Introduction
Liver cancer is one of the most common malignant
tumors with high incidence and mortality, which are ranked sixth
and third worldwide, respectively, according to the GLOBOCAN
estimates published in 2018 (1).
Hepatocellular carcinoma (HCC) accounts for >90% of all primary
liver cancers and occurs predominantly in patients with underlying
chronic liver disease triggered by host and environmental factors
(2). For example, hepatocellular
adenoma (HCA), a rare benign tumor, possesses a high risk of
transformation into HCC (3). Several
therapeutic regimens and drugs, including surgery, radiotherapy,
chemotherapy as well as traditional Chinese medicine have been
applied at different stages of HCC to increase the survival rates
of patients and improve their quality of life. However, as patients
with HCC are usually asymptomatic during the early stages of the
disease and the progression of HCC is rapid, the majority of
patients are incidentally diagnosed at an advanced stage (4). In addition, the present treatment
strategies for advanced and unresectable HCC require further
development. Therefore, it is necessary to identify novel
therapeutic targets for the treatment of patients with HCC.
MicroRNAs (miRNAs, miRs) are a class of non-coding
RNAs ~22 nucleotides in length that regulate post-transcriptional
gene expression (5) and are widely
involved in various processes of carcinogenesis. It is now
generally accepted that the dysregulation of miRNAs with
tumor-suppressive or oncogenic functions may affect the development
of tumors (6). Li et al
(7) demonstrated that miR-34a acted
as a tumor suppressor in breast cancer via the downregulation of
Bcl-2. In addition, another study revealed that increased miR-21
expression was associated with poor prognosis in patients with
breast cancer, indicating that miR-21 acts as an oncogene in breast
cancer (8). Therefore, miRNAs may
serve as promising biomarkers in the diagnosis, recurrence and
prognosis of human cancers.
There is considerable evidence that miRNAs play
important roles in the development and progression of HCC (9). It has been reported that miR-221 is
markedly upregulated in HCC cells, and its overexpression promotes
the proliferation and invasion of HCC cells (10,11).
Furthermore, Zheng et al (12) demonstrated that the expression levels
of miR-490-3p were significantly lower in HCC and HCA tissues
compared with normal liver tissues. However, the role of miR-490-3p
in HCC has not yet been fully elucidated. Therefore, the present
study aimed to investigate the role of miR-490-3p in HCC
tumorigenesis, which may suggest a novel treatment strategy for
HCC.
Materials and methods
Cell culture
Two human HCC cell lines, namely Huh-7 and HEP
3B2.1-7, were obtained from the American Type Culture Collection.
MIHA immortalized human hepatocytes were purchased from Mingzhou
Biotechnology Co., Ltd. (cat. no. MZ-1157). The cells were
maintained in high-glucose Dulbecco's modified Eagle's medium
(DMEM-H; Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.), and 1% penicillin and streptomycin (100 U/ml). Cells were
cultured at 37°C in an atmosphere of 5% CO2 and 95%
air.
Cell transfection and lentiviral
infection
miRNA-490-3p mimics and negative control (NC) mimics
were obtained from Shanghai GenePharma Co., Ltd.. Huh-7 and HEP
3B2.1-7 cells (5×103/per well) were seeded onto 6-well
plates and cultured at 37°C. When the cells reached 70–80%
confluence, they were transfected with miR-490-3p or NC mimics (10
nM) for 6 h using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following transfection, the medium was replaced with fresh DMEM
(Gibco; Thermo Fisher Scientific, Inc.) and the cells were
incubated for an additional 42 h (For transwell assay, cells were
incubated for an additional 18 h). The sequences were as follows:
miR-490-3p mimics, 3′-GUCGUACCUCAGGAGGUCCAAC-5′; NC mimics,
3′-GGGTTACGATTGCCCAGAT-5′.
The tropomodulin 3 (TMOD3) overexpression plasmid
(pLVX–IRES-Puro-TMOD3) was purchased from Shanghai GenePharma Co.,
Ltd.. Subsequently, 293T cells (American Type Culture Collection)
were infected with 1 µg/µl pLVX–IRES-Puro-TMOD3 or empty vector.
Following infection for 72 h, the lentiviral particles were
collected and concentrated. Huh-7 cells of 60–80% confluence were
seeded onto 6-well plates overnight prior to infection.
