According to recent global cancer statistics, there
were an estimated 18.1 million new cancer cases and 9.6 million
cancer-associated deaths in 2018 (1). Accumulating evidence indicates that
miRNAs (miRNAs/miRs) may act as oncogenes or tumor suppressor, and
play critical roles in the occurrence and development of cancer
(2). miRNAs are a class of
non-coding RNA (ncRNA) with a length of ~22 nt. In 1993, Lee et
al (3) first discovered miRNAs
in Caenorhabditis elegans, which prompted intense research
on miRNAs. RNA interference technology (4), which was subsequently discovered in
1998, markedly advanced the research on miRNAs. To date, thousands
of miRNAs have been reported in vertebrates, with 2,654 in humans
(5). Dysregulation of miRNA
expression has become a hallmark of various cancer types (6). Among the miRNAs that are known to be
abnormally expressed, miR-497 is considered to be one of the miRNAs
that play a key role in the pathogenesis of cancer (7).
miR-497 is encoded by the first intron of the
MIR497HG gene located on human chromosome 17p13.1 (8). miR497 belongs to the
miR-15/16/195/424/497 family that have the same 3′-untranslated
(UTR) binding seed sequence (AGCAGCA) (9). During autopsy, it was found that the
expression of miR-497 was similar in various normal tissues,
including the cerebral cortex, frontal cortex, primary visual
cortex, thalamus, heart, lungs, liver, kidneys, spleen, stomach and
skeletal muscle (10). However, an
imbalance in miR-497 expression is closely associated with the
occurrence and development of tumors. Based on a study of the
global miRNA expression profile of primary breast cancer, miR-497
was the first miRNA that was confirmed to be downregulated in
breast cancer (11). Subsequently, a
study of the global miRNA expression profile of gastric cancer was
the first to demonstrate that the expression of miR-497 was
increased in non-tumor tissues (12). Accumulating evidence shows that the
expression of miR-497 is downregulated in several tumor types,
including primary peritoneal carcinoma (13), adrenocortical carcinoma (14,15),
malignant astrocytoma (16),
colorectal cancer (17–21), osteosarcoma (22), cervical cancer (23), liver cancer (24–28),
breast cancer (29–31), neuroblastoma (32), non-small cell lung cancer (33–35),
gastric cancer (36–38), ovarian cancer (39), nasopharyngeal carcinoma (40), osteosarcoma (41,42),
angiosarcoma (43), cervical cancer
(44,45), thyroid cancer (46,47),
cutaneous squamous cell carcinoma (48,49),
melanoma (50), glioma (51), clear cell renal cell carcinoma
(52), anaplastic large cell
lymphoma (53), esophageal carcinoma
(54) and bladder cancer (9), among others. These results suggest that
miR-497 acts as a tumor suppressor. Notably, the expression of
miR-497 has been found to be upregulated in hepatocellular
carcinoma (55), chronic
lymphoblastic leukemia (56), and
head and neck squamous cell carcinoma (57). This finding suggests that miR-497 may
play different roles in different types of tumors. To
comprehensively summarize the regulation of miR-497 expression in
cancer, a total of 285 studies associated with the analysis of
miR-497 have been compiled. The aim of the present review was to
focus on the mechanism of regulation of miR-497 expression at the
pre-transcriptional and transcriptional levels in cancer, and to
emphasize the role of miR-497 in chemotherapeutic drug resistance
and the diagnosis and treatment of malignant tumors. The specific
expression, targets and functions of miR-497 in different cancer
types are shown in Table I.
The biosynthesis of miR-497 is mediated by multiple
steps: i) The MIR497HG gene (NCBI gene ID, 574456) on chromosome 17
produces a long primary miRNA (pri-miRNA) transcript (pri-miR-497)
through RNA polymerase II transcription; ii) pri-miRNA is processed
by the Drosha complex in the nucleus into RNA with a hairpin
structure, which is termed precursor miRNA (pre-miRNA)-497; iii)
pre-miR-497 is transported into the cytoplasm by GTP-binding
nuclear protein Ran and exportin-5; iv) pre-miR-497 is cleaved by
Dicer to produce double strands with a length of ~22nt: miR-497,
miRNA*. miRNA*, known as the passenger strand, is discarded; v) the
double-stranded body is directed into the argonaute (Ago)2 protein
to form a RNA-induced silencing complex (RISC); vi) of the two
miRNA double strands, only one chain (miR-497, guide chain) is
retained in the Ago protein and stably forms RISC, whereas the
other chain is discarded as a passing chain (miR-497*); and vii)
the Ago-miRNA complex mainly binds to the 3′UTR of the target mRNA
in a sequence-specific manner. miRNA target recognition usually
depends on the seed sequence of the miRNA, so one miRNA can target
and regulate multiple mRNAs (58).
Copy number abnormalities are a type of genomic
structural variation, and they may be divided into two levels
according to size, namely the microscopic and submicroscopic
levels. Genomic structural variation at the submicroscopic level
refers to variations in the length of DNA fragments of 1 Kb to 3
Mb, including deletion, insertion, repetition, rearrangement,
inversion and changes in the number of DNA copies, among others. It
has been reported that there is a high frequency of loss of
heterozygosity on chromosome 17p13.1 (miR497 genomic locus), and
the downregulation of miR-497 may be associated with the unique
allele loss pattern in primary peritoneal carcinoma (13). It has also been reported that ~71% of
colon cancer tissues display a decrease in DNA copy number
(78,000–1,000,000) in a certain segment of chromosome 17p13.1, and
the expression level of miR-497 was shown to be significantly
decreased in colon cancer tissue samples, indicating that the
downregulation of miR-497 in colon cancer is closely associated
with the decrease in DNA copy number (17). Deletions and duplications of
chromosomal loci may reduce or increase the copy number of miRNAs,
which may have a significant impact on explaining the genetic
heterogeneity of cancer.
Studies have demonstrated that DNA methylation can
control gene expression, and more specifically, that promoter DNA
hypermethylation can downregulate gene expression (59,60). It
has been observed that the CpG island located upstream of the
pri-miR-497 transcription initiation site is methylated by
monoallelic methylation in the normal colonic mucosa, while in ~75%
of colorectal adenomas, the island is hypermethylated, and this
change is associated with a significant downregulation of miR-497
expression (8). Another study
demonstrated that miR-497 DNA is highly methylated and miR-497
expression is downregulated in hepatocellular carcinoma (HCC).
Bioinformatics analysis revealed that the expression of the
miR-195/497 cluster may be affected not only by its hypermethylated
promoter region, but also by hypermethylated transcription factors
(TFs) neurogenin 2 and DNA damage inducible transcript 3 (25). These results suggest that DNA
methylation can regulate the transcriptional activity of miR-497,
resulting in the downregulation of miR-497 expression in
cancer.
The term lncRNA refers to an ncRNA that is >200
nt in length. lncRNAs may act as endogenous target simulators,
regulating gene expression by competing with miRNAs; this mode of
action is referred to as the ‘miRNA sponge’ method, and lncRNAs
with this function are known as competitive endogenous RNAs
(ceRNAs). This mode is a relatively simple regulatory mechanism in
the function of lncRNAs (61).
The lncRNA X-inactive specific transcript (XIST) is
one of the earliest lncRNAs identified in mammals and plays an
important role in X chromosome inactivation (62). The imbalance of XIST expression may
play the role of an oncogene or tumor suppressor gene in different
malignant tumors (63). In gastric
cancer, the expression of XIST is upregulated and can be inhibited
with miR-497. Further study revealed that XIST promotes the
proliferation and metastasis of gastric cancer cells by regulating
the miR497/metastasis-associated in colon cancer-1 axis (64). It has also been reported that XIST
may act as an miR-497 ‘sponge’, which targets programmed cell death
protein 4 to further regulate the proliferation and migration of
HCC cells (55).
There is a close functional association between the
lncRNA plasmacytoma variant translocation 1 (PVT1) and Myc, which
plays a carcinogenic role in a variety of cancer types (65). It was previously demonstrated that
the expression of PVT1 is specifically increased in osteosarcoma
cells and tissues, and acts as a molecular sponge to inhibit the
expression of miR-497, indicating that PVT1 promotes glucose
metabolism, cell proliferation and the metastasis of osteosarcoma
cells through the miR497/hexokinase 2 pathway (66). It was also demonstrated that the
expression of PVT1 is upregulated in non-small cell lung cancer
(NSCLC) tissues, and that it inhibits cancer cell viability and
induces apoptosis through negative regulation of miR-497. Further
in vivo experiments revealed that PVT1 gene knockout may
inhibit tumor growth and promote miR-497 expression in vivo
(67).
The lncRNA gastric cancer-associated transcript 3
(GACAT3) is located on human Chr2p24.3 and its expression has been
reported to be significantly associated with gastric cancer
progression. Some experiments have proven that GACAT3 directly
binds to miR-497 and negatively regulates the expression of miR-497
to promote the progression of gastric cancer (68). Other experiments demonstrated that
GACAT3 acts as the ceRNA of miR-497, enhancing the expression of
Cyclin D2, which in turn promotes the development of breast cancer
(69).
