG63 osteosarcoma cells decreased gradually with increasing
2-ME doses, whereas the expression of caspase-3 increased gradually
(Fig. 4A), suggesting a
dose-dependent association between Bcl-2, VEGF, caspase-3 and 2-ME
(Fig. 4).
2-ME decreases tumor development in
vivo
MG63 cells were used to establish xenograft models
in vivo and the antitumor activity of 2-ME was evaluated.
Tumor volume was recorded every 5 days during the drug
administration period (Fig. 5A). All
experimental groups representing the different administered doses
of 2-ME exhibited slower growth of transplanted tumors compared
with the control group (Fig. 5A). As
the 2-ME concentration increased, the inhibitory effect became more
potent (Fig. 5A). After 30 days of
drug administration, the mice were sacrificed and the tumor tissues
removed and weighed. The diameter of the largest tumor was 1.02 cm
at the end of the experiment. The tumor weights of all experimental
groups were lower compared with the control group. As the 2-ME
concentration increased, tumor weight increased at a slower rate in
a dose-dependent manner (Fig.
5B).
Pathological features of xenograft
tumors
H&E staining of xenograft tumors revealed that
compared with the control group, the experimental groups
demonstrated different degrees of tumor cell density reduction,
scattered tissue structure (Fig. 6A)
In addition, some cells exhibited nuclear pyknosis and nuclear
lysis, particularly in the group treated with 40 mg/kg of 2-ME,
which demonstrated liquefaction necrosis in the center of the tumor
(Fig. 6A).
In order to investigate the expression levels of
VEGF, Bcl-2 and caspase-3 in vivo, immunohistochemical
staining of xenograft tumors was performed. The expression levels
of Bcl-2 and VEGF in tumor tissues were decreased incrementally
with increasing 2-ME concentrations, whereas the expression levels
of caspase-3 gradually increased (Fig.
6B).
To confirm apoptosis in xenograft tumors, TUNEL
staining was used to demonstrate that the number of apoptotic cells
in the treatment groups were significantly higher than in the
control group. The results also demonstrated that with increasing
2-ME concentrations, the number of apoptotic cells and the degree
of apoptosis gradually increased (Fig.
6C).
2-ME treatment has no liver and kidney
side effects in vivo
In order to investigate the effects of 2-ME on the
liver and kidney functions of OS-bearing nude mice, the serum ALT,
AST, Cr and BUN values were measured. There were no significant
differences between the experimental groups and the control group
(all P>0.05; Table I). Thus, 2-ME
did not impair the liver or kidney functions of nude mice.
 | Table I.Effect of 2-ME on mouse liver and
kidney function. |
Table I.
Effect of 2-ME on mouse liver and
kidney function.
| 2-ME, mg/kg | No. of mice, n | ALT, µ/l | AST, µ/l | BUN, µmol/l | Cr, µmol/l |
|---|
| 0 | 8 | 24.60±2.98 | 107.75±13.46 | 4.82±0.52 | 18.39±4.08 |
| 10 | 8 | 24.43±2.18 | 109.07±14.75 | 5.49±0.69 | 20.25±4.57 |
| 20 | 8 | 24.74±3.43 | 102.18±16.76 | 4.04±0.83 | 19.43±5.45 |
| 30 | 8 | 25.19±2.57 | 111.59±19.51 | 5.35±0.44 | 21.95±4.42 |
Discussion
2-ME inhibits the proliferation of animal and human
tumor cells (35). It is generally
considered that the mechanism of action of 2-ME against cell
proliferation is primarily associated with inhibition of
microtubule function and disruption of normal microtubule function
and stability (36). In the present
study, human OS cell proliferation was inhibited in a
dose-dependent manner following 2-ME treatment, indicating that
2-ME blocks cell proliferation and induces cell cycle arrest and
apoptosis.
In the present study, cell morphology investigations
revealed that with increased concentrations of 2-ME, the number of
OS MG63 cells gradually decreased, and the cell density
significantly decreased. Cells gradually shrunk and became rounded.
Furthermore, the MTT assay demonstrated that 2-ME inhibited the
proliferation of MG63 cells in a time- and dose-dependent manner.
2-ME treatment resulted in an increased number of MG63 cells in the
G0/G1 phase. Using an established nude mouse
xenograft model in vitro, different concentrations of 2-ME
inhibited tumor growth compared with the control group, and there
was a dose-dependent association with 2-ME. The H&E staining
results of nude mice xenografts demonstrated that the experimental
groups exhibited different degrees of tumor cell density reduction
and scattered tissue structure, with some cells exhibiting nuclear
pyknosis and nuclear lysis. Collectively, these results demonstrate
that 2-ME inhibited MG63 OS cell proliferation and xenograft
growth.