Subsequently, cells were infected with the lentiviral particles for
24 h and then the infection medium was replaced with fresh medium.
Puromycin (2.5 µg/ml; Thermo Fisher Scientific, Inc.) was added to
the medium to select stable Huh-7 cells and 48 h following
infection, cells were collected for subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was
then synthesized with the PrimeScript™ RT reagent kit (Takara
Biotechnology Co., Ltd.), according to the manufacturer's protocol.
Subsequently, qPCR was performed using a SYBR® Premix Ex
Taq™ kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. All experiments were carried out in
triplicate and the fold change of gene expression was calculated
using the 2−ΔΔCq method as previously described
(13). The primer sequences used
were as follows: For miR-490-3p, forward,
5′-CAACCTGGAGGACTCCATGC-3′ and reverse,
5′-CTCAACTGGTGTCGTGGAGTC-3′; for U6, forward,
5′-CTCGCTTCGGCAGCACAT-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
for TMOD3, forward, 5′-CCACCAAATCCAACCAATGTAG-3′ and reverse,
5′-TCAGTGCCAGAATCCCAACTC-3′; and for β-actin, forward,
5′-GTCCACCGCAAATGCTTCTA-3′ and reverse, 5′-TGCTGTCACCTTCACCGTTC-3′.
The RT-qPCR conditions used were as follows: 2 min at 94°C,
followed by 35 cycles (30 sec at 94°C and 45 sec at 55°C). U6 and
β-actin were used as the internal controls for miR-490-3p and
TMOD3, respectively.
Cell counting Kit-8 (CCK-8) assay
Cell proliferation was assessed using a CCK-8 assay
kit (Dojindo Molecular Technologies, Inc.) according to the
manufacturer's protocol. Briefly, Huh-7 and HEP 3B2.1-7 cells were
transfected with miR-490-3p or NC mimics for 0, 24, 48 and 72 h.
Then, 10 µl CCK-8 reagent was added to each well and cells were
incubated for an additional 2 h prior measurement of the absorbance
at 450 nm.
Flow cytometric analysis
An Annexin V-FITC/PI Apoptosis kit (Thermo Fisher
Scientific, Inc.) was used to stain apoptotic cells and distinguish
them from normal cells in a subsequent analysis using a flow
cytometer (FACScan; BD Biosciences). Briefly, Huh-7 and HEP 3B2.1-7
cells were washed three times with PBS (Thermo Fisher Scientific,
Inc.) and then fixed with 100 µl binding buffer (Thermo Fisher
Scientific, Inc.). Subsequently, 5 µl propidium iodide and 5 µl
Annexin V-FITC were used to stain the cells for 15 min in the dark.
Finally, cells were analysed with a flow cytometer (Flowjo V10.6.2,
BD Biosciences).
Western blot analysis
Total protein was extracted from cell lines using
RIPA lysis buffer (Beyotime Institute of Biotechnology). Total
protein concentration of the Huh-7 cell extract was determined
using a BCA Protein Assay kit (Sigma-Aldrich; Merck KGaA).
Subsequently, total proteins were separated by 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis and then transferred
onto polyvinylidene difluoride (Thermo Fisher Scientific, Inc.)
membranes. Following blocking with 5% fat-free milk at room
temperature for 1 h, the membranes were incubated with primary
antibodies overnight at 4°C and then washed three times with TBS
supplemented with Tween-20 followed by incubation with a
horseradish peroxidase-conjugated secondary antibody (dilution,
1:5,000; cat. no. ab6721; Abcam) at room temperature for 1 h.
Subsequently, protein bands were visualized using an Enhanced
Chemiluminescence Detection System (Thermo Fisher Scientific,
Inc.). Primary antibodies used in the present study were as
follows: Anti-Bax (dilution, 1:1,000; cat. no. ab32503),
anti-caspase-3 (dilution, 1:1,000; cat. no. ab13847), anti-Bcl-2
(dilution, 1:1,000; cat. no. ab32124), anti-phosphorylated (p)-p38
(dilution, 1:1,000; cat. no. ab170099), anti-p-ERK (dilution,
1:1,000; cat. no. ab229912), anti-p-Akt (dilution, 1:1,000; cat.
no. ab18785), anti-Akt (dilution, 1:1,000; cat. no. ab8805),
anti-p38 (dilution, 1:1,000; cat. no. ab31828), anti-ERK (dilution,
1:1,000; cat. no. ab53277) and anti-β-actin (dilution, 1:1,000;
cat. no. ab8226). All the antibodies were purchased from Abcam. IPP
6.0 (Image-Pro Plus 6.0) was used for the densitometry
analysis.