The lncRNA nuclear paraspeckle assembly transcript1
(NEAT1) is a type of ncRNA, with a length of ~3.2 kb, which is
mainly enriched in the nucleus. In gastric cancer, NEAT1 acts as an
oncogene and can promote the growth of gastric cancer cells by
regulating the miR-497/phosphoinositide-3-kinase regulatory subunit
1 axis (70).
The lncRNA LINC00152, which is located in human
Chr2p11.2, is reported to be upregulated in several types of
cancer. In thyroid cancer, LINC00152 acts as a miR-497 ‘sponge’,
downregulating its downstream target, brain-derived neurotrophic
factor (BDNF), to promote the proliferation and invasion of
papillary thyroid cancer cells (71). In multiple myeloma, LINC00152 acts as
an oncogene and regulates cell growth by targeting miR-497
expression (72).
The lncRNA LINC00662 is located on chromosomal band
19q11 and its length is 2,085 bp. It was reported that the
expression of LINC00662 is increased in lung cancer, nasopharyngeal
carcinoma, oral squamous cell carcinoma and other malignant tumors
(73). LINC00662 has also been
reported to play a carcinogenic role in gastric cancer through the
LINC00662-miR-497-Hippo-yes-associated protein 1 (YAP1) axis
(74).
The lncRNA LINC00978 is located in the 2q13 region
of the human chromosome. It was recently reported that the
expression of LINC00978 is upregulated in lung, breast and gastric
cancer, as well as other malignant tumors, and is associated with a
poor prognosis (75). It was
demonstrated that LINC00978 inhibits the expression of miR-497 by
acting as a ceRNA, and that it targets and suppresses the
expression of neurotrophic receptor tyrosine kinase 3 and promotes
the proliferation of gastric cancer cells (76).
The lncRNA small nucleolar RNA host gene 1 (SNHG1)
is located in 11q12.3 and is widely expressed in cancer tissues.
Recently, new evidence from basic and clinical studies has
suggested that SNHG1 is involved in tumorigenesis and is an
indicator of a poor prognosis in different types of cancer, such as
hepatocellular carcinoma, gastric cancer and epithelial ovarian
cancer (77). A previous study has
found that SNHG1 acts as a miR-497 ‘sponge’ to regulate the
expression of insulin-like growth factor receptor (IGF1-R), and
then promotes the proliferation and invasion of NSCLC (78). Other study has reported that SNHG1
and miR-497/miR-195-5p synergistically regulate the
epithelial-to-mesenchymal transition (EMT) of colorectal cells
(79).
The lncRNA small nucleolar RNA host gene 16 (SNHG16)
(Entrez ID, 100507246) was initially identified as an oncogene in
neuroblastoma. It is reported that the upregulation of SNHG16
expression is associated with the prognosis of patients with
neuroblastoma (80). In thyroid
papillary carcinoma, SNHG16 acts as a miR-497 ‘sponge’, which
targets and regulates the expression of BDNF and YAP1 and plays the
role of an oncogene (81). In
diffuse large B-cell lymphoma, SNHG16 interacts directly with
miR-497 by acting as a ceRNA, which targets the expression of
proto-oncogene serine/threonine-protein kinase, thereby promoting
cell proliferation and inhibiting apoptosis (82).
The lncRNA HOXC13 antisense RNA (HOXC13-AS), located
in 12q13.13, can accelerate the progression of tumors such as those
of breast and nasopharyngeal cancer (83). A previous study has found that
HOXC13-AS promotes the proliferation and growth of breast cancer
cells in vitro and In vivo through the
miR-497/phosphatase and tensin homolog axis (84).
The lncRNA metastasis-associated in lung
adenocarcinoma transcript 1 (MALAT1) is an 8.5-kb lncRNA, located
in 11q13. It was previously demonstrated that MALAT1 is widely
expressed in normal tissues, suggesting that it plays a potentially
important role in development and evolution (88). Experiments have shown that miR-497
suppresses the expression of MALAT1 after transcription, and MALAT1
also competes with miR-497 for its molecular target eukaryotic
initiation factor 4E, to induce cell cycle arrest (53). In addition, MALAT1 directly binds to
splicing factor proline- and glutamine-rich, indicating its
multifaceted role in the pathophysiology of adrenocortical
carcinoma (89).
The lncRNA DLX6 antisense RNA 1 (DLX6-AS1), located
on human chromosome 7q21.3, was firstly identified to be highly
expressed in normal brain tissues (90). A previous study has found that the
DLX6-AS1/miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway is
involved in the pathogenesis of pancreatic cancer (91).
The lncRNA LINC00339 is widely expressed in a
variety of tissues and cells. It was recently reported that
LINC00339 is overexpressed in pancreatic cancer cells and tissues,
and that it increases the expression of IGF1-R by acting as an
miR-497 ‘sponge’, thereby promoting the proliferation, invasion and
metastasis of cancer cells (92).
The lncRNA LINC01410, located on the 9q13
chromosome, may act as an oncogene in gastric cancer by promoting
cell invasion and angiogenesis (93). It was recently reported that the
expression of LINC01410 is increased in pancreatic cancer tissues
and cells, while the downregulation of LINC01410 expression may
inhibit the proliferation and migration of pancreatic cancer cells.
The underlying mechanism may be closely associated with the
regulation of the expression of miR-497 and the interferon
inducible transmembrane protein 3 gene (94).
The lncRNA AC009022.1 (PDXDC2P-NPIPB14P) is an
important member of the lncRNA family, but its role in human tumors
remains elusive. The study by Yu and Zhang (95) was the first to demonstrate that
AC009022.1 enhances the proliferation and invasion of colorectal
cancer cells by inhibiting the expression of miR-497 and promoting
the expression of actin-related protein 3B.
The lncRNA CDKN2B antisense RNA 1 (CDKN2B-AS1) is
located on human chromosome 9p21.3. It has been reported that the
expression of this lncRNA is upregulated in tumor tissues and plays
a carcinogenic role. A previous study has reported that CDKN2B-AS1
acts as an oncogene in the tumorigenesis of laryngeal squamous cell
carcinoma by regulating the miR-497/cyclin-dependent kinase 6 axis
(96).
All the lncRNAs associated with the regulation of
miR-497 expression in cancer are shown in Table II.
circ_0018289 is located in chr10:46968580-46969453,
has a length of 348 bp and plays an important role in the
proliferation, metastasis and invasion of cervical cancer cells.
Through bioinformatics prediction and luciferase reporter gene
detection, it was shown that circ_0018289 directly binds to miR-497
and plays an oncogenic role in cervical cancer (98).
circPVT1 originates from the PVT1 gene locus and is
located in Chr8q24, but unlike lncPVT1, circPVT1 appears to be
transcribed from its promoter. In addition, chromosome 8q24 carries
the oncogene c-Myc (99). A previous
study has reported that circPVT1 acts as an oncogene in head and
neck squamous cell carcinoma and that the
mut-p53/YAP/transcriptional enhanced associate domain complex
regulates its expression in transcription (100). Further investigation revealed that
circPVT1 causes targeted inhibition of miR-497, whereas miR-497
causes targeted regulation of mitotic checkpoint
serine/threonine-protein kinase BUB1 (100). It was recently demonstrated that
circPVT1 can directly bind to miR-497 and reduce the inhibitory
effect of miR-497 on the expression of Bcl-2, thus inhibiting the
apoptosis of NSCLC cells (101).
All the circRNAs associated with the regulation of
miR-497 expression in cancer are shown in Table III.
In eukaryotes, TFs can specifically bind to a
specific sequence upstream of the 5′ end of the gene, thus
affecting the transcription process from DNA to mRNA or miRNA. In
this manner, TFs can regulate the expression of miRNAs positively
or negatively.
The nuclear factor-κB (NF-κB) signaling pathway was
one of the first to be identified pathways associated with miRNA
expression. It was more recently demonstrated that miR-497 directly
targets the regulation of inhibitor κB kinase β, which in turn
affects the expression of NF-κB (102). Another study found that miR-497 is
involved in the regulation of the NF-κB/matrix metallopeptidase
(MMP)-9/prostate-specific antigen signaling pathway in prostate
cancer (103). However, according
to current research, the regulatory association between the NF-κB
signaling pathway and miR-497 is different from those
aforementioned. It was initially observed that activating NF-κB can
increase the level of miR-497 (102). It was recently demonstrated that
the inflammatory cytokine tumor necrosis-factor (TNF)-α inhibits
the expression of miR-497 through negative transcriptional
regulation mediated by NF-κB, and that it upregulates the
expression of spalt-like transcription factor 4, and promotes the
self-renewal and metastasis of HCC cells (104). The aforementioned evidence
indicates that there is a negative feedback loop between miR-497
and the NF-κB signaling pathway that regulates its expression.
YAP1 is a key molecule of the Hippo signaling
pathway and acts as a transcriptional coactivator. In NSCLC and
HCC, miR-497 inhibits the proliferation of cancer cells by
targeting YAP1 (105,106). In papillary thyroid carcinoma,
miR-497 inhibits cancer cell proliferation and invasion by
negatively regulating the expression of YAP1 (107).
