Introduction
Colorectal cancer (CRC) is the second leading cause
of cancer-associated mortality in the USA, with an estimated
145,600 new cases and 51,020 deaths in both men and women in 2019
(1). Although the death rate is
decreasing as a result of increased use of colonoscopy in early
screening, the decline in incidence has tapered in recent years
(2). The pathogenesis of CRC is a
multistep process that originates from premalignant neoplastic
lesions, known as adenomas, and progresses to invasion and further
metastasis (3). Early adenoma
formation is accompanied by the mutation of the tumor suppressor
gene adenomatous polyposis coli (APC), which is present in
~80% of sporadic CRCs and all familial adenomatous polyposis cases
(4). Most somatic APC
mutations result in a truncated Apc protein and contribute in CRC
development (5). Mechanistically,
dysregulation of the Wnt signaling pathway as a result of the
APC mutation leads to dysfunction of the multiprotein
‘destruction complex’ and translocation of β-catenin into the
nucleus to activate transcription factors that belong to the T-cell
factor (TCF)/lymphoid enhancer factor family (6,7). This
gives rise to increased expression of genes that regulate cell
proliferation and apoptosis, such as c-Myc and Axin2.
Mutated APC multiple intestinal neoplasia mouse model
(ApcMin) possess a nonsense mutation at codon 850 of
APC, increasing its predisposition to intestinal adenoma
formation, which is similar to the human somatic mutation that is
associated with the progression of human CRC (8). Therefore, ApcMin mice have been
widely used as a model for the study of intestinal tumorigenesis.
This model is particularly advantageous for assessing the effect of
anticancer agents in the early stages of cancer formation, since
ApcMin mice spontaneously grow detectable intestinal
adenomas within several months (9).
It has been well established that diets high in
fruit and vegetables decrease the risk of developing various types
of cancer, including CRC (10–12). A
class of natural products abundant in these types of foods are
flavonoids, which have been widely reported to have preventive
effects against CRC, as demonstrated in animal studies (13,14) and
population-based studies (15–17). The
chemistry of chalcones attracts much attention owing to its simple
structure, easy synthesis, variable construct derivates and
promising biological functions, including its activity against
inflammation, angiogenesis, bacterial infection, diabetes and
cancer, as well as its role in immunomodulation (18–21).
Chalcones are reported to possess chemopreventive activities in a
variety of solid tumors, such as prostate cancer, melanoma and
colon cancer (22,23). Chalcones serve as precursors for
flavonoid synthesis and are considered promising candidates for
various disease treatments, including dietary cancer prevention
(24,25). Previous in vitro studies have
indicated that chalcones and their derivatives selectively induce
apoptosis and restrain proliferation in human cancer cell lines
(26), such as Caco-2 cells,
particularly when the hydroxyl groups are present in chalcone
molecules (27), and these are
thought to be potent modulators of angiogenesis (19). However, the in vivo anticancer
effects of these chalcone derivatives remain to be fully
elucidated. In the present study, the chemopreventive effects of
4′-hydroxychalcone (4-HC; an α,β-unsaturated ketone with the
chalcone backbone and one hydroxyl-substituent at the 4′ position
of the A ring; Fig. 1A), was
evaluated for the first time on the spontaneous intestinal
tumorigenesis in ApcMin mice. The results of the present
study provide scientific evidence that supports 4-HC as a potential
chemopreventive regimen for intestinal neoplasms originating from
APC mutations.
Materials and methods
Animals and chemicals
A total of 4 male ApcMin (8 weeks old,
average starting weight, 22 g) and 8 female C57BL/6 mice (8 weeks
old, average starting weight, 19 g) were obtained Beijing Vital
River Laboratory Animal Technology Co., Ltd. Animals were housed
under optimal conditions (21°C, 60% relative humidity, 12-h
light/dark cycle, free access to food and water) in the barrier
facility of the Laboratory Animal Center, Sichuan University. The
animal experiments were reviewed and approved by the Animal
Investigation Committee of the West China Second University
Hospital, Sichuan University (Chengdu, China). The 4-HC was
purchased from Selleck Chemicals and was dissolved in DMSO to a
final concentration of 10 mg/ml for storage, and further dissolved
in corn oil prior to administration.