Transwell assay
The migration and invasion abilities of Huh-7 and
HEP 3B2.1-7 cells were evaluated using 24-well Transwell chambers
(Corning, Inc.). Briefly, a total of 1×104 cells/well
were seeded into the upper chamber containing DMEM without serum.
In addition, DMEM supplemented with 10% FBS was added into the
lower chamber to induce cell migration. Following 24-h incubation,
migratory or invaded cells on the lower surface of the membranes
were fixed and stained with 0.2% crystal violet at room temperature
for 30 min. Photomicrographs of the migratory and invaded cells
were captured using a laser confocal microscope (Olympus CX23;
Olympus Corporation) and the cell number was counted in five
randomly selected fields. For the Transwell invasion assay, the
upper chamber was pre-coated with Matrigel (BD Biosciences).
Luciferase activity assay
TargetScan (http://www.targetscan.org/vert_71/) predicted that
TMOD3 is potential target of miR-490-3p. Therefore, the putative
miR-490-3p binding sequence of wild type (WT) TMOD3 (CCAGGUUA) and
a mutant sequence (MT; GGC GGU C) were synthesized and sub-cloned
into the luciferase reporter plasmid pMIR (Shanghai GenePharma Co.,
Ltd.) to obtain pMIR-WT-TMOD3 and p-MIR-MT-TMOD3 plasmids,
respectively. Subsequently, cells were co-transfected with the
luciferase plasmids (WT or MT) and miR-490-3p or NC mimics using
Lipofectamine 2000. Following 48-h incubation, the luciferase
activity in each group was detected using the Dual-Luciferase
Reporter Assay System (Promega Corporation) with Renilla
luciferase activity serving as endogenous control.
Statistical analysis
Experiments were replicated three times and
statistical analyses were conducted using GraphPad Prism 7 software
(GraphPad Inc.). Results are presented as mean ± standard
deviation. The data were compared using one-way ANOVA followed by
Tukey's test, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of miR-490-3p inhibits
the proliferation of HCC cells
The expression of miR-490-3p in human hepatocytes
and HCC cells was evaluated using RT-qPCR. The data indicate the
level of miR-490-3p was significantly lower in HCC cells compared
with that in human hepatocytes (Fig.
1A). In order to explore the role of miR-490-3p in HCC, Huh-7
and HEP 3B2.1-7 cells were transfected with miR-490-3p mimics. As
shown in Fig. 1B, the expression
levels of miR-490-3p were significantly increased in Huh-7 and HEP
3B2.1-7 cells following transfection with miR-490-3p mimics
compared with the NC group. Subsequently, a CCK-8 assay was
performed to investigate the effect of miR-490-3p on the viability
of the HCC cells. The results reveal that miR-490-3p overexpression
significantly reduced the viability of Huh-7 and HEP 3B2.1-7 cells
(Fig. 1C and D). The aforementioned
results indicate that the overexpression of miR-490-3p inhibited
the proliferation of HCC cells.
Overexpression of miR-490-3p induces
Huh-7 and HEP 3B2.1-7 cell apoptosis
Apoptosis is an important biological process in
cancer cells; therefore, the effect of miR-490-3p mimics on the
apoptosis of HCC cells was investigated. As shown in Fig. 2A and B, flow cytometric analysis
reveal that the apoptosis rates of Huh-7 and HEP 3B2.1-7 cells were
significantly increased following transfection with miR-490-3p
mimics compared with those in the corresponding NC group (Fig. 2A and B). In addition, western
blotting results demonstrate that the levels of the pro-apoptotic
proteins Bax and cleaved caspase-3 were significantly increased,
while the expression levels of the anti-apoptotic protein Bcl-2
were significantly decreased in Huh-7 cells following transfection
with miR-490-3p mimics compared with the NC group (Fig. 2C and D). These results suggest that
the overexpression of miR-490-3p induced the apoptosis of Huh-7
cells.