Slug is a type of TF that can promote the occurrence
of EMT, and can increase the migration and invasion ability of
tumor cells. A previous study found that the expression of miR-497
is significantly associated with EMT in breast cancer cells, which
is mediated by the targeted regulation of Slug (108).
Hypoxia inducible factor-1α (HIF-1α) is a subunit of
HIF-1. As a major transcriptional regulator, it can activate the
transcription of >40 genes and participate in the inflammatory
response (109). It was previously
demonstrated that HIF-1α binds to the site of hypoxia response
element 1 in the 1.5-kb region upstream of the miR-497 promoter,
where it directly activates its transcription (110). It was observed that the expression
of miR-497 decreased significantly under hypoxic conditions,
whereas the expression of miR-497 increased after the cells were
restored to a normoxic state for 24 h. Therefore, it may be
inferred that miR-497 is a hypoxia-induced miRNA. It was also been
suggested that miR-497 inhibits angiogenesis in breast cancer by
targeting HIF-1α (111).
Twist is a highly conserved TF that plays an
important role in embryonic development. The expression of Twist is
involved in the occurrence, development, invasion and metastasis of
breast cancer (112). Current
research shows that Twist can promote angiogenesis in pancreatic
cancer by targeting the miR-497/vascular endothelial growth factor
A axis (113).
The SMAD family is composed of several TFs. SMAD3 is
an important transporter in the transforming growth factor-β
(TGF-β) signal transduction pathway, and SMAD7 has been considered
as an important negative feedback regulator of the TGF-β signaling
pathway in the past (114). It has
been demonstrated that miR-497 directly interacts with the 3′-UTR
sequence of SMAD3 transcripts, indicating that it may act as one of
the regulators of the TGF-β signaling pathway (115). In addition, it has been shown that
miR-497 regulates the proliferation and invasion of breast cancer
cells by targeting SMAD7 (116).
Another study demonstrated that miR-497 can enhance the
invasiveness of oral squamous cell carcinoma cells by inhibiting
SMAD7 (117).
Paired box (PAX) proteins are named after the highly
conserved pairing box gene binding region in their structure. PAX2
is located on human chromosome 10 q25 and is highly expressed in
ovarian cancer (118). It was
recently reported that overexpression of miR-497 inhibits
proliferation and induces the apoptosis of ovarian cancer cells by
targeting PAX2 (119).
PBX homeobox 3 (PBX3) belongs to the family of human
PBX TFs. It was previously demonstrated that PBX3 is targeted by
multiple miRNAs, such as let-7c, miR-200b, miR-222 and miR-424, and
is essential for liver tumor-initiating cells (121). In addition, miR-497 was found to
play an anti-tumor role in human multiple myeloma by targeting PBX3
(122).
SRY-box transcription factor 5 (SOX5) is an
important member of the SOX family. The gene is located at 12p12.1
and can directly bind to DNA or bind to other proteins to regulate
gene expression (123). It has been
shown that SOX5 is the direct target of miR-497-5p, indicating that
the overexpression of miR-497 induces cell apoptosis by inhibiting
the expression of the SOX5 gene, which in turn inhibits the
proliferation, migration and invasion of NSCLC (124).
SALL4 is a TF that plays an important role in
maintaining the self-renewal and pluripotency of embryonic stem
cells (104). It has been
demonstrated that miR-497 acts as a tumor suppressor to inhibit the
expression of SALL4 and regulate the metastasis of HCC cells
(104). Further studies
demonstrated that TNF-α activates the NF-κB signaling pathway,
causing p65 to enter the nucleus and bind to the p65 binding site
on the miR-497 promoter, thereby inhibiting the transcription of
miR-497, upregulating the expression of SALL4 and, ultimately,
promoting the proliferation of HCC cells (104).
Estrogen receptor α (ERα) acts as a TF in the
nucleus by regulating the transcription of downstream target genes.
It was recently demonstrated that, in ERα-negative breast cancer,
the low levels of ERα reduce the expression of miR-497, thus
promoting the expression of estrogen-related receptor α, and
enhancing the proliferation, migration and invasion of breast
cancer cells by increasing macrophage migration inhibitory factor
expression and MMP-9 activity (125).
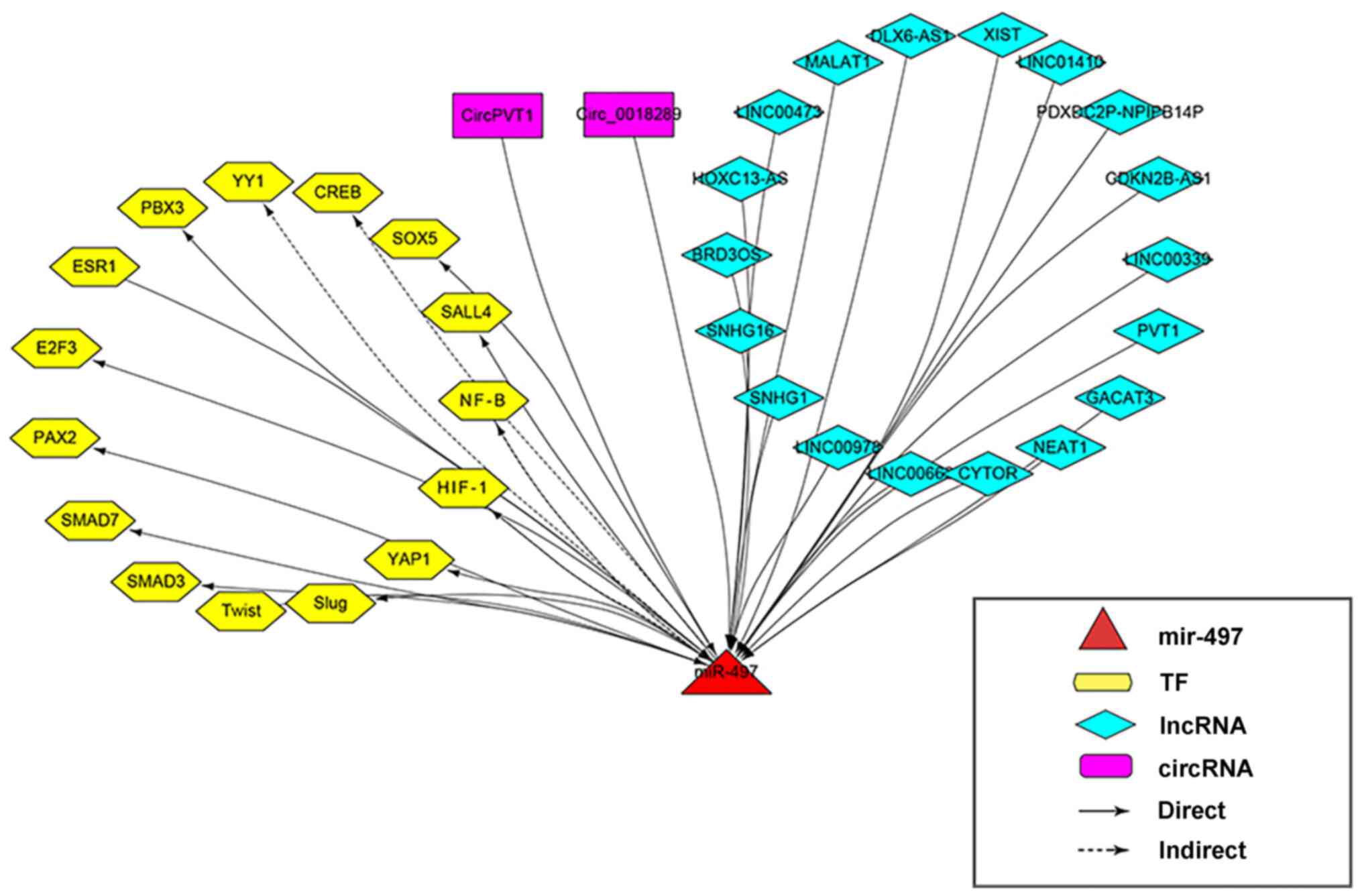
All the TFs associated with the regulation of
miR-497 expression in cancer are shown in Table IV. A map of the
lncRNA-circRNA-TF-miR-497 regulatory network is shown in Fig. 1.
Ideal biomarkers must be highly sensitive, specific
and easy to obtain, so that they can be detected in easily
accessible samples such as blood, saliva or urine. Previous studies
have demonstrated that miRNAs in the serum or plasma may be used as
non-invasive biomarkers for cancer diagnosis (126,127).
Animal experiments have demonstrated that the expression of miR-497
in tissues and in the circulation is positively correlated,
indicating that the expression of miRNAs in the circulation may
reflect their expression in tumor tissues (126). Similar results were obtained from a
series of clinical studies. In patients with gliomas, the
researchers found that miR-497 has diagnostic significance in
preoperative grading, and can distinguish pleomorphic glioblastomas
from low-grade gliomas, with high sensitivity and specificity
(127). It was also reported that
the downregulation of miR-497 expression in cancer tissue and
plasma is consistent in nasopharyngeal carcinoma, indicating that
plasma miR-497 may be used in the diagnosis of this disease
(128). In addition, with the
development of bioinformatics technology, an increasing number of
biomarkers for disease diagnosis and staging have been identified.
