Introduction
Hepatocellular carcinoma (HCC) is a type of tumor
with a high degree of malignancy and vascular invasion
characteristics, such as the formation of a tumor thrombus (TT) in
the portal vein system obstructing the hepatic inflow tract
(1–3). Data from 2015 indicates that worldwide,
the incidence of TTs in HCC is high (44~62.2%) (4). In contrast, the obstruction of the
outflow vasculature by the formation of a tumor thrombus (TT) in
the hepatic vein (HV), inferior vena cava (IVC), or right atrium
(RA) is rare; according to statistics, the worldwide incidence of
TT in the IVC and RA in cases of advanced HCC in 2010 was range
from 1.4 to 4.9% (2,3,5). The
prognosis of these patients is extremely poor, and the median
survival duration of untreated patients is 2–5 months (6,7). To the
best of our knowledge, there is currently no worldwide consensus
regarding the management of HCC with IVC/RA TTs. Such patients are
classified under C-stage in The Barcelona Clinic Liver Cancer
(BCLC) staging (8) due to vascular
invasion; the standard treatment recommended by this staging system
is the use of sorafenib (9).
However, its clinical benefit remains to be highly controversial.
The reported treatment measures for TT in IVC or RA include
surgery, radiotherapy, chemotherapy, radiotherapy combined with
chemotherapy, intervention, targeted therapy or antiangiogenic
drugs and various comprehensive treatments (including combination
treatments, such as surgery combined with radiotherapy, and
transarterial chemoembolization combined with three-dimensional
conformal radiotherapy) (6,9). It is difficult to obtain satisfactory
results with a single treatment. For now, it is necessary to make
the best treatment decisions for the patient based on the general
condition of the patient; the location, size and number of
intrahepatic tumors; extrahepatic metastasis; classification of
tumor thrombosis; patient will; and the equipment of the treatment
facility, among others (2,6,7). To this
end, the present review assessed the literature for the treatment
of TT in the IVC and RA to provide assistance in clinical
practice.
Clinical manifestations of TT in IVC/RA
The clinical manifestations associated with the TT
are associated with the location of the TT, the stability of the TT
and the level of blockage of the vein. Generally, a stable TT that
incompletely blocks the HV and IVC has no special clinical
manifestation. Such TTs are usually found in imaging studies.
Complete obstruction of the HV and IVC may cause Budd-Chiari
syndrome (10). The patient can
present with manifestations such as varicose veins of the
esophagus, fundus, upper extremity in relation to varicose veins of
bilateral upper limbs, thoracic cavity and abdomen, pleural
effusion, lower extremity edema, tachycardia and difficulty
breathing, syncope, repeated pneumonia and sudden death (6,11).
Electrocardiograms can show a complete right bundle branch block.
The clinical manifestations are primarily associated with
post-hepatic portal hypertension caused by the obstruction of
hepatic venous return, low cardiac output caused by obstruction of
IVC blood flow, and tricuspid obstruction or pulmonary embolism
caused by embolization (6,7). The detachment of the embolus can cause
sudden death (6,7,9).
Pathophysiology
Of the patients with end-stage HCC, 0.7-22.0%
(12) have thrombosis of the IVC,
whereas higher rates and different types of vascular invasions have
been demonstrated in autopsy reports. The worldwide incidence rates
of portal vein invasion, HV invasion, IVC invasion and RA invasion
have been reported to be 26.0-80.0, 11.0-23.0, 9.0-26.0 and
2.4-6.3%, respectively (13–17). TT in the RA may be an isolated TT,
but the IVC TT is more often observed to be extending into the RA.
Anthony (15) found that ~78% cases
of TT in the RA originate from the IVC, and ~25% cases of TT in the
RA are large enough to prolapse to the right ventricle and cause
tricuspid stenosis or insufficiency. Furthermore, 2 cases of TT
were reported to have extended through the patent foramen ovale to
the left atrium; and 1 case of TT was reported to have developed
through lung metastasis to the left atrium. Isolated cardiac TT,
involving RA (8%) and RV (9%) is also not uncommon (15). TT can also occasionally occur in the
left atrium (potentially secondary to seeding through the patent
foramen ovale) and multiple chambers. There have also been cases
reported where isolated TTs in the RA occurred as complications
following hepatectomy (18). As the
tumor cells penetrate the vascular endothelial cells, the invading
cells stimulate the formation of the thrombus; furthermore, TT
provides favorable conditions for the rapid proliferation of tumor
cells (19). After HCC invades the
HV and IVC, TT develops centripetally due to the flow of blood, and
generally does not exceed the level of the renal vein. Both
continuous and discontinuous growth patterns of TT with regard to
the primary tumor have been reported (14). A previous study revealed that the
fastest growth rate of TT was 4.0 cm over 1 month, and the slowest
growth rate was 3.0 cm over 6 months. The average reported growth
rate is 3.7 cm over 3.2 months (20). Tumors in the right hepatic lobe
usually invade the right HV and directly affect the IVC. The left
hepatic lobe tumor first invades the left HV and the middle HV,
then enters the IVC, and finally invades the RA (14,17).
The IVC/RA TT is a specific type of blood-rich TT,
which is also supplied by the arterial branch. Literature that
focuses on this type of TT has stated that its blood supply comes
from the left and right hepatic artery branches, as well as the
left and right phrenic arteries, left gastric artery and
intercostal artery (2,21).
Diagnosis
The diagnosis of TT in HV, IVC and RA depends on
various imaging examinations, such as ultrasound, digital
subtraction angiography (DSA), CT scans and MRI.
Ultrasound
For TT in HV and IVC, gray-scale ultrasound showed a
substantial echo mass in the HV and IVC, sometimes extending to the
RA; color Doppler ultrasound showed HV or IVC blood flow to be
narrow or interrupted; HV and IVC masses showed the same changes as
intrahepatic lesions (i.e., hyperechoic enhancement in the arterial
phase and hypoechoic enhancement in the portal and delayed phases)
on ultrasound angiography; transesophageal ultrasound is important
for making surgical decisions, as it can more accurately determine
the location and classification of TT (22).
DSA
A typical angiography of the abdominal cavity and
hepatic artery exhibits ‘thread and streaks sign’ and ‘asymmetric
dumbbell sign’ for TT in the IVC or RA (20). TT in the IVC or RA is associated with
intrahepatic lesions through the HV. In such cases, the patients
often present with different degrees of hepatic artery-HV shunts,
which is also one of the main causes of poor transcatheter arterial
chemoembolization (TACE) treatment effectiveness and poor lipiodol
deposit of tumor and TT (20).