Overexpression of miR-490-3p
attenuates the migration and invasion of HCC cells
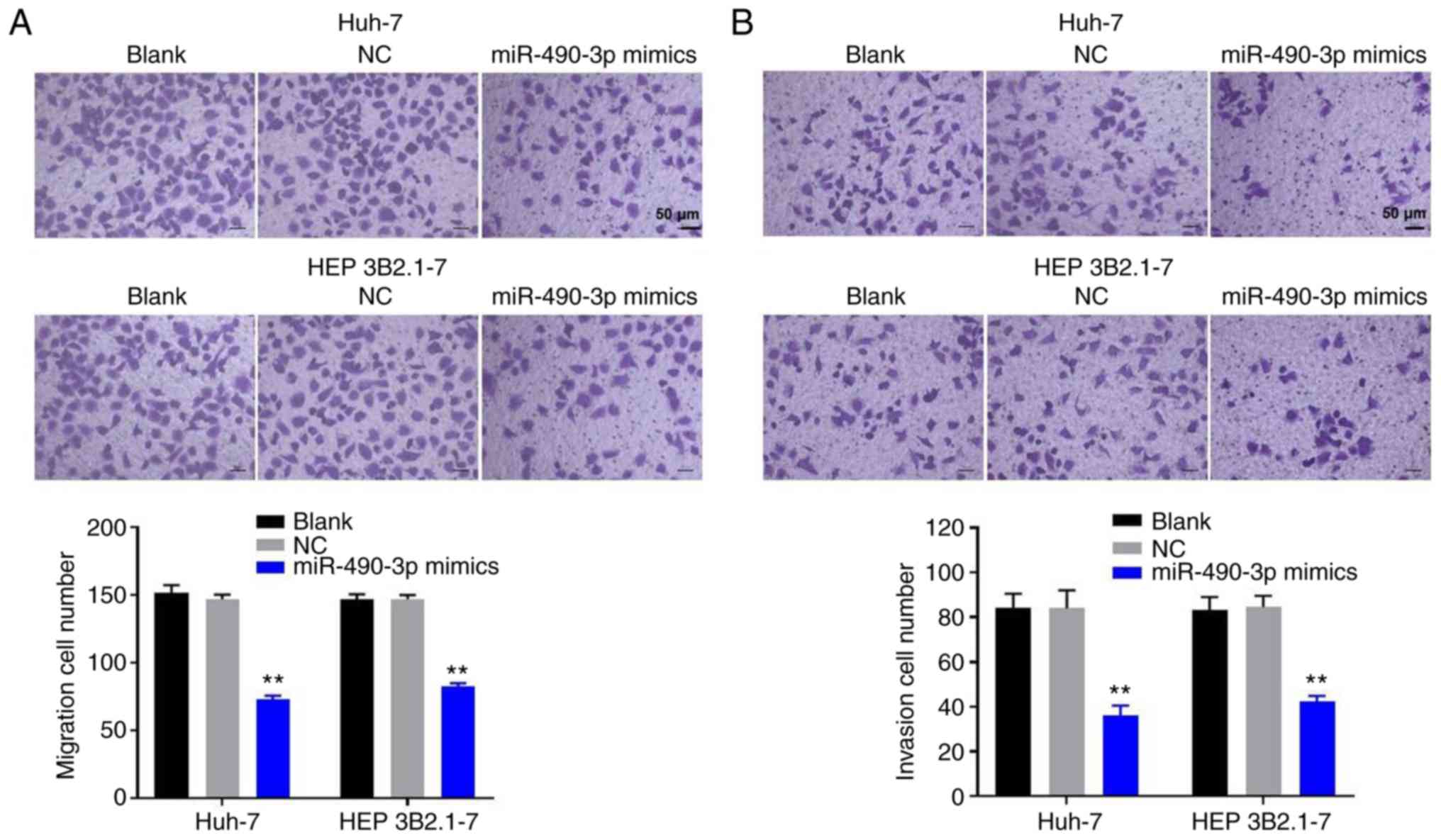
To investigate the role of miR-490-3p in the
migration and invasion of HCC cells, Transwell migration and
invasion assays were performed. The results demonstrate that
miR-490-3p mimics significantly decreased the migration ability of
Huh-7 and HEP 3B2.1-7 HCC cells compared with the respective NC
group (Fig. 3A). In addition, the
Transwell invasion assay indicated that invasion of the HCC cells
was significantly inhibited following transfection with miR-490-3p
mimics (Fig. 3B). The data suggest
that the overexpression of miR-490-3p attenuated the migration and
invasion of HCC cells.