In patients with malignant astrocytoma, the concentration of 7
types of miRNAs (including miR-497) in the serum was found to be
significantly decreased, and the comprehensive expression analysis
of the 7-miRNA panel revealed that it had high sensitivity (88.00%)
and high specificity (97.87%) in predicting malignant astrocytoma
(16). In a study on the plasma
miRNA profile of patients with bladder cancer, it was found that
the expression of miR-497 in the plasma was significantly
decreased, and the serum/plasma miR-497 exhibited relatively high
sensitivity and specificity in the diagnosis of bladder cancer
(129). Zhang et al
(130) identified a group of miRNA
markers, including miR-497, in the serum, which can distinguish
patients with cervical cancer from those with cervical
intraepithelial neoplasia and healthy subjects, with a high
sensitivity and specificity, and may thus be recommended for the
early non-invasive detection of cervical cancer. Furthermore,
receiver-operating characteristic curve analysis demonstrated that
serum miR-497 could distinguish colorectal cancer or precancerous
lesions from normal controls with high sensitivity (80.91%) and
specificity (81.43%), while univariate and multivariate analysis
identified serum miR-497 as an independent prognostic factor for
CRC (131).
One of the most important reasons for the poor
prognosis of postoperative radiotherapy and chemotherapy is drug
resistance, which adversely affects the survival time of the
patients; therefore, it is particularly important to explore
whether miR-497 can improve drug resistance. In osteosarcoma,
miR-497 enhances the sensitivity of osteosarcoma cells to cisplatin
through the phosphoinositide 3-kinase/Akt pathway (132). In ovarian cancer, miR-497 reduces
cisplatin resistance of ovarian cancer cells by targeting mammalian
target of rapamycin/P70S6K1 (133).
In addition, miR-497 exhibits similar results when studying drug
resistance to other common chemotherapeutic drugs. In pancreatic
ductal adenocarcinoma, miR-497 can increase the sensitivity of
pancreatic ductal adenocarcinoma cells to erlotinib and gemcitabine
(134). In lymphoma, the
upregulation of miR-497 expression can enhance the chemosensitivity
of lymphoma cells and significantly increase the survival rate of
the patients (135). In multiple
myeloma, miR-497 can increase the sensitivity of multiple myeloma
cells to bortezomib (136). In
colorectal cancer, miR-497 can enhance the sensitivity of
colorectal cancer cells to oxaliplatin (137). Notably, although the majority of
the studies have demonstrated that miR-497 agonists can increase
the sensitivity of cancer cells to chemotherapeutic drugs (132–137),
some studies have yielded different results (138,139).
A previous study have shown that miR-497 is involved in the
resistance of NSCLC cells to gefitinib through the insulin-like
growth factor-1 receptor bypass pathway (138). Temozolomide (TMZ) is mainly used in
the treatment of gliomas. It has been demonstrated that
overexpression of miR-497 reduces the sensitivity of glioma cells
to TMZ chemotherapy (110). Further
research has found that the downregulation of miR-497 expression
could enhance the cytotoxic effect of TMZ on glioma cells,
suggesting that TMZ combined with miR-497 antagonists may help
reduce the incidence of TMZ resistance in gliomas and increase the
overall response rate of chemotherapy (139).
miR-497 plays an important role in the treatment of
drug-resistant cancer and significantly affects the prognosis of
the patients. Therefore, miR-497 may be a potential biomarker
affecting the prognosis of cancer patients. It was previously
observed that low expression of serum miR-497 is associated with a
poor prognosis in patients with osteosarcoma (140). Subsequently, it was demonstrated
that the expression level of miR-497 increases significantly
following surgical resection of the primary tumor, indicating that
serum miR-497 may be used to evaluate the effect of the surgery,
and even as a prognostic index of malignant astrocytoma (16). It was recently reported that miR-497
in extracellular microvesicles is a predictive biomarker of the
benefits of mitogen-activated protein kinase pathway inhibitor
therapy in patients with metastatic cutaneous malignant melanoma
(141). In addition to the
aforementioned prognostic indicators, survival analysis may also be
used to reflect the prognosis of disease treatment. Through Cox
regression analysis, it was revealed that the downregulation of
miR497 expression was associated with longer survival time in
patients with pancreaticobiliary adenocarcinoma (142). miRNA-seq data analysis of The
Cancer Genome Atlas revealed that miR-497 was negatively correlated
with the survival of patients with oropharyngeal squamous cell
carcinoma, which was verified in an independent cohort study
(143). To further verify the
potential predictive value of miR-497 expression level for
prognosis, two meta-analyses of multiple studies were conducted. In
2018, a meta-analysis of 12 studies (including a total of 989
cancer patients) revealed that tumor patients with a high level of
miR-497 expression exhibited longer OS times (144). In 2019, a meta-analysis of 15
studies (including a total of 1,760 participants) revealed that
lower miR-497 expression levels were significantly correlated with
shorter OS time, but not disease-free survival or relapse-free
survival, indicating that miR-497 was not significantly correlated
with early prediction, but may be of value as a biomarker of
long-term prognosis (145).
The aim of the present review was to summarize the
role of DNA copy number and epigenetics (DNA methylation, lncRNAs,
circRNAs and TFs) in the regulation of miR-497 expression. At the
DNA level, the copy number of DNA is positively correlated with the
expression of miR-497, and DNA methylation is one of the mechanisms
underlying the abnormal expression of miR-497 in cancer. At the
transcriptional level, lncRNAs and circRNAs act as miRNA sponges
and inhibit miR-497 expression. In addition, the expression of
miR-497 can not only be regulated by TFs, but can also be targeted
to regulate the expression of TFs, in which there is even a
feedback loop between miR-497 and certain TFs (e.g., NF-κB and
HIF-1α). Furthermore, the present review also summarized the
potential of serum/plasma miR-497 as a non-invasive diagnostic
marker, as a factor improving chemotherapeutic drug resistance and
as a prognostic marker. Although the regulatory mechanisms of
miR-497 expression in cancer were preliminarily summarized, the
complex networks involved in the regulation of this expression are
still incompletely understood. Moreover, due to the complexity of
miRNA biosynthesis and the diversity of the target genes involved,
it is necessary to further elucidate the role of miR-497 in tumors.
The guidelines for diagnosis and treatment of primary liver cancer
in China (2019 edition) points out that the current liver cancer
detection kit based on a circulating miRNA model has been verified
by multicenter clinical trials (in a cohort with 1,812 patients),
has been registered with three types of medical devices by the
State Drug Administration and has been put into clinical
application (146). Although
serum/plasma miR-497 may be used as a biomarker for diagnosis, it
has not been verified in a large number of samples. Notably, most
studies have demonstrated that miR-497 agonists can increase the
sensitivity of cancer cells to chemotherapeutic drugs, and some
studies have reported that the combination with miR-497 antagonists
may help reduce the incidence of TMZ resistance in gliomas; thus,
different drugs must to be used according to the different types of
cancer. In addition, novel drugs for miR-497 must be developed.
Understanding the regulatory mechanism of miR-497 expression will
provide new approaches to further study the function of miR-497 and
develop new drugs. In conclusion, the varying levels of miR-497
expression among different cancers and healthy patients plays an
important role in the diagnosis, treatment and prognosis of several
tumors, and it is worthwhile to study the main mechanism underlying
this imbalance, as it may prove to be of value in the clinical
setting.
Not applicable.
The present study was supported by grants from the
Natural Science Foundation of China (grant no. 81641110) and the
Guangdong Province Natural Science Foundation (grant no.
2015A030313725).
Not applicable.
GL analyzed the data and wrote the manuscript. ZX,
SL and HL collected the data. KH and GX were involved in the
conception and design of the study and revised the manuscript. All
authors have read and approved the final version of the
manuscript.
Not applicable.
Not applicable.
All the authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature.
391:806–811. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kozomara A, Birgaoanu M and
Griffiths-Jones S: miRBase: From microRNA sequences to function.
Nucleic Acids Res. 47:D155–D162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joyce BT, Zheng Y, Zhang Z, Liu L,
Kocherginsky M, Murphy R, Achenbach CJ, Musa J, Wehbe F, Just A, et
al: miRNA-processing gene methylation and cancer risk. Cancer
Epidemiol Biomarkers Prev. 27:550–557. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang G, Xiong G, Cao Z, Zheng S, You L,
Zhang T and Zhao Y: miR-497 expression, function and clinical
application in cancer. Oncotarget. 7:55900–55911. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Menigatti M, Staiano T, Manser CN,
Bauerfeind P, Komljenovic A, Robinson M, Jiricny J, Buffoli F and
Marra G: Epigenetic silencing of monoallelically methylated miRNA
loci in precancerous colorectal lesions. Oncogenesis. 2:e562013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Itesako T, Seki N, Yoshino H, Chiyomaru T,
Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa M
and Enokida H: The microRNA expression signature of bladder cancer
by deep sequencing: The functional significance of the miR-195/497
cluster. PLoS One. 9:e843112014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Danaher RJ, Miller CS, Berger JR,
Nubia VG, Wilfred BS, Neltner JH, Norris CM and Nelson PT:
Expression of miR-15/107 family microRNAs in human tissues and
cultured rat brain cells. Genomics Proteomics Bioinformatics.