2-ME induced both endogenous and exogenous apoptotic
pathways in the present study, this may explain the broad-spectrum
activity of 2-ME and the effects of 2-ME on a variety of cellular
processes, including microtubule destruction, initiation of signal
transduction pathways and production of reactive oxygen species,
ultimately inducing apoptosis (37).
Flow cytometry and TUNEL staining were used in the present study to
detect apoptosis of tumor cells and xenograft tumor tissues,
respectively. The results of the present study demonstrated that
2-ME induced apoptosis of MG63 cells and xenograft tumor cells in a
dose-dependent manner.
In the present study. western blotting and RT-qPCR
demonstrated that with increased 2-ME doses, the expression of
caspase-3 in MG63 OS cells gradually increased and the expression
of Bcl-2 and VEGF gradually decreased, suggesting that the
expression of the aforementioned proteins depended on the dose of
2-ME. Immunohistochemical staining of nude mice xenografts
demonstrated that with increased 2-ME concentrations, the
expression of caspase-3 in tumor tissues gradually increased, and
the expression of Bcl-2 and VEGF gradually decreased. In addition,
apoptosis detected by TUNEL staining of xenograft tumors
demonstrated that 2-ME induced apoptosis of MG63 OS cells and
transplanted tumor tissues. The mechanism of apoptosis may be
associated with Bcl-2 and caspase-3.
In contrast with other chemotherapeutic drugs, 2-ME
has no issues with toxicity, such as gastrointestinal discomfort,
hair loss and leukopenia (38). Even
if the effective therapeutic dose of 2-ME is increased 12-fold,
there has been no report of death due to treatment, only temporary
weight loss and reversible changes in liver function, but no
pathological changes (39). As 2-ME
is loosely bound to estrogen, it does not have the carcinogenic
risk of estrogen (40). In the
present study, measurements of liver and kidney functional
indicators revealed that 2-ME did not impair the liver and renal
function of tumor-bearing nude mice suggesting that the anticancer
activity of 2-ME has no short-term side effects.
A recent study has demonstrated that oleuropein
alone or in combination with 2-ME, has anticancer effects on highly
metastatic 143B OS cells and induces tumor cell autophagy (41). Notably, a synergistic effect between
oleuropein and 2-ME on 143B OS cells was detected (41).
In summary, 2-ME had a strong inhibitory effect on
OS both in vitro and in vivo. The mechanism by which
2-ME exerts its side effects may be associated with VEGF, Bcl-2 and
caspase-3 with little or no side effects, which is of clinical
significance for the treatment of patients with OS. To the best of
our knowledge, no clinical trials on the treatment of OS with 2-ME
have yet been conducted; therefore, further studies are
warranted.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study is available from the corresponding author on reasonable
request.
Authors' contributions
HT conceived and analyzed the experiments, HT, WX,
LJ and XT performed the experiments and analysis. YZ analyzed the
experimental data. HT wrote the manuscript and reviewed it for
intellectual content. FT was involved in drafting the manuscript
and revising it critically for important intellectual content, made
substantial contributions to conception and design and acquisition
of data, gave final approval of the version to be published and
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All authors
have read and approved the manuscript.
Ethics approval and consent to
participate
The animal experiments performed were approved by
the Experimental Animal Care Committee (approval no. S01315022I) of
Renmin Hospital, Wuhan University (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miwa S, Shirai T, Yamamoto N, Hayashi K,
Takeuchi A, Igarashi K and Tsuchiya H: Current and emerging targets
in immunotherapy for osteosarcoma. J Oncol. 2019:70350452019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Yang J, Zhao N, Wang C, Kamar S,
Zhou Y, He Z, Yang J, Sun B, Shi X, et al: Progress in the
chemotherapeutic treatment of osteosarcoma. Oncol Lett.
16:6228–6237. 2018.PubMed/NCBI
|
|
3
|
Teter Z, Śliwczyński A, Brzozowska M,
Świerkowski M, Jacyna A, Pinkas J, Sierocka A, Marczak M,
Dańska-Bidzińska A, Bidziński M and Wierzba W: The assessment of
overall survival (OS) after adjuvant chemotherapy for patients with
malignant endometrial cancer in Poland. Ginekol Pol. 88:296–301.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Ren M, Zhao X, Wang A and Wang J:
Emerging roles of circular RNAs in osteosarcoma. Med Sci Monit.
24:7043–7050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou X, Zhou L, Zhu W, Mao Y and Chen L:
Effectiveness of 2-methoxyestradiol in alleviating angiogenesis
induced by intracranial venous hypertension. J Neurosurg.
