|
1
|
Yu Y, Luo W, Yang ZJ, Chi JR, Li YR, Ding
Y, Ge J, Wang X and Cao XC: miR-190 suppresses breast cancer
metastasis by regulation of TGF-β-induced epithelial-mesenchymal
transition. Mol Cancer. 17:702018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu BW, Yu ZH, Chen AX, Chi JR, Ge J, Yu Y
and Cao XC: Estrogen receptor-α-miR-1271-SNAI2 feedback loop
regulates transforming growth factor-β-induced breast cancer
progression. J Exp Clin Cancer Res. 38:1092019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van de Water W, Bastiaannet E, Dekkers OM,
de Craen AJ, Westendorp RG, Voogd AC, van de Velde CJ and Liefers
GJ: Adherence to treatment guidelines and survival in patients with
early-stage breast cancer by age at diagnosis. Br J Surg.
99:813–820. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duffy SW, Morrish OWE, Allgood PC, Black
R, Gillan MGC, Willsher P, Cooke J, Duncan KA, Michell MJ, Dobson
HM, et al: Mammographic density and breast cancer risk in breast
screening assessment cases and women with a family history of
breast cancer. Eur J Cancer. 88:48–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Derks MGM, Bastiaannet E, van de Water W,
de Glas NA, Seynaeve C, Putter H, Nortier JWR, Rea D, Hasenburg A,
Markopoulos C, Dirix LY, et al: Impact of age on breast cancer
mortality and competing causes of death at 10 years follow-up in
the adjuvant TEAM trial. Eur J Cancer. 99:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McPherson K, Steel CM and Dixon JM: ABC of
breast diseases. Breast cancer-epidemiology, risk factors, and
genetics. BMJ. 309:1003–1006. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ursin G, Ma H, Wu AH, Bernstein L, Salane
M, Parisky YR, Astrahan M, Siozon CC and Pike MC: Mammographic
density and breast cancer in three ethnic groups. Cancer Epidemiol
Biomarkers Prev. 12:332–338. 2003.PubMed/NCBI
|
|
9
|
Vachon CM, van Gils CH, Sellers TA, Ghosh
K, Pruthi S, Brandt KR and Pankratz VS: Mammographic density,
breast cancer risk and risk prediction. Breast Cancer Res.
9:2172007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Huang R, Ma L, Liu S and Zong X:
Locoregional surgical treatment improves the prognosis in primary
metastatic breast cancer patients with a single distant metastasis
except for brain metastasis. Breast. 45:104–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mamounas EP, Anderson SJ, Dignam JJ, Bear
HD, Julian TB, Geyer CE Jr, Taghian A, Wickerham DL and Wolmark N:
Predictors of locoregional recurrence after neoadjuvant
chemotherapy: Results from combined analysis of National Surgical
Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol.
30:3960–3966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kapoor PM, Lindstrom S, Behrens S, Wang X,
Michailidou K, Bolla MK, Wang Q, Dennis J, Dunning AM, Pharoah PDP,
et al: Assessment of interactions between 205 breast cancer
susceptibility loci and 13 established risk factors in relation to
breast cancer risk, in the Breast Cancer Association Consortium.
Int J Epidemiol. 49:216–232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: An evolving concept. Nat Rev Cancer. 12:133–143.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W, Qin Y, Wang D, Zhou L, Liu Y, Chen
S, Yin L, Xiao Y, Yao XH, Yang X, et al: CCL20 triggered by
chemotherapy hinders the therapeutic efficacy of breast cancer.
PLoS Biol. 16:e20058692018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sueoka T, Koyama K, Hayashi G and Okamoto
A: Chemistry-driven epigenetic investigation of histone and DNA
modifications. Chem Rec. 18:1727–1744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Long H, Chang C, Zhao M and Lu Q:
Crosstalk between metabolism and epigenetic modifications in
autoimmune diseases: A comprehensive overview. Cell Mol Life Sci.