Adenoma burden assessment
Male ApcMin mice were bred with C57BL/6 mice
and the offspring were genotyped by polymerase chain reaction (PCR)
using the following primers according to the manufacturer's
instructions (Sigma-Aldrich: Merck KGaA): IMR0033,
5′-GCCATCCCTTCACGTTAG-3′; IMR0034, 5′-TTCCACTTTGGCATAAGGC-3′; and
IMR0758, 5′-TTCTGAGAAAGACAGAAGTTA-3′. Male 8-week-old ApcMin
mice were treated with vehicle (DMSO in corn oil; n=11) or 10
mg/kg/day 4-HC (n=12) by oral gavage every day for 12 weeks. The
dose of 4-HC used was considered based on a previous study
(28) and the final concentration
was tested across 3 different concentrations (4-week-old mice were
treated with 5, 10 or 20 mg/kg of 4-HC for 8 weeks; Fig. S1). At the end of the experimental
period, 20-week-old mice were euthanized by CO2
asphyxiation, with a flow rate of 30% displacement of the cage
volume per min, followed by cervical dislocation. Small intestines
and colons were removed and opened longitudinally along the
mesenchymal side. A stereoscopic dissection microscope (Stemi
2000-c; Carl Zeiss AG) was utilized to assess the tumor burden by
counting the number of adenomas and determining their dimensions
(AxioVision Application, version 4.6, Cal Zeiss Microscopy).
Cardiac puncture was performed as soon as mice were euthanized and
mouse blood was collected. Complete blood cell counts were
performed by the clinical laboratory at West China Second
University Hospital.
Tissue processing, immunofluorescence
staining and TUNEL
Adenoma-containing mouse intestines were prepared
using the Swiss-roll technique and fixed in 4% paraformaldehyde
overnight at 4°C. Tissues were then embedded in paraffin and
subsequently cut into 5-µm-thick sections. Paraffin-embedded slides
were deparaffinized in room temperature with xylene and rehydrated
with a graded ethanol series (100, 100, 95, 95 and 50%).
Histological analysis was performed using hematoxylin and eosin
staining (5 min each in room temperature). For tissue
immunofluorescence staining, antigen retrieval was performed in
citrate buffer (Sigma-Aldrich; Merck KGaA) with heating for 13.5
min in a microwave oven at 95°C. The samples were then incubated in
blocking buffer consisting of PBS supplemented with 3.5% normal
goat serum (Thermo Fisher Scientific, Inc.) at room temperature for
30 min. Samples were then incubated in a humidified chamber
overnight at 4°C with primary antibodies diluted in blocking
buffer. The primary antibodies included: β-catenin (1:200; cat. no.
ab32572) and proliferation marker protein Ki-67 (1:500; cat. no.
ab15580) (both from Abcam). Following three washes in PBS and one
wash in blocking buffer, tissue samples were incubated with goat
anti-rabbit Alexa Fluor 488-conjugated secondary antibody in room
temperature for 20–30 min (1:400; cat. no. A11008; Thermo Fisher
Scientific, Inc.) and then washed three times in PBS, followed by
the addition of Hoechst 33342 nuclear stain (15 min at room
temperature) and coverslip mounting with SlowFade Gold Antifade
reagent (Thermo Fisher Scientific, Inc.).
To determine if 4-HC affects apoptosis of intestinal
adenomas, TUNEL staining was performed using the DeadEnd™
Fluorometric TUNEL System (Promega Corporation) according to
manufacturer's instructions. A total of 12 polyps from 4 mice in
each treatment group were selected for TUNEL staining, and five
fields per slide were examined to quantify the TUNEL-positive
cells.
Image acquisition and
quantification
Bright-field microscopy images were acquired with an
Optronics MicroFire charge-coupled device camera on a Leica DM2000
Upright Compound Microscope (Leica Microsystems, Inc.).
Fluorescence microscopy images were acquired using a Nikon A1R
confocal microscope (×20 magnification used; p to ×60/1.4 oil
immersion objective lens; Nikon Corporation). Images were analyzed
using ImageJ software (v2.0.0-rc-69/1.52p; National Institutes of
Health) (29). Quantification of
Ki-67-, TUNEL- and β-catenin-positive cells was performed by
measuring the area of target fluorescence and normalizing it to the
area of nuclear fluorescence, and was expressed as fold change over
the vehicle treated group.