CT
Plain CT scans demonstrate a low-density or
iso-density mass, with a CT value of 26–52 HU, which is equal to
the density of the cardiac tissue. Although a low-density line is
observed at the edge of the TT, distinction is difficult. Enhanced
CT shows a filling defect in the invaded vascular lumen, which can
extend up to the RA; the vascular lumen is observed to be
irregularly narrow and locally compressed or surrounded by the
tumor. As enhanced CT for IVC TT can manifest as undeveloped
inferior vena cava, it needs to be distinguished from the early
stage of the portal vein or when the IVC is compressed. In general,
patients with suspected TT in the HV or IVC must also undergo a
5-min delayed CT scan to confirm the diagnosis and eliminate any
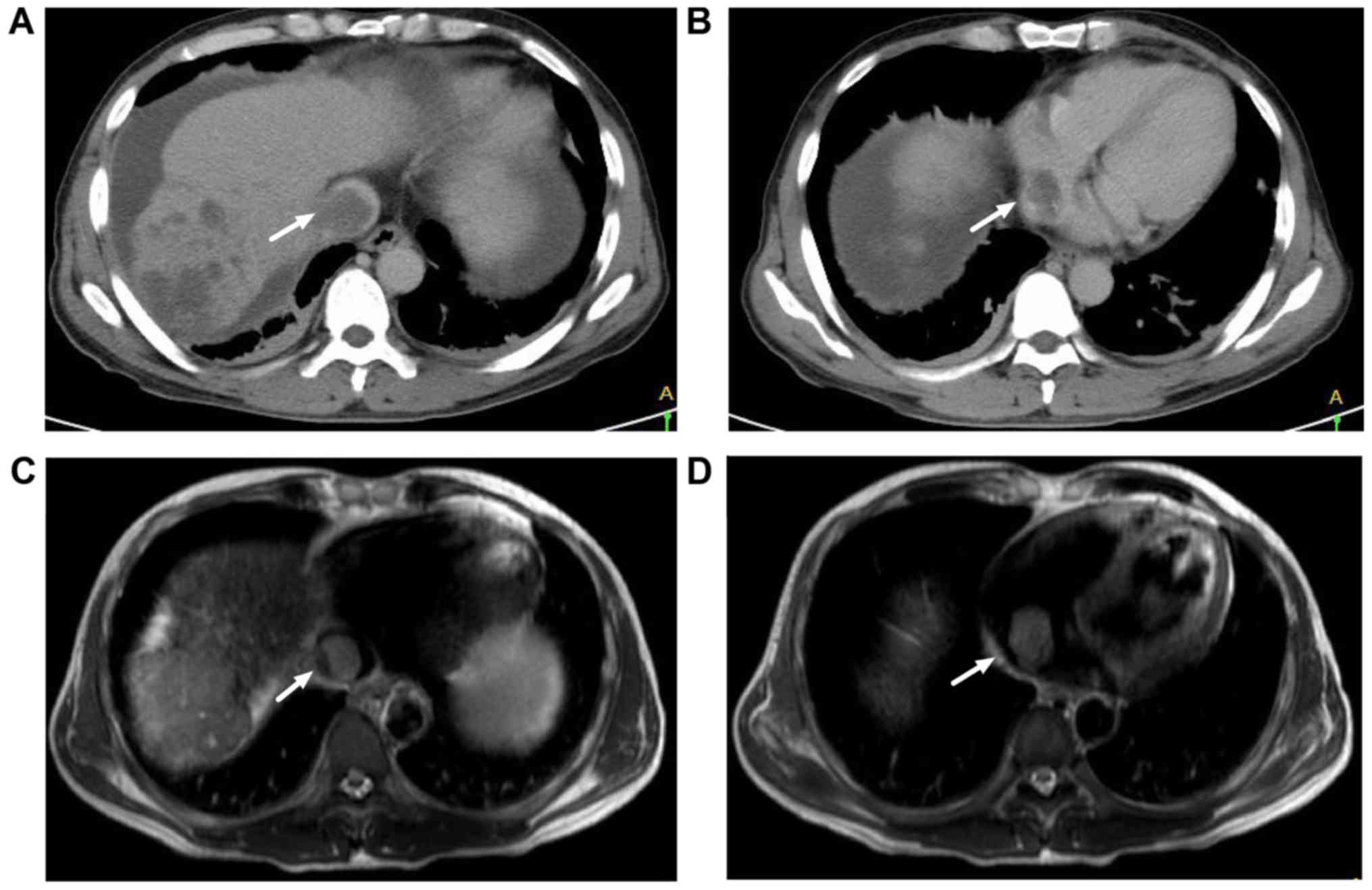
misinterpretation caused by uneven iodine contrast agent (20). Representative CT findings of HCC with
IVC TT and RA TT are presented in Fig.
1A and B.
MRI
TT in the IVC and RA observed via MRI was clearer
compared with that observed via the CT scan, and imaging in the
coronal plane demonstrated the location and length of the TT, which
provided the necessary basis for developing a surgical plan.
T1-weighted imaging showed a low signal block of TT in the HV and
IVC cavity. T2-weighted imaging manifested as a solid high-signal
block of TT in the cavity owing to the flowing void effect of the
HV and IVC. The intrahepatic nodule also showed a high signal.
Furthermore, scanning with enhanced arterial phase showed
mild-to-moderate inhomogeneous abnormal enhancement of the block in
the IVC, that with enhanced portal vein phase showed a decreased
degree of block shadow enhancement, and the delayed phase showed
slight enhancement. The intrahepatic lesions were consistent with
those of TT in the IVC (20).
Representative MRI findings of HCC with IVC TT and RA TT are
presented in Fig. 1C and D.
Treatment
Previous treatments for HCC with TT in the IVC and
RA have been conservative, and the majority of clinical treatments
include best supportive care. However, with the improvement in the
current understanding of the disease and the investigation into
associated active treatment measures, an increasing number of
clinicians have recognized the necessity of active treatment. The
characteristics and clinical results of these studies are
summarized in Tables I–IV. Chun et al (6) first studied the differences in survival
time between patients treated with the best supportive palliative
care (including the control of tumor-related symptoms,
psychological counseling and spiritual help) and those undergoing
active treatment [mainly including chemotherapy (TACE), surgery and
radiotherapy, among others], with a median survival time of 2 and 4
months, respectively. Although the difference is small, it was
still statistically significant.
 | Table I.Active surgical treatment outcome for
hepatocellular carcinoma with tumor thrombus in the inferior vena
cava or right atrium. |
Table I.