12:19–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan L, Huang X, Shao Q, Huang M, Deng L,
Wu Q, Zeng Y and Shao J: MicroRNA miR-21 overexpression in human
breast cancer is associated with advanced clinical stage, lymph
node metastasis and patient poor prognosis. RNA. 14:2348–2360.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo J, Miao Y, Xiao B, Huan R, Jiang Z,
Meng D and Wang Y: Differential expression of microRNA species in
human gastric cancer versus non-tumorous tissues. J Gastroen
Hepatol. 24:652–657. 2009. View Article : Google Scholar
|
|
13
|
Flavin RJ, Smyth PC, Laios A, O'Toole SA,
Barrett C, Finn SP, Russell S, Ring M, Denning KM, Li J, et al:
Potentially important microRNA cluster on chromosome 17p13.1 in
primary peritoneal carcinoma. Mod Pathol. 22:197–205. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Özata DM, Caramuta S, Velázquez-Fernández
D, Akçakaya P, Xie H, Höög A, Zedenius J, Bäckdahl M, Larsson C and
Lui W: The role of microRNA deregulation in the pathogenesis of
adrenocortical carcinoma. Endocr-Relat Cancer. 18:643–655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caramuta S, Lee L, Ozata DM, Akçakaya P,
Xie H, Höög A, Zedenius J, Bäckdahl M, Larsson C and Lui WO:
Clinical and functional impact of TARBP2 over-expression in
adrenocortical carcinoma. Endocr Relat Cancer. 20:551–564. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang C, Wang C, Chen X, Chen S, Zhang Y,
Zhi F, Wang J, Li L, Zhou X, Li N, et al: Identification of seven
serum microRNAs from a genome-wide serum microRNA expression
profile as potential noninvasive biomarkers for malignant
astrocytomas. Int J Cancer. 132:116–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo ST, Jiang CC, Wang GP, Li YP, Wang CY,
Guo XY, Yang RH, Feng Y, Wang FH, Tseng HY, et al: MicroRNA-497
targets insulin-like growth factor 1 receptor and has a tumour
suppressive role in human colorectal cancer. Oncogene.
32:1910–1920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Jiang CF, Li DM, Ge X, Shi ZM, Li
CY, Liu X, Yin Y, Zhen L, Liu LZ and Jiang BH: MicroRNA-497
inhibits tumor growth and increases chemosensitivity to
5-fluorouracil treatment by targeting KSR1. Oncotarget.
7:2660–2671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang N, Shen Q and Zhang P: miR-497
suppresses epithelial-mesenchymal transition and metastasis in
colorectal cancer cells by targeting fos-related antigen-1. Onco
Targets Ther. 9:6597–6604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Y, Chen J, Gao C, Zhu D, Xu X, Wu C and
Jiang J: MicroRNA-497 inhibits tumor growth through targeting
insulin receptor substrate 1 in colorectal cancer. Oncol Lett.
14:6379–6386. 2017.PubMed/NCBI
|
|
21
|
Hong S, Yan Z, Wang H, Ding L and Bi M:
Up-regulation of microRNA-497-5p inhibits colorectal cancer cell
proliferation and invasion via targeting PTPN3. Biosci Rep.
39:BSR201911232019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Namløs HM, Meza-Zepeda LA, Barøy T,
Østensen IHG, Kresse SH, Kuijjer ML, Serra M, Bürger H,
Cleton-Jansen AM and Myklebost O: Modulation of the osteosarcoma
expression phenotype by microRNAs. PLoS One. 7:e480862012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo M, Shen D, Zhou X, Chen X and Wang W:
MicroRNA-497 is a potential prognostic marker in human cervical
cancer and functions as a tumor suppressor by targeting the
insulin-like growth factor 1 receptor. Surgery. 153:836–847. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie Y, Wei RR, Huang GL, Zhang MY, Yuan YF
and Wang HY: Checkpoint kinase 1 is negatively regulated by miR-497
in hepatocellular carcinoma. Med Oncol. 31:8442014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He XX, Kuang SZ, Liao JZ, Xu CR, Chang Y,
Wu YL, Gong J, Tian DA, Guo AY and Lin JS: The regulation of
microRNA expression by DNA methylation in hepatocellular carcinoma.
Mol Biosyst. 11:532–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furuta M, Kozaki K, Tanimoto K, Tanaka S,
Arii S, Shimamura T, Niida A, Miyano S and Inazawa J: The
tumor-suppressive miR-497-195 cluster targets multiple cell-cycle
regulators in hepatocellular carcinoma. PLoS One. 8:e601552013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y,
Li PY, Wang M, Lin JS and He XX: miR-497 suppresses angiogenesis
and metastasis of hepatocellular carcinoma by inhibiting VEGFA and
AEG-1. Oncotarget. 6:29527–29542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding Q, He K, Luo T, Deng Y, Wang H, Liu
H, Zhang J, Chen K, Xiao J, Duan X, et al: SSRP1 contributes to the
malignancy of hepatocellular carcinoma and is negatively regulated
by miR-497. Mol Ther. 24:903–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen L, Li J, Xu L, Ma J, Li H, Xiao X,
Zhao J and Fang L: miR-497 induces apoptosis of breast cancer cells
by targeting Bcl-w. Exp Ther Med. 3:475–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang
Y, Zou C, Zhang X, Liu S, Wang X, et al: Analysis of miR-195 and
miR-497 expression, regulation and role in breast cancer. Clin
Cancer Res. 17:1722–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei C, Luo Q, Sun X, Li D, Song H, Li X,
Song J, Hua K and Fang L: MicroRNA-497 induces cell apoptosis by
negatively regulating Bcl-2 protein expression at the
posttranscriptional level in human breast cancer. Int J Clin Exp
Pathol. 8:7729–7739. 2015.PubMed/NCBI
|
|
32
|
Creevey L, Ryan J, Harvey H, Bray IM,
Meehan M, Khan AR and Stallings RL: MicroRNA-497 increases
apoptosis in MYCN amplified neuroblastoma cells by targeting the
key cell cycle regulator WEE1. Mol Cancer. 12:232013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao WY, Wang Y, An ZJ, Shi CG, Zhu GA,
Wang B, Lu MY, Pan CK and Chen P: Downregulation of miR-497
promotes tumor growth and angiogenesis by targeting HDGF in
non-small cell lung cancer. Biochem Biophys Res Commun.
435:466–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin Q, Han Y, Zhu D, Li Z, Shan S, Jin W,
Lu Q and Ren T: miR-145 and miR-497 suppress TGF-β-induced
epithelial-mesenchymal transition of non-small cell lung cancer by
targeting MTDH. Cancer Cell Int. 18:1052018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang X, Wang L, Liu W and Li F:
MicroRNA-497-5p inhibits proliferation and invasion of non-small
cell lung cancer by regulating FGF2. Oncol Lett. 17:3425–3431.
2019.PubMed/NCBI
|
|
36
|
Li W, Jin X, Deng X, Zhang G, Zhang B and
Ma L: The putative tumor suppressor microRNA-497 modulates gastric
cancer cell proliferation and invasion by repressing eIF4E. Biochem
Biophys Res Commun. 449:235–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie G, Ke Q, Ji YZ, Wang AQ, Jing M and
Zou LL: FGFR1 is an independent prognostic factor and can be
regulated by miR-497 in gastric cancer progression. Braz J Med Biol
Res. 52:e78162018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng L, Cheng K, Zang R, Wang Q and Wang
J: miR-497-5p inhibits gastric cancer cell proliferation and growth
through targeting PDK3. Biosci Rep. 39:BSR201906542019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang W, Ren F, Wu Q, Jiang D, Li H, Peng
Z, Wang J and Shi H: MicroRNA-497 inhibition of ovarian cancer cell
migration and invasion through targeting of SMAD specific E3
ubiquitin protein ligase 1. Biochem Biophys Res Commun.
449:432–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang S, Mo Y, Midorikawa K, Zhang Z, Huang
G, Ma N, Zhao W, Hiraku Y, Oikawa S and Murata M: The potent tumor
suppressor miR-497 inhibits cancer phenotypes in nasopharyngeal
carcinoma by targeting ANLN and HSPA4L. Oncotarget. 6:35893–35907.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ruan WD, Wang P, Feng S, Xue Y and Zhang
B: MicroRNA-497 inhibits cell proliferation, migration, and
invasion by targeting AMOT in human osteosarcoma cells. Onco
Targets Ther. 9:303–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun Z, Li A, Yu Z, Li X, Guo X and Chen R:
MicroRNA-497-5p suppresses tumor cell growth of osteosarcoma by
targeting ADP ribosylation factor-like protein 2. Cancer Biother
Radiopharm. 32:371–378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Y, Kuang D, Zhao X, Chen D, Wang X,
Yang Q, Wan J, Zhu Y, Wang Y, Zhang S, et al: miR-497-5p inhibits
cell proliferation and invasion by targeting KCa3.1 in
angiosarcoma. Oncotarget. 7:58148–58161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang H, Wu XL, Wu KH, Zhang R, Ju LL, Ji
Y, Zhang YW, Xue SL, Zhang YX, Yang YF, et al: MicroRNA-497
regulates cisplatin chemosensitivity of cervical cancer by
targeting transketolase. Am J Cancer Res. 6:2690–2699.