miR-490-3p directly targets TMOD3
To further investigate the mechanisms by which
miR-490-3p affects HCC tumorigenesis, TargetScan analysis was
performed to identify the potential targets of miR-490-3p. The
screening results indicate that TMOD3 is a potential target of
miR-490-3p (Fig. 4A). A dual
luciferase reporter system assay was then used to confirm the
association between miR-490-3p and TMOD3. As shown in Fig. 4B, luciferase activity was
significantly reduced in cells co-transfected with miR-490-3p
mimics and TMOD3-WT compared with that in the corresponding NC
group. However, miR-490-3p mimics did not affect the luciferase
activity of TMOD3-MT. Furthermore, RT-qPCR and western blot assays
showed that miR-490-3p mimics significantly downregulated the
expression levels of TMOD3 in Huh-7 cells compared with those in
the NC group (Fig. 4C-E).
Furthermore, the level of phosphorylation of Akt in Huh-7 cells was
decreased by miR-490-3p mimics (Fig. 4D
and F). These data confirm that TMOD3 is a direct target of
miR-490-3p.
miR-490-3p acts as a tumor suppressor
in Huh-7 cells via the negative regulation of TMOD3
Although the aforementioned results verify that
TMOD3 is a direct binding target of miR-490-3p, the underlying
mechanisms of miR-490-3p in HCC tumorigenesis remain largely
unknown. Therefore, TMOD3 was overexpressed in Huh-7 cells to
further explore the mechanism (Fig.
5A). CCK-8 assay revealed that miR-490-3p mimics significantly
reduced the viability of Huh-7 cells, while this effect was
markedly attenuated following the overexpression of TMOD3 (Fig. 5B). In addition, miR-490-3p
mimics-induced apoptosis was significantly decreased in Huh-7 cells
following TMOD3 overexpression (Fig. 5C
and D). Furthermore, western blot assay demonstrated that
miR-490-3p mimics significantly downregulated the levels of p-p38
and p-ERK in Huh-7 cells, (Fig.
5E-G). Additionally, the miR-490-3p mimics-induced
downregulation of p38 and ERK phosphorylation was significantly
attenuated by TMOD3 overexpression (Fig.
5E-G). These data indicate that miR-490-3p acted as a tumor
suppressor in Huh-7 cells via the negative regulation of TMOD3,
p-p38 and p-ERK.
 | Figure 5.miR-490-3p acts as a tumor suppressor
in Huh-7 cells via the negative regulation of TMOD3. Huh-7 cells
were transfected with TMOD3-OE or/and miR-490-3p mimics for 72 h.
(A) The successful overexpression of TMOD3 in cells following
transfection with TMOD3 OE plasmid was confirmed with reverse
transcription-quantitative PCR. (B) Cell viability was detected
using Cell Counting Kit-8 assay. (C) Apoptotic cells were detected
with Annexin V-PI double staining and flow cytometric analysis. (C)
Representative flow cytometry images and (D) quantified apoptosis
data are shown. (E) Levels of p-p38, p38, p-ERK and ERK in Huh-7
cells were determined using western blotting. (F and G) Relative
levels of p-p38 and p-ERK were quantified via normalization to p38
and ERK, respectively. *P<0.05, **P<0.01 vs. NC group;
##P<0.01 vs. miR-490-3p mimics group. miR, microRNA;
TMOD3, tropomodulin 3; OE, overexpression; NC, negative control;
TMOD3 OE-NC, empty plasmid; p, phosphorylated. |
Discussion
A multitude of miRNAs have already been considered
as therapeutic targets for HCC in preclinical models, thus
providing a credible bench-to-bedside connection. For example,
Gougelet et al (14) showed
that miR-34a exhibited an oncogenic role in liver cancer, as its
downregulation inhibited the proliferation of hepatocytes. In
addition, Pineau et al (15)
demonstrated that miR-221 served an important role in
hepatocarcinogenesis and its overexpression promoted the
proliferation of HCC cells. By contrast, Zheng et al
(12) suggested that the expression
levels of miR-490-3p were significantly downregulated in HCA and
HCC tissues. These findings prompted the further investigation of
the role of miR-490-3p in HCC cells in the present study. The
results indicate that the upregulation of miR-490-3p inhibited the
proliferation and invasion of HCC cells.