Active surgical treatment outcome for
hepatocellular carcinoma with tumor thrombus in the inferior vena
cava or right atrium.
| Author, year | Patients, n | Extent of
resection | Mortality, % | MST, months | 1-year OS, % | 3-year OS, % | (Refs.) |
|---|
| Wang et al,
2013 | 25 | R0:25 | 0.0 | 19.0 | 68.0 | 22.5 |
(7) |
| Li et al,
2013 | 13 | NA | 0.0 | 22.0 | 52.1 | 25.1 | (26) |
| Wakayama et
al, 2013 | 13 | R0:5 | 0.0 | 30.8 | 80.0 | 30.0 | (29) |
|
|
| R1/2:8 |
| 10.5 | 29.2 |
|
|
| Kokudo et
al, 2014 | 13 | R0:9 | 7.6 | 16.7 | 76.9 | 15.4 | (36) |
|
|
| R1/2:4 |
|
|
|
|
|
| Kokudo et
al, 2017 | 71 | NA | 9.9 | 16.4 | 63.2 | 33.1 | (37) |
| Liu et al,
2012 | 65 | NA | 0.0 | 17.0 |
|
| (62) |
 | Table IV.Characteristics of included
trails. |
Table IV.
Characteristics of included
trails.
| A, Surgical |
|---|
|
|---|
| Author, year | Country | Mean age ± SD
(range), years | Male, % | PVTT, % | Extrahepatic mets,
% | AFP, µg/l
(%)a | Child-Pugh
classification, A/B | Previous
treatment | Combined
modality | HBV infection,
% | Tumor size ± SD, cm
(%)b | (Refs.) |
|---|
| Wang et al,
2013 | China | 48.5±11.6 | 96.0 | 48 | NA | >1,000.0
(68.0) | 100/0 | NA | TACE, RT | 100 | >10.00
(88.0) |
(7) |
| Li et al,
2013 | China | 49.7 (35–72) | 84.6 | NA | NA | NA | 100/0 | RT | NA | NA | ≥10.00 (69.2) | (26) |
| Wakayama et
al, 2013 | Japan | 63.4±11.8
(37–86) | 92.3 | NA | 61.5 | NA | 100/0 | NA | TACE, RT | 53.8 | 11.8±4.3 | (29) |
| Kokudo et
al, 2014 | Japan | 61.8
(57.4-66.2) | 77.0 | NA | 76.9 | 22,812.0 | 85/15 | NA | Surgery, TACE | 46 | 8.79 | (36) |
| Kokudo et
al, 2017 | Japan | NA | NA | NA | NA | NA | 100/0 | NA | NA | NA | NA | (37) |
| Liu et al,
2012 | China | 52.6±4.6 | 83.1 | NA | 0.0 | 391.8 | NA | NA | TACE, RFA | NA | ≥10.00 (50.8) | (62) |
|
| B, RT |
|
| Author,
year | Country | Mean age ± SD
(range), years | Male, % | PVTT, % | Extrahepatic
mets, % | AFP, µg/l
(%)a | Child-Pugh
classification, A/B | Previous
treatment | Combined
modality | HBV infection,
% | Tumor size ± SD,
cm (%)b | (Refs.) |
|
| Koo et al,
2010 | Korea | 54±8 | 90.5 | 45.2 | NA | >1,000
(47.60) | 61.9/38.1 | NA | TACE | 83.3 | 10.0±4.0 | (47) |
| Igaki et al,
2008 | Japan | 70 (45–81) | 88.9 | NA | NA | >1,000
(16.70) | 44.4/55.6 | Surgery, TACE, PEI,
RFA | NA | NA | NA | (63) |
| Hou et al,
2012 | China | NA | NA | NA 100 | NA | NA | NA | Surgery, TACE
Surgery, TACE | NA | NA | NA | (43) |
| Komatsu et
al, 2011 | Japan | 68.5 (45–83) | 75 | 58.8 | NA | >1,000
(31.25) | 75/18.75 | NA | RFA in 1
patient | NA | NA | (42) |
|
| C, TACE |
|
| Author,
year | Country | Mean age ± SD
(range), years | Male, % | PVTT, % | Extrahepatic
mets, % | AFP, µg/l
(%)a | Child-Pugh
classification, A/B | Previous
treatment | Combined
modality | HBV infection,
% | Tumor size ± SD,
cm (%)b | (Refs.) |
|
| Chung et al,
2014 | Korea | 56.5±10.6 | 82.3 | 79 | 37 | >400 (74.2) | 76.2/23.8 | NA | NA | NA | ≥10.0 (64.5) | (46) |
| Chern et al,
2009 | China | 57 (34–74) | 76.9 | 57.7 | NA | >400 (46.1) | NA | NA | NA | 80.8 | 10.9 | (45) |
| Koo et al,
2010 | Korea | 51±12 | 89.7 | 51.7 | NA | >1,000
(51.7) | 58.6/41.4 | NA | NA | 75.9 | 12.9±3.8 | (47) |
| Kim et al,
2013 | Korea | 55.3±9.4 | 90 | 52 | 35 | >200 (67.0) | 100/0 | NA | NA | 83.0 | ≥10.0 (60.0) | (64) |
|
|
| 53.7±9.6 | 89 | 66 | 43 | >200 (81.0) | 100/0 | NA | NA | 85.0 | ≥10.0 (60.0) |
|
| Liu et al,
2012 | China | 50.9±5.4 | 82 | NA | 0 | 383.3 | NA | NA | Chemotherapy
Systemic | 82.0 | >10.0
(54.0) | (62) |
Surgical treatment
HCC with TT in the IVC and RA is generally
considered to be an advanced cancer, with patients generally being
in poor condition, and may be associated with systemic multiple
metastases, making surgical treatment more difficult and limiting
patient survival time. However, with continuous advancements in
surgical technology and an increase in the current understanding of
such TTs, aggressive treatments, particularly including surgical
treatment for selected patients, have been conducted at the most
advanced Asian medical centers, which can prolong the survival time
of patients and improve their quality of life.
Previous studies have reported a median survival
time of 7–8 months after surgery (23,24), but
in recent years, with the improvement in surgical techniques,
preoperative interventions, and comprehensive postoperative
treatment interventions, the median survival time of patients
undergoing surgery has markedly improved, with studies reporting a
survival time of 10.5-30.8 months (7,25). Wang
et al (7) retrospectively
analyzed the treatment outcome of 56 patients with advanced HCC.
The 1-, 3- and 5-year survival rates in the surgical group were
68.0, 22.5 and 13.5%, respectively, with a median survival time of
19 months. In contrast, the 1- and 3-year survival rates in the
TACE group were 15 and 5%, respectively, with a median survival
time of 4.5 months. Thus, the survival rate in the surgical group
was significantly higher than that in the TACE group.