2016.PubMed/NCBI
|
|
45
|
Chen Y, Du J, Wang Y, Shi H, Jiang Q,
Wang' Y, Zhang H, Wei Y, Xue W, Pu Z, et al: MicroRNA-497-5p
induces cell cycle arrest of cervical cancer cells in s phase by
targeting CBX4. Onco Targets Ther. 12:10535–10545. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang P, Meng X, Huang Y, Lv Z, Liu J, Wang
G, Meng W, Xue S, Zhang Q, Zhang P, et al: MicroRNA-497 inhibits
thyroid cancer tumor growth and invasion by suppressing BDNF.
Oncotarget. 8:2825–2834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhuang J, Ye Y, Wang G, Ni J, He S, Hu C,
Xia W and Lv Z: MicroRNA-497 inhibits cellular proliferation,
migration and invasion of papillary thyroid cancer by directly
targeting AKT3. Mol Med Rep. 16:5815–5822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mizrahi A, Barzilai A, Gur-Wahnon D,
Ben-Dov IZ, Glassberg S, Meningher T, Elharar E, Masalha M,
Jacob-Hirsch J, Tabibian-Keissar H, et al: Alterations of microRNAs
throughout the malignant evolution of cutaneous squamous cell
carcinoma: The role of miR-497 in epithelial to mesenchymal
transition of keratinocytes. Oncogene. 37:218–230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wei XH, Gu XL, Zhou XT, Ma M and Lou CX:
miR-497 promotes the progression of cutaneous squamous cell
carcinoma through FAM114A2. Eur Rev Med Pharmacol Sci.
22:7348–7355. 2018.PubMed/NCBI
|
|
50
|
Chai L, Kang XJ, Sun ZZ, Zeng MF, Yu SR,
Ding Y, Liang JQ, Li TT and Zhao J: miR-497-5p, miR-195-5p and
miR-455-3p function as tumor suppressors by targeting hTERT in
melanoma A375 cells. Cancer Manag Res. 10:989–1003. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu F, Ye Y, Zhang H, He X, Sun X, Yao C,
Mao H, He X, Qian C, Wang B, et al: miR-497/Wnt3a/c-jun feedback
loop regulates growth and epithelial-to-mesenchymal transition
phenotype in glioma cells. Int J Biol Macromol. 120:985–991. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qu F, Ye J, Pan X, Wang J, Gan S, Chu C,
Chu J, Zhang X, Liu M, He H and Cui X: MicroRNA-497-5p
down-regulation increases PD-L1 expression in clear cell renal cell
carcinoma. J Drug Target. 27:67–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hoareau-Aveilla C, Quelen C, Congras A,
Caillet N, Labourdette D, Dozier C, Brousset P, Lamant L and
Meggetto F: miR-497 suppresses cycle progression through an axis
involving CDK6 in ALK-positive cells. Haematologica. 104:347–359.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang L, Li K, Lin X, Yao Z, Wang S, Xiong
X, Ning Z, Wang J, Xu X, Jiang Y, et al: Metformin induces human
esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1
axis. Cancer Lett. 450:22–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Y, Zhu Z, Huang S, Zhao Q, Huang C,
Tang Y, Sun C, Zhang Z, Wang L, Chen H, et al: lncRNA XIST
regulates proliferation and migration of hepatocellular carcinoma
cells by acting as miR-497-5p molecular sponge and targeting PDCD4.
Cancer Cell Int. 19:1982019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Maura F, Cutrona G, Mosca L, Matis S,
Lionetti M, Fabris S, Agnelli L, Colombo M, Massucco C, Ferracin M,
et al: Association between gene and miRNA expression profiles and
stereotyped subset #4 B-cell receptor in chronic lymphocytic
leukemia. Leuk Lymphoma. 56:3150–3158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wald AI, Hoskins EE, Wells SI, Ferris RL
and Khan SA: Alteration of microRNA profiles in squamous cell
carcinoma of the head and neck cell lines by human papillomavirus.
Head Neck. 33:504–512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Matsuyama H and Suzuki HI: Systems and
synthetic microRNA biology: From biogenesis to disease
pathogenesis. Int J Mol Sci. 21:1322019. View Article : Google Scholar
|
|
59
|
Daura-Oller E, Cabre M, Montero MA,
Paternain JL and Romeu A: Specific gene hypomethylation and cancer:
New insights into coding region feature trends. Bioinformation.
3:340–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Esteller M: Relevance of DNA methylation
in the management of cancer. Lancet Oncol. 4:351–358. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang X, Wang W, Zhu W, Dong J, Cheng Y,
Yin Z and Shen F: Mechanisms and functions of long non-coding RNAs
at multiple regulatory levels. Int Mol Sci. 20:55732019. View Article : Google Scholar
|
|
62
|
Loda A and Heard E: Xist RNA in action:
Past, present, and future. PLoS Genet. 15:e10083332019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang Z, Jiang X, Jiang X and Zhao H:
X-inactive-specific transcript: A long noncoding RNA with complex
roles in human cancers. Gene. 679:28–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ma L, Zhou Y, Luo X, Gao H, Deng X and
Jiang Y: Long non-coding RNA XIST promotes cell growth and invasion
through regulating miR-497/MACC1 axis in gastric cancer.
Oncotarget. 8:4125–4135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li M, Tang X, Fu Y, Wang T and Zhu J:
Regulatory mechanisms and clinical applications of the long
non-coding RNA PVT1 in cancer treatment. Front Oncol. 9:7872019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song J, Wu X, Liu F, Li M, Sun Y, Wang Y,
Wang C, Zhu K, Jia X, Wang B and Ma X: Long non-coding RNA PVT1
promotes glycolysis and tumor progression by regulating miR-497/HK2
axis in osteosarcoma. Biochem Biophys Res Commun. 490:217–2124.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Guo D, Wang Y, Ren K and Han X: Knockdown
of LncRNA PVT1 inhibits tumorigenesis in non-small-cell lung cancer
by regulating miR-497 expression. Exp Cell Res. 362:172–179. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Feng L, Zhu Y, Zhang Y and Rao M: LncRNA
GACAT3 promotes gastric cancer progression by negatively regulating
miR-497 expression. Biomed Pharmacother. 97:136–142. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhong H, Yang J, Zhang B, Wang X, Pei L,
Zhang L, Lin Z, Wang Y and Wang C: LncRNA GACAT3 predicts poor
prognosis and promotes cell proliferation in breast cancer through
regulation of miR-497/CCND2. Cancer Biomark. 22:787–797. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xia T, Chen J, Wu K, Zhang J and Yan Q:
Long noncoding RNA NEAT1 promotes the growth of gastric cancer
cells by regulating miR-497-5p/PIK3R1 axis. Eur Rev Med Pharmacol
Sci. 23:6914–6926. 2019.PubMed/NCBI
|
|
71
|
Sun Z, Guo X, Zang M, Wang P, Xue S and
Chen G: Long non-coding RNA LINC00152 promotes cell growth and
invasion of papillary thyroid carcinoma by regulating the
miR-497/BDNF axis. J Cell Physiol. 234:1336–1345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yu T, Xu Z, Zhang X, Men L and Nie H: Long
intergenic non-protein coding RNA 152 promotes multiple myeloma
progression by negatively regulating microRNA-497. Oncol Rep.
40:3763–3771. 2018.PubMed/NCBI
|
|
73
|
Xu D, Chen Y, Yuan C, Zhang S and Peng W:
Long non-coding RNA LINC00662 promotes proliferation and migration
in oral squamous cell carcinoma. Onco Targets Ther. 12:647–656.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu Z, Yao Y, Huang S, Li L, Jiang B, Guo
H, Lei W, Xiong J and Deng J: LINC00662 promotes gastric cancer
cell growth by modulating the Hippo-YAP1 pathway. Biochem Bioph Res
Commun. 505:843–859. 2018. View Article : Google Scholar
|
|
75
|
Xu X, Gu J, Ding X, Ge G, Zang X, Ji R,
Shao M, Mao Z, Zhang Y, Zhang J, et al: LINC00978 promotes the
progression of hepatocellular carcinoma by regulating EZH2-mediated
silencing of p21 and E-cadherin expression. Cell Death Dis.