75:3353–3369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dziaman T, Gackowski D, Guz J, Linowiecka
K, Bodnar M, Starczak M, Zarakowska E, Modrzejewska M, Szpila A,
Szpotan J, et al: Characteristic profiles of DNA epigenetic
modifications in colon cancer and its predisposing
conditions-benign adenomas and inflammatory bowel disease. Clin
Epigenetics. 10:722018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhol CS, Panigrahi DP, Praharaj PP,
Mahapatra KK, Patra S, Mishra SR, Behera BP and Bhutia SK:
Epigenetic modifications of autophagy in cancer and cancer
therapeutics. Semin Cancer Biol. 66:22–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su J, Huang YH, Cui X, Wang X, Zhang X,
Lei Y, Xu J, Lin X, Chen K, Lv J, et al: Homeobox oncogene
activation by pan-cancer DNA hypermethylation. Genome Biol.
19:1082018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zummeren MV, Kremer WW, Leeman A, Bleeker
MCG, Jenkins D, Sandt MV, Doorbar J, Heideman DAM, Steenbergen RDM,
Snijders PJF, et al: HPV E4 expression and DNA hypermethylation of
CADM1, MAL, and miR124-2 genes in cervical cancer and precursor
lesions. Mod Pathol. 31:1842–1850. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Angulo JC, Andres G, Ashour N,
Sanchez-Chapado M, Lopez JI and Ropero S: Development of castration
resistant prostate cancer can be predicted by a DNA
hypermethylation profile. J Urol. 195:619–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdel-Hafiz HA and Horwitz KB: Role of
epigenetic modifications in luminal breast cancer. Epigenomics.
7:847–862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Han S, Li Y, Liu Y, Zhang D, Li Y
and Zhang J: MicroRNA-20a contributes to cisplatin-resistance and
migration of OVCAR3 ovarian cancer cell line. Oncol Lett.
14:1780–1786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Safdari Y, Khalili M, Ebrahimzadeh MA,
Yazdani Y and Farajnia S: Natural inhibitors of PI3K/AKT signaling
in breast cancer: Emphasis on newly-discovered molecular mechanisms
of action. Pharmacol Res. 93:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Avanzato D, Pupo E, Ducano N, Isella C,
Bertalot G, Luise C, Pece S, Bruna A, Rueda OM, Caldas C, et al:
High USP6NL levels in breast cancer sustain chronic AKT
phosphorylation and GLUT1 stability fueling aerobic glycolysis.
Cancer Res. 78:3432–3444. 2018.PubMed/NCBI
|
|
27
|
Khor TO, Gul YA, Ithnin H and Seow HF:
Positive correlation between overexpression of phospho-BAD with
phosphorylated Akt at serine 473 but not threonine 308 in
colorectal carcinoma. Cancer Lett. 210:139–150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Long YH, Wang SQ, Zhang YY, Li YF,
Mi JS, Yu CH, Li DY, Zhang JH and Zhang XJ: JMJD6 regulates histone
H2A.X phosphorylation and promotes autophagy in triple-negative
breast cancer cells via a novel tyrosine kinase activity. Oncogene.
38:980–997. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Long YH, Wang SQ, Li YF and Zhang
JH: Phosphorylation of H2A.XTyr39 positively regulates
DNA damage response and is linked to cancer progression. FEBS J.
283:4462–4473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma A, Singh K and Almasan A: Histone
H2AX phosphorylation: A marker for DNA damage. Methods Mol Biol.