It has been reported that miR-490-3p expression is
associated with several types of cancer. Tian et al
(16) demonstrated that miR-490-3p
increased the cisplatin sensitivity of ovarian cancer cells via the
negative regulation of ATP binding cassette subfamily C member 2
expression. In addition, Kang et al (17) revealed an inhibitory effect of
miR-490-3p on esophageal squamous cell carcinoma tissues and cells,
which proceeded via the targeting of high mobility group A2, while
Qu et al (18) found that
decreased levels of miR-490-3p were associated with poor clinical
outcome in Helicobacter pylori-induced gastric cancer.
Furthermore, in another study, upregulated miR-490-3p expression
inhibited the growth and invasiveness of colorectal cancer cells by
inhibiting the expression of the oncogene voltage dependent anion
channel 1 (19). In the present
study, the overexpression of miR-490-3p significantly inhibited the
proliferation, migration and invasion of HCC cells. Furthermore,
the overexpression of miR-490-3p significantly induced apoptosis in
HCC cells via upregulation of the protein expression levels of Bax
and cleaved caspase-3. Overall, consistent with the aforementioned
previous studies, the present data indicate that miR-490-3p may be
an important biomarker in HCC.
It is well documented that miRNAs exert their
biological functions by negatively regulating the expression of
target genes. To investigate the underlying mechanism of miR-490-3p
in the progression of HCC, a TargetScan analysis and luciferase
reporter assay were performed to predict and identify,
respectively, the potential target genes of miR-490-3p. The results
indicate that TMOD3 is a target of miR-490-3p. TMOD3, a unique
tropomodulin, belongs to the pointed-end capping protein family,
which is involved in the regulation of the actin filament structure
in diverse cell types (20). It has
been reported that TMOD3 negatively regulates endothelial cell
migration and serves a crucial role in asymmetric division during
oocyte maturation (21,22). Furthermore, Jin et al
(23) demonstrated that TMOD3
promotes liver cancer progression via activation of the MAPK/ERK
signalling pathway. Consistent with this, Zheng et al
(24) confirmed that the
EGFR-PI3K-AKT signalling pathway is activated by TMOD3 during
hepatocarcinogenesis. Functions of TMOD3 have been observed in
cancers other than liver cancer (25,26). In
a study conducted by Zhan et al (25), an interaction with TMOD3 was found to
be involved in the protumorigenic activity of lysyl oxidase-like 2
in esophageal cancer cells. In another study, conducted by Paul
et al (26), TMOD3 was
identified as a protein associated with etoposide chemoresistance
in non-small cell lung carcinoma cells. To the best of our
knowledge, the present study is the first to reveal the association
between miR-490-3p overexpression and TMOD3 in HCC cells.
In conclusion, the results of the present study
suggested that miR-490-3p inhibits the proliferation and invasion
of HCC cells via the downregulation TMOD3. Therefore, miR-490-3p
may be a potential biomarker and therapeutic target for the
diagnosis and treatment of HCC, respectively.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW, YY, and PG analyzed and interpreted the
experimental data, and were major contributors to the development
of the first draft of the present manuscript. GY designed the
study, analyzed the data, reviewed and approved the final draft of
the manuscript prior to submission. All authors approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wallace MC, Preen D, Jeffrey GP and Adams
LA: The evolving epidemiology of hepatocellular carcinoma: A global
perspective. Expert Rev Gastroenterol Hepatol. 9:765–779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blanc JF, Frulio N, Chiche L, Bioulac-Sage
P and Balabaud C: Hepatocellular adenoma management: Advances but
still a long way to go. Hepat Oncol. 2:171–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Omyła-Staszewska J and Deptała A:
Effective therapeutic management of hepatocellular carcinoma-on the
basis of a clinical case. Contemp Oncol (Pozn). 16:60–63.
2012.PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Yuan L, Luo J, Gao J, Guo J and Xie
X: miR-34a inhibits proliferation and migration of breast cancer
through down-regulation of Bcl-2 and SIRT1. Clin Exp Med.
13:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Z, Zhang X, Wang G and Zheng H: Role
of microRNAs in hepatocellular carcinoma. Hepat Mon. 14:e186722014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garofalo M, Di Leva G, Romano G, Nuovo G,
Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P,
et al: miR-221&222 regulate TRAIL resistance and enhance
tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell.
16:498–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
le Sage C, Nagel R, Egan DA, Schrier M,
Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafrè SA, et
al: Regulation of the p27(Kip1) tumor suppressor by miR-221 and
miR-222 promotes cancer cell proliferation. EMBO J. 26:3699–3708.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng J, Sadot E, Vigidal JA, Klimstra DS,
Balachandran VP, Kingham TP, Allen PJ, D'Angelica MI, DeMatteo RP,
Jarnagin WR and Ventura A: Characterization of hepatocellular
adenoma and carcinoma using microRNA profiling and targeted gene
sequencing. PLoS One. 13:e02007762018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gougelet A, Sartor C, Bachelot L, Godard
C, Marchiol C, Renault G, Tores F, Nitschke P, Cavard C, Terris B,
et al: Antitumour activity of an inhibitor of miR-34a in liver
cancer with β-catenin-mutations. Gut. 65:1024–1034. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pineau P, Volinia S, McJunkin K, Marchio
A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM and
Dejean A: miR-221 overexpression contributes to liver
tumorigenesis. Proc Natl Acad Sci USA. 107:264–269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian J, Xu YY, Li L and Hao Q: miR-490-3p
sensitizes ovarian cancer cells to cisplatin by directly targeting
ABCC2. Am J Transl Res. 9:1127–1138. 2017.PubMed/NCBI
|
|
17
|
Kang NN, Ge SL, Zhang RQ, Huang YL, Liu SD
and Wu KM: miR-490-3p inhibited the proliferation and metastasis of
esophageal squamous cell carcinoma by targeting HMGA2. Eur Rev Med
Pharmacol Sci. 22:8298–8305. 2018.PubMed/NCBI
|
|
18
|
Qu M, Li L and Zheng WC: Reduced
miR-490-3p expression is associated with poor prognosis of
Helicobacter pylori induced gastric cancer. Eur Rev Med
Pharmacol Sci. 21:3384–3388. 2017.PubMed/NCBI
|
|
19
|
Liu X, He B, Xu T, Pan Y, Hu X, Chen X and
Wang S: miR-490-3p functions as a tumor suppressor by inhibiting
oncogene VDAC1 expression in colorectal cancer. J Cancer.
9:1218–1230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamashiro S, Gokhin DS, Kimura S, Nowak RB
and Fowler VM: Tropomodulins: Pointed-end capping proteins that
regulate actin filament architecture in diverse cell types.
Cytoskeleton (Hoboken). 69:337–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fischer RS, Fritz-Six KL and Fowler VM:
Pointed-end capping by tropomodulin3 negatively regulates
endothelial cell motility. J Cell Biol. 161:371–380. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jo YJ, Jang WI, Kim NH and Namgoong S:
Tropomodulin-3 is essential in asymmetric division during mouse
oocyte maturation. Sci Rep. 6:292042016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin C, Chen Z, Shi W and Lian Q:
Tropomodulin 3 promotes liver cancer progression by activating the
MAPK/ERK signaling pathway. Oncol Rep. 41:3060–3068.
2019.PubMed/NCBI
|
|
24
|
Zheng H, Yang Y, Hong YG, Wang MC, Yuan
SX, Wang ZG, Bi FR, Hao LQ, Yan HL and Zhou WP: Tropomodulin 3
modulates EGFR-PI3K-AKT signaling to drive hepatocellular carcinoma
metastasis. Mol Carcinog. 58:1897–1907. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhan XH, Jiao JW, Zhang HF, Xu XE, He JZ,
Li RL, Zou HY, Wu ZY, Wang SH, Wu JY, et al: LOXL2 upregulates
phosphorylation of ezrin to promote cytoskeletal reorganization and
tumor cell invasion. Cancer Res. 79:4951–4964. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paul D, Chanukuppa V, Reddy PJ, Taunk K,
Adhav R, Srivastava S, Santra MK and Rapole S: Global proteomic
profiling identifies etoposide chemoresistance markers in non-small
cell lung carcinoma. J Proteomics. 138:95–105. 2016. View Article : Google Scholar : PubMed/NCBI
|