10:7522019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bu J, Lv W, Liao Y, Xiao X and Lv B: Long
non-coding RNA LINC00978 promotes cell proliferation and
tumorigenesis via regulating microRNA-497/NTRK3 axis in gastric
cancer. Int J Biol Macromol. 123:1106–1114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dong B, Chen X, Zhang Y, Zhu C and Dong Q:
The prognostic value of lncRNA SNHG1 in cancer patients: A
meta-analysis. BMC Cancer. 19:7802019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li Z, Lu Q, Zhu D, Han Y, Zhou X and Ren
T: Lnc-SNHG1 may promote the progression of non-small cell lung
cancer by acting as a sponge of miR-497. Biochem Bioph Res Commun.
506:632–640. 2018. View Article : Google Scholar
|
|
79
|
Bai J, Xu J, Zhao J and Zhang R: lncRNA
SNHG1 cooperated with miR-497/miR-195-5p to modify
epithelial-mesenchymal transition underlying colorectal cancer
exacerbation. J Cell Physiol. 235:1453–1468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Christensen LL, True K, Hamilton MP,
Nielsen MM, Damas ND, Damgaard CK, Ongen H, Dermitzakis E, Bramsen
JB, Pedersen JS, et al: SNHG16 is regulated by the Wnt pathway in
colorectal cancer and affects genes involved in lipid metabolism.
Mol Oncol. 10:1266–1282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wen Q, Zhao L, Wang T, Lv N, Cheng X,
Zhang G and Bai L: LncRNA SNHG16 drives proliferation and invasion
of papillary thyroid cancer through modulation of miR-497. Onco
Targets Ther. 12:699–708. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhu Q, Li Y, Guo Y, Hu L, Xiao Z, Liu X,
Wang J, Xu Q and Tong X: Long non-coding RNA SNHG16 promotes
proliferation and inhibits apoptosis of diffuse large B-cell
lymphoma cells by targeting miR-497-5p/PIM1 axis. J Cell Mol Med.
23:7395–7405. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu N, Wang Z, Liu D and Xie P:
HOXC13-AS-miR-122-5p-SATB1-C-Myc feedback loop promotes migration,
invasion and EMT process in glioma. Onco Targets Ther.
12:7165–7173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li X, Wang Q, Rui Y, Zhang C, Wang W, Gu
J, Tang J and Ding Y: HOXC13-AS promotes breast cancer cell growth
through regulating miR-497-5p/PTEN axis. J Cell Physiol.
234:22343–22351. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang L, Wang Y, Li X, Xia X, Li N, He R,
He H, Han C and Zhao W: ZBTB7A Enhances Osteosarcoma
Chemoresistance by Transcriptionally Repressing
lncRNALINC00473-IL24 Activity. Neoplasia. 19:908–918. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bai J, Zhao W, Li W, Ying Z and Jiang D:
Long noncoding RNA LINC00473 indicates a poor prognosis of breast
cancer and accelerates tumor carcinogenesis by competing endogenous
sponging miR-497. Eur Rev Med Pharmaco. 23:3410–3420. 2019.
|
|
87
|
He Z: LINC00473/miR-497-5p regulates
esophageal squamous cell carcinoma progression through targeting
PRKAA1. Cancer Biother Radiopharm. 34:650–659. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li Z, Zhu Q, Zhang H, Hu Y, Wang G and Zhu
Y: MALAT1: A potential biomarker in cancer. Cancer Manag Res.
10:6757–6768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hassan N, Zhao J, Glover A, Robinson BG
and Sidhu SB: Reciprocal interplay of miR-497 and MALAT1 promotes
tumourigenesis of adrenocortical cancer. Endocr Relat Cancer.
26:677–688. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhao P, Guan H, Dai Z, Ma Y, Zhao Y and
Liu D: Long noncoding RNA DLX6-AS1 promotes breast cancer
progression via miR-505-3p/RUNX2 axis. Eur J Pharmacol.
865:1727782019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang J, Ye Z, Mei D, Gu H and Zhang J:
Long noncoding RNA DLX6-AS1 promotes tumorigenesis by modulating
miR-497-5p/FZD4/FZD6/Wnt/β-catenin pathway in pancreatic cancer.
Cancer Manag Res. 11:4209–4221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang R, Hao S, Yang L, Xie J, Chen S and
Gu G: LINC00339 promotes cell proliferation and metastasis in
pancreatic cancer via miR-497-5p/IGF1R axis. J BUON. 24:729–738.
2019.PubMed/NCBI
|
|
93
|
Zhang J, Chen Z, Chen D, Tian X, Wang C,
Zhou Z, Gao Y, Xu Y, Chen C, Zheng Z, et al:
LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer
angiogenesis and metastasis. Oncogene. 37:2660–2675. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Cai M, Xu L, Shen L and Zhang J: The
expression of long non-coding RNA-LINC01410 in pancreatic cancer
and its effect on proliferation and migration of pancreatic cancer
cells. Zhonghua Yi Xue Za Zhi. 99:1406–1411. 2019.(In Chinese).
PubMed/NCBI
|
|
95
|
Yu C and Zhang F: LncRNA AC009022.1
enhances colorectal cancer cells proliferation, migration, and
invasion by promoting ACTR3B expression via suppressing miR-497-5p.
J Cell Biochem. 121:1934–1944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cui X, Yu T, Shang J, Xiao D and Wang X:
Long Non-Coding RNA CDKN2B-AS1 facilitates laryngeal squamous cell
cancer through regulating miR-497/CDK6 Pathway. Onco Targets Ther.
12:8853–8862. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yin Y, Long J, He Q, Li Y, Liao Y, He P
and Zhu W: Emerging roles of circRNA in formation and progression
of cancer. J Cancer. 10:5015–5021. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Gao Y, Zhang M, Xu B, Han L, Lan S, Chen
J, Dong Y and Cao L: Circular RNA expression profiles reveal that
hsa_circ_0018289 is up-regulated in cervical cancer and promotes
the tumorigenesis. Oncotarget. 8:86625–86633. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Adhikary J, Chakraborty S, Dalal S, Basu
S, Dey A and Ghosh A: Circular PVT1: An oncogenic non-coding RNA
with emerging clinical importance. J Clin Pathol. 72:513–519. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Verduci L, Ferraiuolo M, Sacconi A, Ganci
F, Vitale J, Colombo T, Paci P, Strano S, Macino G, Rajewsky N and
Blandino G: The oncogenic role of circPVT1 in head and neck
squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD
transcription-competent complex. Genome Biol. 18:2372017.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Qin S, Zhao Y, Lim G, Lin H and Zhang X
and Zhang X: Circular RNA PVT1 acts as a competing endogenous RNA
for miR-497 in promoting non-small cell lung cancer progression.
Biomed Pharmacother. 111:244–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mechtler P, Singhal R, Kichina JV, Bard
JE, Buck MJ and Kandel ES: MicroRNA analysis suggests an additional
level of feedback regulation in the NF-κB signaling cascade.
Oncotarget. 6:17097–17106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kong X, Duan L, Qian X, Xu D, Liu H, Zhu Y
and Qi J: Tumor-suppressive microRNA-497 targets IKKβ to regulate
NF-κB signaling pathway in human prostate cancer cells. Am J Cancer
Res. 5:1795–1804. 2015.PubMed/NCBI
|
|
104
|
Zhao B, Wang Y, Tan X, Ke K, Zheng X, Wang
F, Lan S, Liao N, Cai Z, Shi Y, et al: Inflammatory
Micro-environment contributes to stemness properties and metastatic
potential of HCC via the NF-κB/miR-497/SALL4 Axis. Mol Ther
Oncolytics. 15:79–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Huang C, Ma R, Yue J, Li N, Li Z and Qi D:
miR-497 Suppresses YAP1 and Inhibits Tumor Growth in Non-Small Cell
Lung Cancer. Cell Physiol Biochem. 37:342–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang L, Yu Z, Xian Y and Lin X:
microRNA-497 inhibits cell proliferation and induces apoptosis by
targeting YAP1 in human hepatocellular carcinoma. FEBS Open Bio.
6:155–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Cheng H, Dong H, Feng J, Tian H, Zhang H
and Xu L: miR-497 inhibited proliferation, migration and invasion
of thyroid papillary carcinoma cells by negatively regulating YAP1
expression. Onco Targets Ther. 11:4711–4721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wu Z, Li X, Cai X, Huang C and Zheng M:
miR-497 inhibits epithelial mesenchymal transition in breast
carcinoma by targeting Slug. Tumour Biol. 37:7939–7950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lin D and Wu J: Hypoxia inducible factor
in hepatocellular carcinoma: A therapeutic target. World J
Gastroenterol. 21:12171–12178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lan J, Xue Y, Chen H, Zhao S, Wu Z, Fang
J, Han C and Lou M: Hypoxia-induced miR-497 decreases glioma cell
sensitivity to TMZ by inhibiting apoptosis. FEBS Lett.
588:3333–3339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu Z, Cai X, Huang C, Xu J and Liu A:
miR-497 suppresses angiogenesis in breast carcinoma by targeting
HIF-1α. Oncol Rep. 35:1696–1702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Qiao W, Jia Z, Liu H, Liu Q, Zhang T, Guo
W, Li P, Deng M and Li S: Prognostic and clinicopathological value
of Twist expression in breast cancer: A meta-analysis. PLoS One.