125:746–753. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang MZ, Liu YF, Ding N, Zhao PX, Zhang
X, Liu MY, Adzavon YM, Huang JN, Long X, Wang XJ, et al:
2-Methoxyestradiol improves the apoptosis level in keloid
fibroblasts through caspase-dependent mechanisms in vitro. Am J
Transl Res. 10:4017–4029. 2018.PubMed/NCBI
|
|
7
|
Dikshit A, Hales K and Hales DB: Whole
flaxseed diet alters estrogen metabolism to promote
2-methoxtestradiol-induced apoptosis in hen ovarian cancer. J Nutr
Biochem. 42:117–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song LM, Wang GD, Wang HP and Xing NZ:
Effect of 2-methoxyestradiol combined with quercetin on prostate
cancer in vitro. Zhonghua Yi Xue Za Zhi. 96:95–99. 2016.(In
Chinese). PubMed/NCBI
|
|
9
|
Shi X, Wang Z, Xu F, Lu X, Yao H, Wu D,
Sun S, Nie R, Gao S, Li P, et al: Design, synthesis and
antiproliferative effect of 17β-amide derivatives of
2-methoxyestradiol and their studies on pharmacokinetics. Steroids.
128:6–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang SH, Cho HT, Devi S, Zhang Z, Escuin
D, Liang Z, Mao H, Brat DJ, Olson JJ, Simons JW, et al: Antitumor
effect of 2-methoxyestradiol in a rat orthotopic brain tumor model.
Cancer Res. 66:11991–11997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nolte EM, Joubert AM, Lakier R, van
Rensburg A and Mercier AE: Exposure of breast and lung cancer cells
to a novel estrone analog prior to radiation enhances
Bcl-2-mediated cell death. Int J Mol Sci. 19:28872018. View Article : Google Scholar
|
|
12
|
Smolle MA, Leithner A, Posch F, Szkandera
J, Liegl-Atzwanger B and Pichler M: MicroRNAs in different
histologies of soft tissue sarcoma: A comprehensive review. Int J
Mol Sci. 18:19602017. View Article : Google Scholar
|
|
13
|
Zhao ZW, Yang LL, Ji JS, Zheng LY, Fang SJ
and Wang JL: Effects and mechanism ofitraconazole on prostate
cancer PC-3 cell apoptosis. Zhonghua Yi Xue Za Zhi. 96:3160–3163.
2016.(In Chinese). PubMed/NCBI
|
|
14
|
Sun J, Zhang X, Sun Y, Tang ZS and Guo DY:
Effects of hylomecon vernalis ethanol extracts on cell cycle and
apoptosis of colon cancer cells. Mol Med Rep. 15:3485–3492. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee J, Jung MK, Park HJ, Kim KE and Cho D:
Erdr1 suppresses murine melanoma growth via regulation of
apoptosis. Int J Mol Sci. 17:1072016. View Article : Google Scholar
|
|
16
|
van Vuuren RJ, Botes M, Jurgens T, Joubert
AM and van den Bout I: Novel sulphamoylated 2-methoxy estradiol
derivatives inhibit breast cancer migration by disrupting
microtubule turnover and organization. Cancer Cell Int. 19:12019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maran A, Zhang M, Kennedy AM, Sibonga JD,
Rickard DJ, Spelsberg TC and Turner RT: 2-methoxyestradiol induces
interferon gene expression and apoptosis in osteosarcoma cells.
Bone. 30:393–398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo X, Chen C, Liu X, Hou P, Guo X, Ding
F, Wang Z, Hu Y, Li Z and Zhang Z: High oral bioavailability of
2-methoxyestradiol in PEG-PLGA micelles-microspheres for cancer
therapy. Eur J Pharm Biopharm. 117:116–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu J, Zhang W, Zhang Y, Wang Y, Liu M and
Liu Y: Effects of Spica prunellae on caspase-3-associated
proliferation and apoptosis in human lung cancer cells in vitro. J
Cancer Res Ther. 14:760–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nan Y, Wang S and Jia W: Caspase
independent cleavages of TDP-43 generates 35 kD fragment that cause
apoptosis of breast cancer cells. Biochem Biophys Res Commun.
497:51–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grunewald S, Fitzl G and Springsguth C:
Induction of ultra-morphological features of apoptosis in mature
and immature sperm. Asian J Androl. 19:533–537. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choudhary GS, Al-Harbi S and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 1219:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Treffkorn S and Mayer G: Conserved versus
derived patterns of controlled cell death during the embryonic
development of two species of Onychophora (velvet worms). Dev Dyn.