920:613–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Connor MJ: Targeting the DNA damage
response in cancer. Mol Cell. 60:547–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weyemi U, Redon CE, Sethi TK, Burrell AS,
Jailwala P, Kasoji M, Abrams N, Merchant A and Bonner WM: Twist1
and Slug mediate H2AX-regulated epithelial-mesenchymal transition
in breast cells. Cell Cycle. 15:2398–2404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
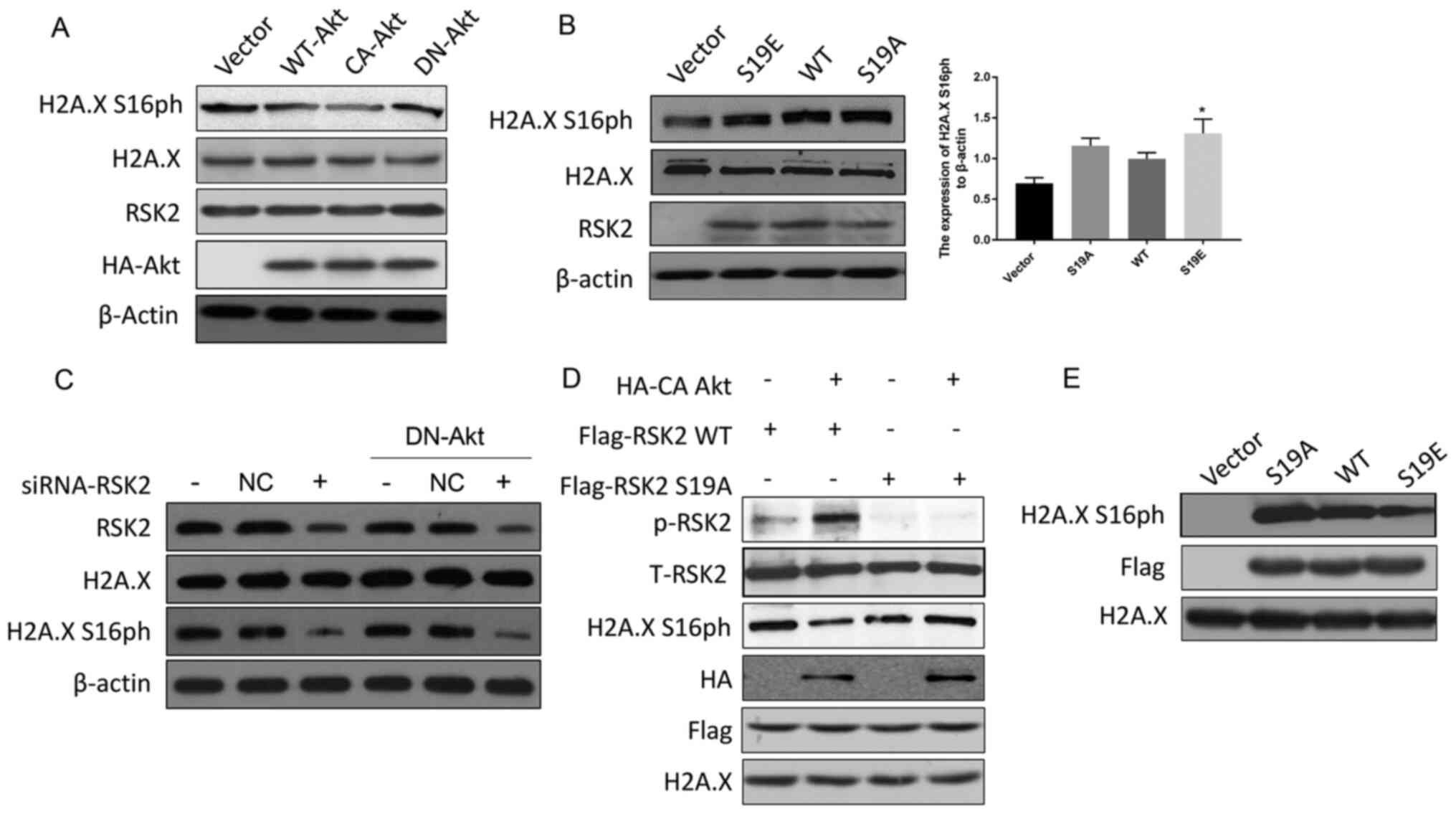
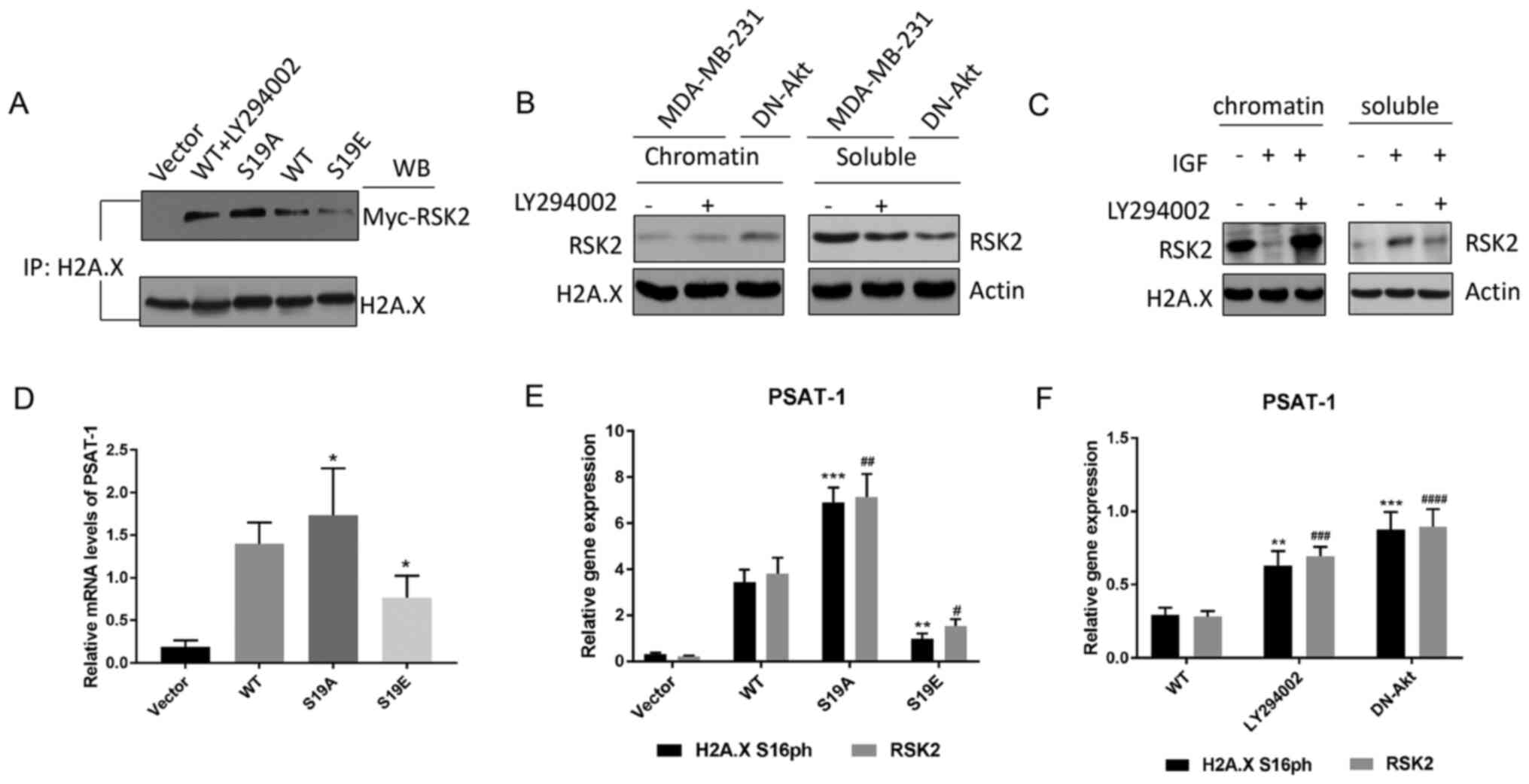
Zhu F, Zykova TA, Peng C, Zhang J, Cho YY,
Zheng D, Yao K, Ma WY, Lau AT, Bode AM and Dong Z: Phosphorylation
of H2AX at Ser139 and a new phosphorylation site Ser16 by RSK2
decreases H2AX ubiquitination and inhibits cell transformation.
Cancer Res. 71:393–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qiu Q, Jiang J, Lin L, Cheng S, Xin D,
Jiang W, Shen J and Hu Z: Downregulation of RSK2 influences the
biological activities of human osteosarcoma cells through
inactivating AKT/mTOR signaling pathways. Int J Oncol.
48:2508–2520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee YJ, Bae JH, Kim SA, Kim SH, Woo KM,
Nam HS, Cho MK and Lee SH: Cariporide Enhances the DNA damage and
apoptosis in acid-tolerable malignant mesothelioma H-2452 cells.
Mol Cells. 40:567–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR.
Methods. 25:402–408. 2002. View Article : Google Scholar
|
|
37
|
Bassett JJ, Bong A HL, Janke EK,
Robitaille M, Roberts-Thomson SJ, Peters AA and Monteith GR:
Assessment of cytosolic free calcium changes during
ceramide-induced cell death in MDA-MB-231 breast cancer cells
expressing the calcium sensor GCaMP6m. Cell Calcium. 72:39–50.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Q, Wen L, Meng Z and Chen Y: Blockage
of endoplasmic reticulum stress attenuates nilotinib-induced
cardiotoxicity by inhibition of the Akt-GSK3β-Nox4 signaling. Eur J
Pharmacol. 822:85–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ravez S, Spillier Q, Marteau R, Feron O
and Frederick R: Challenges and opportunities in the development of
serine synthetic pathway inhibitors for cancer therapy. J Med Chem.