12:e1861912017. View Article : Google Scholar
|
|
113
|
Liu A, Huang C, Cai X, Xu J and Yang D:
Twist promotes angiogenesis in pancreatic cancer by targeting
miR-497/VEGFA axis. Oncotarget. 7:25801–25814. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Gu YY, Liu XS, Huang XR, Yu XQ and Lan HY:
Diverse role of TGF-β in kidney disease. Front Cell Dev Biol.
8:1232020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Jafarzadeh M, Soltani BM, Dokanehiifard S,
Kay M, Aghdami N and Hosseinkhani S: Experimental evidences for
hsa-miR-497-5p as a negative regulator of SMAD3 gene expression.
Gene. 586:216–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Liu J, Zhou Y, Shi Z, Hu Y, Meng T, Zhang
X, Zhang S and Zhang J: microRNA-497 modulates breast cancer cell
proliferation, invasion, and survival by targeting SMAD7. DNA Cell
Biol. 35:521–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hu J, Xu J and Ge W: miR-497 enhances
metastasis of oral squamous cell carcinoma through SMAD7
suppression. Am J Transl Res. 8:3023–3031. 2016.PubMed/NCBI
|
|
118
|
Al-Hujaily EM, Tang Y, Yao DS, Carmona E,
Garson K and Vanderhyden BC: Divergent roles of PAX2 in the
etiology and progression of ovarian cancer. Cancer Prev Res
(Phila). 8:1163–1173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lin Z, Zhao J, Wang X, Zhu X and Gong L:
Overexpression of microRNA-497 suppresses cell proliferation and
induces apoptosis through targeting paired box 2 in human ovarian
cancer. Oncol Rep. 36:2101–2107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang Y, Zhang Z, Li Z, Gong D, Zhan B,
Man X and Kong C: MicroRNA-497 inhibits the proliferation,
migration and invasion of human bladder transitional cell carcinoma
cells by targeting E2F3. Oncol Rep. 36:1293–1300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Han H, Du Y, Zhao W, Li S, Chen D, Zhang
J, Liu J, Suo Z, Bian X, Xing B and Zhang Z: PBX3 is targeted by
multiple miRNAs and is essential for liver tumour-initiating cells.
Nat Commun. 6:82712015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yu T, Zhang X, Zhang L, Wang Y, Pan H, Xu
Z and Pang X: MicroRNA-497 suppresses cell proliferation and
induces apoptosis through targeting PBX3 in human multiple myeloma.
Am J Cancer Res. 6:2880–2889. 2016.PubMed/NCBI
|
|
123
|
Ma S, Chan YP, Woolcock B, Hu L, Wong KY,
Ling MT, Bainbridge T, Webber D, Chan TH, Guan XY, et al: DNA
fingerprinting tags novel altered chromosomal regions and
identifies the involvement of SOX5 in the progression of prostate
cancer. Int J Cancer. 124:2323–2332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li G, Wang K, Wang J, Qin S, Sun X and Ren
H: miR-497-5p inhibits tumor cell growth and invasion by targeting
SOX5 in non-small-cell lung cancer. J Cell Biochem.
120:10587–10595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Han L, Liu B, Jiang L, Liu J and Han S:
MicroRNA-497 downregulation contributes to cell proliferation,
migration, and invasion of estrogen receptor alpha negative breast
cancer by targeting estrogen-related receptor alpha. Tumour Biol.
37:13205–13214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Waters PS, McDermott AM, Wall D, Heneghan
HM, Miller N, Newell J, Kerin MJ and Dwyer RM: Relationship between
circulating and tissue microRNAs in a murine model of breast
cancer. PLoS One. 7:e504592012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Regazzo G, Terrenato I, Spagnuolo M,
Carosi M, Cognetti G, Cicchillitti L, Sperati F, Villani V,
Carapella C, Piaggio G, et al: A restricted signature of serum
miRNAs distinguishes glioblastoma from lower grade gliomas. J Exp
Clin Cancer Res. 35:1242016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Murata M: Inflammation and cancer. Environ
Health Prev Med. 23:502018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Du M, Shi D, Yuan L, Li P, Chu H, Qin C,
Yin C, Zhang Z and Wang M: Circulating miR-497 and miR-663b in
plasma are potential novel biomarkers for bladder cancer. Sci Rep.
5:104372015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang Y, Zhang D, Wang F, Xu D, Guo Y and
Cui W: Serum miRNAs panel (miR-16-2*, miR-195, miR-2861, miR-497)
as novel non-invasive biomarkers for detection of cervical cancer.
Sci Rep. 5:179422015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zou G, Wang R and Wang M: Clinical
response and prognostic significance of serum miR-497 expression in
colorectal cancer. Cancer Biomark. 25:11–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Shao X, Miao M, Xue J, Xue J, Ji X and Zhu
H: The Down-regulation of MicroRNA-497 contributes to cell growth
and cisplatin resistance through PI3K/Akt pathway in osteosarcoma.
Cell Physiol Biochem. 36:2051–2062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Xu S, Fu G, Tao Z, OuYang J, Kong F, Jiang
B, Wan X and Chen K: miR-497 decreases cisplatin resistance in
ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget.
6:26457–26471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Xu J, Wang T, Cao Z, Huang H, Li J, Liu W,
Liu S, You L, Zhou L, Zhang T and Zhao Y: miR-497 downregulation
contributes to the malignancy of pancreatic cancer and associates
with a poor prognosis. Oncotarget. 5:6983–6993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Troppan K, Wenzl K, Pichler M, Pursche B,
Schwarzenbacher D, Feichtinger J, Thallinger GG, Beham-Schmid C,
Neumeister P and Deutsch A: miR-199a and miR-497 are associated
with better overall survival due to increased chemosensitivity in
diffuse large B-cell lymphoma patients. Int J Mol Sci.
16:18077–18095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tian F, Zhan Y, Zhu W, Li J, Tang M, Chen
X and Jiang J: MicroRNA-497 inhibits multiple myeloma growth and
increases susceptibility to bortezomib by targeting Bcl-2. Int J
Mol Med. 43:1058–1066. 2019.PubMed/NCBI
|
|
137
|
Poel D, Boyd LNC, Beekhof R, Schelfhorst
T, Pham TV, Piersma SR, Knol JC, Jimenez CR, Verheul HMW and
Buffart TE: Proteomic analysis of miR-195 and miR-497 replacement
reveals potential candidates that increase sensitivity to
oxaliplatin in MSI/P53wt colorectal cancer cells. Cells.
8:11112019. View Article : Google Scholar
|
|
138
|
Ma W, Kang Y, Ning L, Tan J, Wang H and
Ying Y: Identification of microRNAs involved in gefitinib
resistance of non-small-cell lung cancer through the insulin-like
growth factor receptor 1 signaling pathway. Exp Ther Med.
14:2853–2862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhu D, Tu M, Zeng B, Cai L, Zheng W, Su Z
and Yu Z: Up-regulation of miR-497 confers resistance to
temozolomide in human glioma cells by targeting mTOR/Bcl-2. Cancer
Med. 6:452–462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Pang P, Shi X, Huang W and Sun K: miR-497
as a potential serum biomarker for the diagnosis and prognosis of
osteosarcoma. Eur Rev Med Pharmacol Sci. 20:3765–3769.
2016.PubMed/NCBI
|
|
141
|
Svedman FC, Lohcharoenkal W, Bottai M,
Brage SE, Sonkoly E, Hansson J, Pivarcsi A and Eriksson H:
Extracellular microvesicle microRNAs as predictive biomarkers for
targeted therapy in metastastic cutaneous malignant melanoma. PLoS
One. 13:e2069422018. View Article : Google Scholar
|
|
142
|
Sandhu V, Bowitz Lothe IM, Labori KJ,
Lingjærde OC, Buanes T, Dalsgaard AM, Skrede ML, Hamfjord J,
Haaland T, Eide TJ, et al: Molecular signatures of mRNAs and miRNAs
as prognostic biomarkers in pancreatobiliary and intestinal types
of periampullary adenocarcinomas. Mol Oncol. 9:758–771. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Wong N, Khwaja SS, Baker CM, Gay HA,
Thorstad WL, Daly MD, Lewis JS Jr and Wang X: Prognostic microRNA
signatures derived from The Cancer Genome Atlas for head and neck
squamous cell carcinomas. Cancer Med. 5:1619–1628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Feng J, Gu X, Liu L, Lu M, Ma X, Cao Y,
Jiang R, Wang B and Zhao Q: Prognostic role of microRNA-497 in
cancer patients: A Meta-analysis. J Cancer. 9:3334–3342. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu Z, Wu S, Wang L, Kang S, Zhao B, He F,
Liu X, Zeng Y and Liu J: Prognostic value of MicroRNA-497 in
various cancers: A systematic review and Meta-analysis. Dis
Markers. 2019:24912912019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Department of Medical Administration,
National Health and Health Commission of the People's Republic of
China: Guidelines for diagnosis and treatment of primary liver
cancer in China (2019 edition). Zhonghua Gan Zang Bing Za Zhi.
28:112–128. 2020.(In Chinese). PubMed/NCBI
|