Li et al (26)
reported a classification of HCC with TTs in the IVC/RA to serve as
a guide for surgical treatment, wherein based on the anatomical
location of the TT relative to the heart, it was divided into three
types: Type I, TT is in the IVC below the diaphragm; Type II, the
TT extends above the diaphragm but outside the heart; and Type III,
the TT extends into the right atrium.
Type I TT can be completely removed with the primary
intrahepatic lesion in case of complete hepatic blood flow
blockage. First, the hepatic inflow vasculature is blocked and the
IVC is clamped under the diaphragm. Subsequently, the invaded HV
and IVC are cut longitudinally by the naked eye, and the primary
intrahepatic lesion and the TT are both removed by the naked eye.
Finally, the IVC is washed and the wall is sutured.
Type II TT is extended into the thoracic cavity, but
does require a median sternotomy and thoracotomy (27,28).
This TT can be removed by making an incision in the diaphragm
anterior to the IVC TT, exposing the TT above the diaphragm.
Subsequently, the hepatic blood inflow is blocked and the blood
vessels are clamped on top of the TT, following which, the tumor
and the TT are removed under direct vision. Finally, the IVC wall,
the pericardium and the diaphragm are sutured.
When the TT extends into the RA, a combination of
cardiothoracic and hepatobiliary surgery is required for
hepatectomy and RA TT resection. To remove the TT extending into
the RA, a thoracic surgeon requires surgery under extracorporeal
circulation followed by a laparotomy, which requires a sternotomy.
Following hepatic transection, the superior vena cava and IVC
thrombus are clamped and blood flow is bypassed to the ascending
aorta after oxygenation ex vivo; the RA is incised and the
TT is excised en bloc under direct vision.
Given the high risk of surgery, strict patient
selection measures must be taken. The surgery should be performed
only for those with Child-Pugh A HCC (29). Postoperative failure is mainly
observed in the form of local recurrence and distant metastasis.
Some complications associated with surgery include heart failure,
respiratory failure, infection and pulmonary embolism. Prevention
of postoperative recurrence is the focus of postoperative
management in HCC with TT in the IVC and RA. Measures to prevent
postoperative recurrence include postoperative oral sorafenib
administration (30–32) and adjuvant TACE (33,34).
Radiation therapy
With the advancement of radiotherapy, it is possible
to increase the dose to the target volumes, excluding
radiosensitive organs such as the stomach, small intestine, kidneys
and spinal cord, which can tolerate lower radiation doses, using
three-dimensional conformal radiotherapy (3DCRT), three-dimensional
conformal intensity-modulated radiotherapy, stereotactic
radiotherapy (SBRT) (35), and
particle radiotherapy. Both external and internal irradiation are a
treatment option for patients with HCC exhibiting TT, particularly
for patients who cannot receive, or are unwilling to receive
surgery (29,36,37).
Radiation therapy is particularly suitable when the tumor is
located on top of the liver, where the lesion cannot be detected by
ultrasound, or when the lesion is located near a large blood
vessel, making thermal ablation impossible (38).
A meta-analysis and systematic review of external
radiotherapy for the treatment of the TT in the IVC or RA in 2018
(39) showed that the median total
radiotherapy dose was 48–60 Gy, with a median survival time of 13.2
months (range, 5.6-25.4 months), 1-year overall survival (OS) rate
of 53.6% [95% confidence interval (CI), 45.7-61.3%], 2-year OS rate
of 36.9% (95% CI, 29.8-44.8%), total effective rate of 59.2% (95%
CI, 39.0-76.7%), and disease-free survival rate of 83.8% (95% CI,
64.5-93.7%). Furthermore, a study by Matsuo et al (40) included 87 patients, and the total
dose in 43 patients in the SBRT group was 45–55 Gy/10-15 f. The
efficiency and 1-year OS rates in the SBRT group were 67.0 and
49.3%, respectively. In the 54 patients enrolled in the 3DCRT
group, the total dose of planning target volume was 45–50 Gy/15-25
f and the effective rate and 1-year survival rate were 46.0 and
29.3%, respectively.
Komatsu et al (25) compared the efficacy between new
proton radiotherapy (21 patients) and surgical resection (19
patients) in the treatment of HCC with TT in the IVC. The study
found that for stage IIIB patients [All patients were staged
according to the Union for International Cancer control/American
Joint Committee on Cancer TNM staging system, 7th edition (41)], proton radiotherapy has a significant
survival advantage. The median survival time in the two groups was
748 and 272 days, respectively. For stage IV patients, no
significant difference in survival rates was observed. Furthermore,
Komatsu et al (42)
investigated the effectiveness of proton radiotherapy for patients
with HCC exhibiting TT in the IVC. The 1- and 3-year survival rates
of 16 patients undergoing proton radiotherapy were 61.1 and 36.7%,
respectively. The survival time was 24.2 months.
The designation of the irradiation area is still
controversial, and it should be determined individually. The
majority of studies support the use of radiotherapy when only
including the TT, and if the primary tumor is close to the TT and
the lesion is small, which may include the primary tumor. The dose
of radiotherapy is associated with the choice of radiotherapy
technique and the purpose of the radiotherapy. Currently, an
optimal radiotherapy dose is under debate, and retrospective
studies that have focused on this have shown that the high dose of
radiotherapy is positively associated with prognosis.
There is also some correlation between the efficacy
of radiotherapy and the location of TT. Hou et al (43) retrospectively studied 181 patients
with HCC undergoing external radiation therapy (EBRT). The median
radiotherapy dose in their study was 50 Gy. It was revealed that
the median survival times of patients with portal vein, portal
trunk, IVC and IVC TTs were 10.2, 7.4, 17.4 and 8.5 months,
respectively. The efficacy of radiotherapy in patients with IVC TT
is significantly higher than that in patients with portal vein
tumor thrombosis (PVTT) and other types of TT.
Radiotherapy-induced liver damage is a dose-limiting
liver radiation injury (40,44). As the majority of patients with HCC
have a long history of liver cirrhosis, radiotherapy-induced liver
damage requires sufficient attention. It primarily manifests as
hepatomegaly after 2–12 months of radiation therapy, with a
>5-fold increase in benign ascites and transaminase, an increase
in the Child-Pugh score by ≥2 points, and stomach and duodenal
ulcers. Vascular TT, poor liver function and a very large
proportion of irradiated normal liver tissue are associated with a
high risk of radiotherapy-induced liver damage. The key to avoiding
radiation-induced liver disease is to design a radiotherapy plan
wherein the dose exposure to the normal liver is limited to the
tolerance range.