246:403–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rogers C, Fernandes-Alnemri T, Mayes L,
Alnemri D, Cingolani G and Alnemri ES: Cleavage of DFNA5 by
caspase-3 during apoptosis mediates progression to secondary
necrotic/pyroptotic cell death. Nat Commun. 8:141282017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cianciulli A, Porro C, Calvello R, Trotta
T and Panaro MA: Resistance to apoptosis in Leishmania
infantum-infected human macrophages: A critical role for
anti-apoptotic Bcl-2 protein and cellular IAP1/2. Clin Exp Med.
18:251–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masuda H, Hirose J, Omata Y, Tokuyama N,
Yasui T, Kadono Y, Miyazaki T and Tanaka S: Anti-apoptotic Bcl-2
family member Mcl-1 regulates cell viability and bone-resorbing
activity of osteoclasts. Bone. 58:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Villanova L, Careccia S, De Maria R and
Fiori ME: Micro-economics of apoptosis in cancer: NcRNAs modulation
of BCL-2 family members. Int J Mol Sci. 19:9582018. View Article : Google Scholar
|
|
28
|
Alzate JM, Montoya-Florez LM, Pérez JE,
Rocha NS and Pedraza-Ordonez FJ: The role of the multi-drug
resistance 1, p53, b cell lymphoma 2, and BCL 2-associated X genes
in the biologic behavior and chemotherapeutic resistance of canine
transmissible venereal tumors. Vet Clin Pathol. 48:730–739. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ebrahim AS, Sabbagh H, Liddane A, Raufi A,
Kandouz M and Al-Katib A: Hematologic malignancies: Newer
strategies to counter the BCL-2 protein. J Cancer Res Clin Oncol.
142:2013–2022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiu XG, Chen YD, Yuan J, Zhang N, Lei T,
Liu J and Yang M: Functional BCL-2 rs2279115 promoter noncoding
variant contributes to glioma predisposition, especially in males.
DNA Cell Biol. 38:85–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghasemi A, Khanzadeh T, di Heydarabad M,
Khorrami A, Jahanban Esfahlan A, Ghavipanjeh S, Gholipour Belverdi
M, Darvishani Fikouhi S, Darbin A, Najafpour M and Azimi A:
Evaluation of BAX and BCL-2 gene expression and apoptosis induction
in acute lymphoblastic leukemia cell line CCRFCEM after High- dose
prednisolone treatment. Asian Pac J Cancer Prev. 19:2319–2323.
2018.PubMed/NCBI
|
|
32
|
Bui NL, Pandey V, Zhu T, Ma L, Basapp a
and Lobie PE: Bad phosphorylation as a target of inhibition in
oncology. Cancer Lett. 415:177–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Zhao D, Zhuang J, Zhang F and Xu C:
Caspase-8 and caspase-9 functioned differently at different stages
of the cyclic stretch-induced apoptosis in human periodontal
ligament cells. PLoS One. 11:e01682682016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watts FM Jr, Pouland T, Bunce RA, Berlin
KD, Benbrook DM, Mashayekhi M, Bhandari D and Zhou D: Activity of
oxygen-versus sulfur-containing analogs of the Flex-Het anticancer
agent SHetA2. Eur J Med Chem. 158:720–732. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao L, Zou X, Jin C, Fan J, Guo Z, Lin Q,
Li J and Fu D: The roles of ID-1 in human pancreatic ductal
adenocarcinoma and the therapeutic effects of 2-methoxyestradiol.
Carcinogenesis. 39:728–737. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Minorics R and Zupko I: Steroidal
anticancer agents: An overview of estradiol-related compounds.
Anticancer Agents Med Chem. 18:652–666. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lundström-Stadelmann B, Rufener R, Ritler
D, Zurbriggen R and Hemphill A: The importance of being
parasiticidal an update on drug development for the treatment of
alveolar echinococcosis. Food Waterborne Parasitol. 15:e000402019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen Z, Wu Y, Chen X, Chang X, Zhou Q,
Zhou J, Ying H, Zheng J, Duan T and Wang K: Decreased maternal
serum 2-methoxyestradiol levels are associated with the development
of preeclampsia. Cell Physiol Biochem. 34:2189–2199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Caporarello N, Lupo G, Olivieri M,
Cristaldi M, Cambria MT, Salmeri M and Anfuso CD: Classical VEGF,
Notch and ang signalling in cancer angiogenesis, alternative
approaches and future directions (Review). Mol Med Rep.
16:4393–4402. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Przychodzen P, Wyszkowska R,
Gorzynik-Debicka M, Kostrzewa T, Kuban-Jankowska A and
Gorska-Ponikowska M: Anticancer potential of oleuropein, the
polyphenol of olive oil, with 2-methoxyestradiol, separately or in
combination, in human osteosarcoma cells. Anticancer Res.
39:1243–1251. 2019. View Article : Google Scholar : PubMed/NCBI
|