60:1227–1237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang Q, Jiang W and Hou P: Emerging role
of PI3K/AKT in tumor-related epigenetic regulation. Semin Cancer
Biol. 59:112–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng CH, Wang JB, Lin MQ, Zhang PY, Liu
LC, Lin JX, Lu J, Chen QY, Cao LL, Lin M, et al: CDK5RAP3
suppresses Wnt/β-catenin signaling by inhibiting AKT
phosphorylation in gastric cancer. J Exp Clin Cancer Res.
37:592018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Searle EJ, Telfer BA, Mukherjee D, Forster
DM, Davies BR, Williams KJ, Stratford IJ and Illidge TM: Akt
inhibition improves long-term tumour control following radiotherapy
by altering the microenvironment. EMBO Mol Med. 9:1646–1659. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guerrero-Zotano A, Mayer IA and Arteaga
CL: PI3K/AKT/mTOR: Role in breast cancer progression, drug
resistance, and treatment. Cancer Metastasis Rev. 35:515–524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xie S, Yu X, Li Y, Ma H, Fan S, Chen W,
Pan G, Wang W, Zhang H, Li J and Lin Z: Upregulation of lncRNA
ADAMTS9-AS2 promotes salivary adenoid cystic carcinoma metastasis
via PI3K/Akt and MEK/Erk signaling. Mol Ther. 26:2766–2778. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Starska K, Forma E, Lewy-Trenda I,
Stasikowska-Kanicka O, Skora M and Brys M: Fibroblast growth factor
receptor 1 and 3 expression is associated with regulatory PI3K/AKT
kinase activity, as well as invasion and prognosis, in human
laryngeal cancer. Cell Oncol (Dordr). 41:253–268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Redon CE, Nakamura AJ, Martin OA, Parekh
PR, Weyemi US and Bonner WM: Recent developments in the use of
γ-H2AX as a quantitative DNA double-strand break biomarker. Aging
(Albany NY). 3:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weyemi U, Redon CE, Choudhuri R, Aziz T,
Maeda D, Boufraqech M, Parekh PR, Sethi TK, Kasoji M, Abrams N, et
al: The histone variant H2A.X is a regulator of the
epithelial-mesenchymal transition. Nat Commun. 7:107112016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dong C, Sun J, Ma S and Zhang G:
K-ras-ERK1/2 down-regulates H2A.XY142ph through WSTF to
promote the progress of gastric cancer. BMC Cancer. 19:5302019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ge Y, Liu BL, Cui JP and Li SQ: Livin
promotes colon cancer progression by regulation of
H2A.XY39ph via JMJD6. Life Sci. 234:1167882019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yao K, Peng C, Zhang Y, Zykova TA, Lee MH,
Lee SY, Rao E, Chen H, Ryu J, Wang L, et al: RSK2 phosphorylates
T-bet to attenuate colon cancer metastasis and growth. Proc Natl
Acad Sci USA. 114:12791–12796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lara R, Mauri FA, Taylor H, Derua R, Shia
A, Gray C, Nicols A, Shiner RJ, Schofield E, Bates PA, et al: An
siRNA screen identifies RSK1 as a key modulator of lung cancer
metastasis. Oncogene. 30:3513–3521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kang S, Elf S, Lythgoe K, Hitosugi T,
Taunton J, Zhou W, Xiong L, Wang D, Muller S, Fan S, et al: p90
ribosomal S6 kinase 2 promotes invasion and metastasis of human
head and neck squamous cell carcinoma cells. J Clin Invest.
120:1165–1177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ravez S, Spillier Q, Marteau R, Feron O
and Frédérick R: Challenges and opportunities in the development of
serine synthetic pathway inhibitors for cancer therapy. J Med Chem.
60:1227–1237. 2017. View Article : Google Scholar : PubMed/NCBI
|