TACE
Transvascular interventional therapy is an important
palliative treatment for patients with HCC who cannot undergo
surgery. TACE is recognized as the most commonly used treatment.
Previously, treatment for HCC with TT in the IVC and RA was
contraindicated; however, a small number of literature reports have
shown that TACE can improve the patient survival rate compared with
optimal supportive care.
The effective rate of TACE has been reported to be
13.8-53.8%, with a median survival period of 4.2-10.9 months. Chern
et al (45) studied TACE in
26 patients with advanced HCC exhibiting TT in the IVC or RA. The
complete response rate of TACE in their study was 53.8%, and the
median survival time was 4.2 months (range, 1.5-76.7 months). The
1-, 2- and 3-year survival rates were 41, 25 and 7%, respectively.
Furthermore, they verified that a smaller diameter of the
polyethylene glycol embolic particle sphere (47–180 µm) was better
than a larger diameter (>180 µm), and the response rate was
significantly higher in the former than in the latter. Chung et
al (46) aimed to elucidate the
treatment outcomes of TACE in patients with HCC exhibiting HV
and/or IVC invasion. TACE response rates for primary tumors and TTs
in HV or IVC were 55.6 and 13.0%, respectively. The median OS time
was 10.9 months (range, 0.1-23.0 months). Koo et al
(47) also evaluated the effects of
TACE in patients with HCC exhibiting TT in the IVC, and reported a
response rate and progression-free survival rate of 13.8 and 37.9%,
respectively, in the TACE group. The 1-year survival rate was 17.2%
in the TACE group, and the median survival time was 4.7 months.
TACE enabled selective chemoembolization of
angiographically-confirmed or -suspected blood vessels involved in
the blood supply to the lesion, with embolization of the collateral
artery (right inferior phrenic artery, left gastric artery branch)
first, followed by embolization of the hepatic artery. The embolic
material includes chemotherapeutic drug-iodinated oil-mixed
emulsion, polyvinyl alcohol particles, or a gelatin sponge. The
chemotherapeutic drugs used include epirubicin,
hydroxycamptothecin, oxaliplatin, lobaplatin and fluorouracil
(45,47,48).
Complications of TACE for IVC/RA tumors are
pulmonary embolism and high-risk ischemic hepatic necrosis. These
primarily manifest as fever, abdominal pain, vomiting and transient
deterioration of liver function.
Drugs and other treatments
A small number of studies have reported the use of
sorafenib and thalidomide to treat HCC with TT in the HV, IVC and
RA (49–51). Simão et al (52) reported a rare clinical case that had
a complete response to sorafenib with thrombosis in IVC and RA,
with no recurrence after 3 years of treatment. Existing evidence
from the European, Australasian and Asia-Pacific clinical trials
concerning the use of sorafenib suggests that the drug is
efficacious in the majority of HCC cases, with a median OS of 6.5
months in the treatment group compared with 4.2 months observed in
the placebo group (53,54). There are also vast clinical data for
the use of sorafenib in the treatment of advanced HCC; however,
data concerning its use in the subgroup of patients with TT in the
HV, IVC and RA are scarce. It is unclear whether medical therapy
has been studied prospectively in this population, and this
requires big data analysis. Chang et al (50) reported a case of three patients who
received medical treatment with low-dose thalidomide and additional
treatment via TACE and documented an OS time of >15 months. To
the best of our knowledge, Li et al (55) reported the first case of primary
intrahepatic lesions and IVC and RA TTs treated via percutaneous
microwave ablation, wherein the patient survived for 16 months.
Comprehensive treatment
In a study by Duan et al (56), 11 patients with HCC exhibiting TTs in
IVC and RA were enrolled. The 1- and 3-year survival rates of
patients undergoing TACE combined with external radiotherapy were
54.5 and 27.3%, respectively. The median survival time was 21
months. Koo et al (47)
performed a retrospective analysis among 42 patients with TTs in
the HV, IVC and RA, and subsequently reported the effective and
progression-free survival rates of patients undergoing TACE with
CRT and TACE alone to be 42.9, 71.4, 13.8 and 37.9%, respectively.
The OS rates were 11.7 and 4.7 months, respectively. A survival
analysis (57) for TACE combined
with bare stent implantation versus I-125 particle stent
implantation showed a median survival duration of 93 and 203 days,
respectively, which resolved 97% of lower extremity edema.
Conclusion
HCC is the fourth most common malignant tumor in
China and the third highest cause of cancer-associated mortality,
which seriously threatens the health and life of humans (58). HCC is a highly malignant tumor that
is often associated with intrahepatic vascular invasion (PVTT),
which is an important prognostic factor, whereas the extrahepatic
vascular invasions such as HVTT, IVC TT and RA TT are far less
common when compared with PVTT. HVTT, IVC TT and RA TT have a worse
prognosis than PVTT. Jun et al (59) have demonstrated that the newly
revised UICC staging system (60) is
later than the IVA period, and that HV invasion, IVC invasion, PVTT
and multiple liver cancer nodules are independent risk factors for
RA TT. These patients are in the terminal stage of disease, often
combined with the formation of TT in the portal vein, multiple
intrahepatic metastases, lung metastases, etc. Doppler ultrasound,
CT and MRI, and magnetic resonance angiography can be used to
detect the size, location, length and degree of TT directly and
clearly, which guide the selection of treatment modalities,
assessment of the degree of treatment difficulty and risks, and
preparation of counter-measures.
The present study assessed the clinical
manifestations, pathophysiology, imaging diagnosis techniques and
associated positive treatments for HCC with TT in IVC or RA.
Previously, these patients only underwent the best supportive
treatments, which included the control of tumor-related symptoms,
psychological counseling and spiritual help, and their survival
time was 2–3 months. At present, with the increasing number of case
reports and retrospective analysis of small data, active treatments
such as surgery, radiotherapy, intervention, drugs and
comprehensive treatment have been demonstrated to improve survival
time. Jun et al (59) have
also demonstrated that with a Cancer of the Liver Italian Program
score ≤3 (61), active treatment can
prolong the survival time of patients with HCC with RA TT. The
current data in small studies (Tables
I–III) demonstrate that
surgical treatments have the greatest survival benefits, with a
median survival time of 10.5-30.8 months, which is higher than the
median survival time reported for radiotherapy (5.6-25.4 months).
The therapeutic effect of TACE is poor, with a median survival time
of 4.7-10.9 months. Liu et al (62) retrospectively analyzed 115 cases of
HCC with TT in the HV, IVC and RA, and reported the median survival
time with surgical treatment and TACE to be 17 and 8 months,
respectively, which is consistent with the aforementioned results.
Although other treatments such as sorafenib and thalidomide
administration may also be effective, they have only been reported
in a small number of cases, with little evidence. In addition, such
advanced stage patients often undergo comprehensive treatment, such
as surgery combined with radiotherapy, radiotherapy combined with
TACE, and surgery combined with sorafenib and other treatment
models. Koo et al (47)
retrospectively analyzed 42 cases of HCC with TT in the HV, IVC,
and RA, reported the effective and progression-free survival rates
for TACE with CRT to be 42.9 and 71.4%, respectively, which were
higher than those for TACE alone.
 | Table III.Active TACE treatment outcome for
hepatocellular carcinoma with tumor thrombus in the inferior vena
cava or right atrium. |
Table III.
Active TACE treatment outcome for
hepatocellular carcinoma with tumor thrombus in the inferior vena
cava or right atrium.
| Author, year | Patients, n | TACE cycles | Response rate,
% | MST, months | 1-year OS, % | 3-year OS, % | (Refs.) |
|---|
| Chung et al,
2014 | 62 | 1 | NA | 10.9 | 45.8 | NA | (46) |
| Chern et al,
2009 | 26 | Repeat for 6–8
weeks | 53.8 |
4.2 | 41.0 | 7 | (45) |
| Koo et al,
2010 | 29 | Repeat for 6–8
weeks | 13.8 |
4.7 | 17.2 | NA | (47) |
| Kim et al,
2013 | 60 | 1 | 18.0 |
6.7 | 37.0 | 13 | (64) |
|
| 47 | 1 | 53.0 |
9.7 | NA | NA |
|
| Liu et al,
2012 | 50 | 4-6 | NA |
8.0 | NA | NA | (62) |
Despite this, there remains to be a lack of
consensus on the treatment of HCC with TT in the IVC or RA. The
treatment of such patients is recommended for clinical research,
and it is expected that the current understanding of treatment of
such patients will be improved in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XN designed the study. XN, JZ and YX wrote the
paper, performed the literature search and analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent for publication of images without
any potential identifying information was provided by the
patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TT
|
tumor thrombus
|
|
IVC
|
inferior vena cava
|
|
RA
|
right atrium
|
|
HCC
|
hepatocellular carcinoma
|
|
HV
|
hepatic vein
|
|
DSA
|
digital subtraction angiography
|
|
TACE
|
transcatheter arterial
chemoembolization
|
|
3DCRT
|
three-dimensional conformal
radiotherapy
|
|
SBRT
|
stereotactic radiotherapy
|
|
PVTT
|
portal venous tumor thrombus
|
|
OS
|
overall survival
|
References
|
1
|
Georgen M, Regimbeau JM, Kianmanesh R,
Marty J, Farges O and Belghiti J: Removal of hepatocellular
carcinoma extending in the right atrium without extracorporal
bypass. J Am Coll Surg. 195:892–894. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee I, Chung JW, Kim HC, Yin YH, So YH,
Jeon UB, Jae HJ, Cho BH and Park JH: Extrahepatic collateral artery
supply to the tumor thrombi of hepatocellular carcinoma invading
inferior vena cava: The prevalence and determinant factors. J Vasc
Interv Radiol. 20:22–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okuda K: Hepatocellular carcinoma:
Clinicopathological aspects. J Gastroenterol Hepatol. 12:S314–S318.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang ZM, Lai EC, Zhang C, Yu HW, Liu Z,
Wan BJ, Liu LM, Tian ZH, Deng H, Sun QH and Chen XP: The strategies
for treating primary hepatocellular carcinoma with portal vein
tumor thrombus. Int J Surg. 20:8–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kudo M, Iznmi N, Kokudo N, Matsui O,
Sakamoto M, Nakashima O, Kojiro M and Makuuchi M; HCC Expert Panel
of Japan Society of Hepatology, : Management of hepatocellular
carcinoma in Japan: Consensus-based clinical practice guidelines
proposed by the Japan Society of Hepatology (JSH) 2010 updated
version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun YH, Ahn SH, Park JY, Kim DY, Han KH,
Chon CY, Byun SJ and Kim SU: Clinical characteristics and treatment
outcomes of hepatocellular carcinoma with inferior vena cava/heart
invasion. Anticancer Res. 31:4641–4646. 2011.PubMed/NCBI
|
|
7
|
Wang Y, Yuan L, Ge RL, Sun Y and Wei G:
Survival benefit of surgical treatment for hepatocellular carcinoma
with inferior vena cava/right atrium tumor thrombus: Results of a
retrospective cohort study. Ann Surg Oncol. 20:914–922. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sonsuz A: Barcelona clinic liver cancer
(BCLC) staging: Does it cover all our expectation. J Gastrointest
Cancer. 48:260–261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakamoto K and Nagano H: Outcomes of
surgery for hepatocellular carcinoma with tumor thrombus in the
inferior vena cava or right atrium. Surg Today. 48:819–824. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun JH, Zhang YL, Nie CH, Chen LM, He JD,
Wang WL and Zheng SS: Long-term survival after chemoembolization of
metastatic right atrial tumor thrombus as a presenting feature of
hepatocellular carcinoma: A case study. Oncol Lett. 3:975–977.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawakami M, Koda M, Mandai M, Hosho K,
Murawaki Y, Oda W and Hayashi K: Isolated metastases of
hepatocellular carcinoma in the right atrium: Case report and
review of the literature. Oncol Lett. 5:1505–1508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohwada S, Tanahashi Y, Kawashima Y, Satoh
Y, Nakamura S, Kobayashi I, Ohya T, Ishikawa S, Ohtaki A, Iino Y,
et al: Surgery for tumor thrombi in the right atrium and inferior
vena cava of patients with recurrent hepatocellular carcinoma.
Hepatogastroenterology. 41:154–157. 1994.PubMed/NCBI
|
|
13
|
Nakashima T, Okuda K, Kojiro M, Jimi A,
Yamaguchi R, Sakamoto K and Ikari T: Pathology of hepatocellular
carcinoma in Japan. 232 Consecutive cases autopsied in ten years.
Cancer. 51:863–877. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kojiro M, Nakahara H, Sugihara S, Murakami
T, Nakashima T and Kawasaki H: Hepatocellular carcinoma with
intra-atrial tumor growth. A clinicopathologic study of 18 autopsy
cases. Arch Pathol Lab Med. 108:989–992. 1984.PubMed/NCBI
|
|
15
|
Anthony PP: Primary carcinoma of the
liver: A study of 282 cases in Ugandan Africans. J Pathol.
110:37–48. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Macdonald RA: Primary carcinoma of the
liver; a clinicopathologic study of one hundred eight cases. Ama
Arch Intern Med. 99:266–279. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koo J, Fung K, Siu KF, Lee NW, Lett Z, Ho
J, Wong J and Ong GB: Recovery of malignant tumor cells from the
right atrium during hepatic resection for hepatocellular carcinoma.
Cancer. 52:1952–1956. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sung AD, Cheng S, Moslehi J, Scully EP,
Prior JM and Loscalzo J: Hepatocellular carcinoma with
intracavitary cardiac involvement: A case report and review of the
literature. Am J Cardiol. 102:643–645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng HY, Wang XY, Zhao GL and Chen D:
Imaging findings and transcatheter arterial chemoembolization of
hepatic malignancy with right atrial embolus in 46 patients. World
J Gastroenterol. 14:3563–3568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyayama S, Matsui O, Taki K, Minami T,
Ryu Y, Ito C, Nakamura K, Inoue D, Notsumata K, Toya D, et al:
Extrahepatic blood supply to hepatocellular carcinoma: Angiographic
demonstration and transcatheter arterial chemoembolization.
Cardiovasc Intervent Radiol. 29:39–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tse HF, Lau CP, Lau YK and Lai CL:
Transesophageal echocardiography in the detection of inferior vena
cava and cardiac metastasis in hepatocellular carcinoma. Clin
Cardiol. 19:211–213. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okada S: How to manage hepatic vein tumour
thrombus in hepatocellular carcinoma. J Gastroenterol Hepatol.
15:346–348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asahara T, Itamoto T, Katayama K, Nakahara
H, Hino H, Yano M, Ono E, Dohi K, Nakanishi T, Kitamoto M, et al:
Hepatic resection with tumor thrombectomy for hepatocellular
carcinoma with tumor thrombi in the major vasculatures.
Hepatogastroenterology. 46:1862–1869. 1999.PubMed/NCBI
|
|
25
|
Komatsu S, Kido M, Asari S, Toyama H,
Ajiki T, Demizu Y, Terashima K, Okimoto T, Sasaki R and Fukumoto T:
Particle radiotherapy, a novel external radiation therapy, versus
liver resection for hepatocellular carcinoma accompanied with
inferior vena cava tumor thrombus: A matched-pair analysis.
Surgery. 162:1241–1249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li AJ, Zhou WP, Lin C, Lang XL, Wang ZG,
Yang XY, Tang QH, Tao R and Wu MC: Surgical treatment of
hepatocellular carcinoma with inferior vena cava tumor thrombus: A
new classification for surgical guidance. Hepatobiliary Pancreat
Dis Int. 12:263–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyazaki M, Ito H, Nakagawa K, Shimizu H,
Yoshidome H, Shimizu Y, Ohtsuka M, Togawa A and Kimura F: An
approach to intrapericardial inferior vena cava through the
abdominal cavity, without median sternotomy, for total hepatic
vascular exclusion. Hepatogastroenterology. 48:1443–1446.
2001.PubMed/NCBI
|
|
28
|
Mizuno S, Kato H, Azumi Y, Kishiwada M,
Hamada T, Usui M, Sakurai H, Tabata M, Shimpo H and Isaji S: Total
vascular hepatic exclusion for tumor resection: A new approach to
the intrathoracic inferior vena cava through the abdominal cavity
by cutting the diaphragm vertically without cutting the
pericardium. J Hepatobiliary Pancreat Sci. 17:197–202. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wakayama K, Kamiyama T, Yokoo H, Kakisaka
T, Kamachi H, Tsuruga Y, Nakanishi K, Shimamura T, Todo S and
Taketomi A: Surgical management of hepatocellular carcinoma with
tumor thrombi in the inferior vena cava or right atrium. World J
Surg Oncol. 11:2592013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bi X, Gao J and Cai J: Sorafenib versus
transarterial chemoembolization as adjuvant therapies for patients
with hepatocellular carcinoma and microvascular invasion. J Clin
Oncol. 37 (Suppl 4):S2442019. View Article : Google Scholar
|
|
31
|
Li J, Hou Y, Cai XB and Liu B: Sorafenib
after resection improves the outcome of BCLC stage C hepatocellular
carcinoma. World J Gastroenterol. 22:4034–4040. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Gao J, Zheng SM, Wang Y, Xiang X,
Cheng Q and Zhu J: The efficacy of sorafenib in preventing
hepatocellular carcinoma recurrence after resection: A systematic
review and meta-analysis. Rev Esp Enferm Dig. 112:201–210. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Ke Q, Lin N, Zeng Y and Liu J:
Does postoperative adjuvant transarterial chemoembolization benefit
for all patients with hepatocellular carcinoma combined with
microvascular invasion: A meta-analysis. Scand J Gastroenterol.
54:528–537. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang XP, Liu YC, Chen ZH, Sun JX, Wang K,
Chai ZT, Shi J, Guo WX, Wu MC, Lau WY and Cheng SQ: Postoperative
adjuvant transarterial chemoembolization improves outcomes of
hepatocellular carcinoma associated with hepatic vein invasion: A
propensity score matching analysis. nn Surg Oncol. 26:1465–1473.
2019. View Article : Google Scholar
|
|
35
|
Uemoto K, Doi H, Shiomi H, Yamada K,
Tatsumi D, Yasumoto T, Takashina M, Koizumi M and Oh RJ: Clinical
assessment of micro-residual tumors during stereotactic body
radiation therapy for hepatocellular carcinoma. Anticancer Res.
38:945–954. 2018.PubMed/NCBI
|
|
36
|
Kokudo T, Hasegawa K, Yamamoto S, Shindoh
J, Takemura N, Aoki T, Sakamoto Y, Makuuchi M, Sugawara Y and
Kokudo N: Surgical treatment of hepatocellular carcinoma associated
with hepatic vein tumor thrombosis. J Hepatol. 61:583–588. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kokudo T, Hasegawa K, Matsuyama Y,
Takayama T, Izumi N, Kadoya M, Kudo M, Kubo S, Sakamoto M,
Nakashima O, et al: Liver resection for hepatocellular carcinoma
associated with hepatic vein invasion: A Japanese nationwide
survey. Hepatology. 66:510–517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kouloulias V, Mosa E, Georgakopoulos J,
Platoni K, Brountzos I, Zygogianni A, Antypas C, Kosmidis P,
Mystakidou K, Tolia M, et al: Three-dimensional conformal
radiotherapy for hepatocellular carcinoma in patients unfit for
resection, ablation, or chemotherapy: A retrospective study.
ScientificWorldJournal. 2013:7801412013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rim CH, Kim CY, Yang DS and Yoon WS:
External beam radiation therapy to hepatocellular carcinoma
involving inferior vena cava and/or right atrium: A meta-analysis
and systemic review. Radiother Oncol. 129:123–129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsuo Y, Yoshida K, Nishimura H, Ejima Y,
Miyawaki D, Uezono H, Ishihara T, Mayahara H, Fukumoto T, Ku Y, et
al: Efficacy of stereotactic body radiotherapy for hepatocellular
carcinoma with portal vein tumor thrombosis/inferior vena cava
tumor thrombosis: Evaluation by comparison with conventional
three-dimensional conformal radiotherapy. J Radiat Res. 57:512–523.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Komatsu S, Fukumoto T, Demizu Y, Miyawaki
D, Terashima K, Niwa Y, Mima M, Fujii O, Sasaki R, Yamada I, et al:
The effectiveness of particle radiotherapy for hepatocellular
carcinoma associated with inferior vena cava tumor thrombus. J
Gastroenterol. 46:913–920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hou JZ, Zeng ZC, Zhang JY, Fan J, Zhou J
and Zeng MS: Influence of tumor thrombus location on the outcome of
external-beam radiation therapy in advanced hepatocellular
carcinoma with macrovascular invasion. Int J Radiat Oncol Biol
Phys. 84:362–368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Doi H, Beppu N, Kitajima K and Kuribayashi
K: Stereotactic body radiation therapy for liver tumors: Current
status and perspectives. Anticancer Res. 38:591–599.
2018.PubMed/NCBI
|
|
45
|
Chern MC, Chuang VP, Chern MC, Lin ZH and
Lin YM: Erratum to: Transcatheter arterial chemoembolization for
advanced hepatocellular carcinoma with inferior vena cava and right
atrial tumors. Cardiovasc Intervent Radiol. 32:13212009. View Article : Google Scholar
|
|
46
|
Chung SM, Yoon CJ, Lee SS, Hong S, Chung
JW, Yang SW, Seong NJ, Jang ES, Kim JW and Jeong SH: Treatment
outcomes of transcatheter arterial chemoembolization for
hepatocellular carcinoma that invades hepatic vein or inferior vena
cava. Cardiovasc Intervent Radiol. 37:1507–1515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Koo JE, Kim JH, Lim YS, Park SJ, Won HJ,
Sung KB and Suh DJ: Combination of transarterial chemoembolization
and three-dimensional conformal radiotherapy for hepatocellular
carcinoma with inferior vena cava tumor thrombus. Int J Radiat
Oncol Biol Phys. 78:180–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sengodan P, Grewal H and Gandhi S:
Invasive hepatocellular carcinoma with recurrent pulmonary
embolism: Use of AngioVac cannula thrombectomy device for
mechanical aspiration. J Invasive Cardiol. 26:E100–E103.
2014.PubMed/NCBI
|
|
49
|
Nishikawa H, Kita R, Kimura T and Osaki Y:
Transcatheter arterial embolic therapies for hepatocellular
carcinoma: A literature review. Anticancer Res. 34:6877–6886.
2014.PubMed/NCBI
|
|
50
|
Chang JY, Ka WS, Chao TY, Liu TW, Chuang
TR and Chen LT: Hepatocellular carcinoma with intra-atrial tumor
thrombi. A report of three cases responsive to thalidomide
treatment and literature review. Oncology. 67:320–326. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stotz M, Gerger A, Haybaeck J, Kiesslich
T, Bullock MD and Pichler M: Molecular targeted therapies in
hepatocellular carcinoma: Past, present and future. Anticancer Res.
35:5737–5744. 2015.PubMed/NCBI
|
|
52
|
Simão A, Silva R, Correia L, Caseiro Alves
F, Carvalho A and Nascimento Costa JM: Advanced stage
hepatocellular carcinoma with multiple metastasis and vascular
thrombosis: A case of complete response to sorafenib. Acta Med
Port. 29:139–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li W, Wang Y, Gao W and Zheng J: HCC with
tumor thrombus entering the right atrium and inferior vena cava
treated by percutaneous ablation. BMC Surg. 17:212017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Duan F, Yu W, Wang Y, Liu FY, Song P, Wang
ZJ, Yan JY, Yuan K and Wang MQ: Trans-arterial chemoembolization
and external beam radiation therapy for treatment of hepatocellular
carcinoma with a tumor thrombus in the inferior vena cava and right
atrium. Cancer Imaging. 15:72015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang QH, Zhang W, Liu QX, Liu LX, Wu LL,
Wang JH, Yan ZP and Luo JJ: TACE combined with implantation of
irradiation stent versus TACE combine with bare stent for HCC
complicated by IVCTT. Cardiovasc Intervent Radiol. 39:1280–1288.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jun CH, Sim DW, Kim SH, Hong HJ, Chung MW,
Cho SB, Park CH, Joo YE, Kim HS, Choi SK and Rew JS: Risk factors
for patients with stage IVB hepatocellular carcinoma and extension
into the heart: Prognostic and therapeutic implications. Yonsei Med
J. 55:379–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y
and Makuuchi M: Staging of hepatocellular carcinoma: Assessment of
the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772
patients in Japan. Ann Surg. 245:909–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Daniele B, Annunziata M, Barletta E,
Tinessa V and Di Maio M: Cancer of the Liver Italian Program (CLIP)
score for staging hepatocellular carcinoma. Hepatol Res. 37 (Suppl
2):S206–S209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu J, Wang Y, Zhang D, Liu B and Ou Q:
Comparison of survival and quality of life of hepatectomy and
thrombectomy using total hepatic vascular exclusion and
chemotherapy alone in patients with hepatocellular carcinoma and
tumor thrombi in the inferior vena cava and hepatic vein. Eur J
Gastroenterol Hepatol. 24:186–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Igaki H, Nakagawa K, Shiraishi K, Shiina
S, Kokudo N, Terahara A, Yamashita H, Sasano N, Omata M and Ohtomo
K: Three-dimensional conformal radiotherapy for hepatocellular
carcinoma with inferior vena cava invasion. Jpn J Clin Oncol.
38:438–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim HC, Lee JH, Chung JW, Kang B, Yoon JH,
Kim YJ, Lee HS, Jae HJ and Park JH: Transarterial chemoembolization
with additional cisplatin infusion for hepatocellular carcinoma
invading the hepatic vein. J Vasc Interv Radiol. 24:274–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|