Tissue mRNA expression analysis by
reverse transcription-quantitative PCR (RT-qPCR)
Adenoma tissue samples were collected and stored in
RNAlater (Qiagen GmbH) overnight, and total RNA was extracted using
the RNeasy Mini kit (Qiagen GmbH) according to the manufacturer's
instructions. For cDNA synthesis, 2–5 µg of the total RNA was
reverse transcribed using a SuperScript III Reverse Transcriptase
kit according to the manufacturer's instructions (Invitrogen;
Thermo Fisher Scientific, Inc.). qPCR was performed using SYBR
Green-based detection (cat. no. 4364346, Invitrogen; Thermo Fisher
Scientific, Inc.) and a Mastercycler (Eppendorf) with the following
thermocycling conditions: 2 min of initial denaturation at 94°C,
followed by 25–30 cycles of denaturation at 94°C for 30 sec,
annealing at 55°C for 30 sec and elongation at 72°C for 60 sec, and
then 10 min of final extension at 94°C. The gene targets and
corresponding primers used in this experiment included: Mouse
c-Myc, forward, 5′-ATGCCCCTCAACGTGAACTTC-3′, and reverse,
5′-CGCAACATAGGATGGAGAGCA-3′; mouse Axin2, forward,
5′-TGACTCTCCTTCCAGATCCCA-3′, and reverse,
5′-TGCCCACACTAGGCTGACA-3′); and mouse CD44, forward,
5′-TCGATTTGAATGTAACCTGCCG-3′, and reverse,
5′-CAGTCCGGGAGATACTGTAGC-3′. Technical triplicates were used, and
data were normalized to the housekeeping gene GAPDH
(forward, 5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse,
5′-TGTAGACCATGTAGTTGAGGTCA-3′), and the relative abundance of
transcripts was calculated by the comparative 2−ΔΔCq
method (30).
Statistical analysis
All data are presented as the mean ± SEM analyzed
using GraphPad Prism v7 (GraphPad Software, Inc.). Data for
continuous variables involving two groups were analyzed by unpaired
Student's t-test. For multiple-time-point comparisons (Figs. 1C and S1A), two-way ANOVA followed by Sidak post
hoc test was performed using built-in functions in GraphPad Prism
v7 (GraphPad Software, Inc.). For multigroup comparisons (Fig. S1B), one-way ANOVA followed by
Tukey's post hoc test was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
General in vivo observations
To determine the dose of 4-HC used in the present
study, a toxicity test was performed. The 4-week-old C57BL/6 mice
were orally treated with vehicle or gradient doses of 4-HC (5, 10
or 20 mg/kg/day) for 8 weeks. The body weight change was similar
among mice treated with vehicle, 5 mg/kg/day 4-HC and 10 mg/kg/day,
but the body weight of mice treated with 20 mg/kg/day 4-HC was
significantly lower than that of mice treated with vehicle over
time (Fig. S1A and B). Therefore,
10 mg/kg/day of 4-HC administration was considered to exert no
marked toxicity and was selected for further treatment for the
tumor burden assessment of ApcMin animals.
A total of 24 male ApcMin animals were
randomly allocated for treatment with vehicle or 10 mg/kg/day of
4-HC by oral administration from 8 to 20 weeks (Fig. 1B). ApcMin mice treated with
4-HC exhibited significantly less body weight loss than those
treated with vehicle (Fig. 1C). Mice
treated with 4-HC weighed 4% more at 7 weeks of treatment (P=0.049)
and 11% more at 12 weeks (P<0.01). Body weight loss in both
groups occurred at the first week of treatment due to inadaptation
to oral gavage handling, and also at a late timepoint (8–12 weeks
of treatment) owing to the increased intestinal adenoma burden and
subsequent anemia. Similarly, the endpoint hemoglobin level in
4-HC-treated mice was significantly higher compared with that in
control mice (P=0.01; Fig. 1D).
Oral administration of 4-HC prevents
spontaneous intestinal polyposis in ApcMin mice
Most adenomas in ApcMin mice developed in the
small intestine, with fewer in the colon, and were identified
histologically as adenomatous polyps using H-E stained slides. At
the age of 20 weeks, mice in the control group developed an average
of 6.9, 25.7 and 12.5 polyps in the colon, distal small intestine
(DSI) and proximal small intestine (PSI), respectively; whereas
4-HC treatment led to a significant reduction (45%) in the number
of colon adenomas (P<0.01) (Fig. 2A
and B), and also a decrease in colon adenoma size (35%
reduction) compared with the control treatment (P<0.05; Fig. 2D). Similarly, 4-HC strongly decreased
the number of polyps by 35% (P<0.01) and 33% (P=0.03) in the DSI
and PSI (Fig. 2C), respectively. A
prominent decrease in polyp size was also observed in the DSI, with
a 39% reduction in adenoma surface area (P=0.01; Fig. 2E). Hematoxylin and eosin staining of
Swiss-rolled intestines exhibited similar phenotypes, and
histological analysis showed comparable dysplasia and invasion in
the polyps in both groups (Fig. 2F and
G).
 | Figure 2.Oral administration of 4-HC prevents
spontaneous intestinal polyposis in ApcMin mice. (A)
Representative images of colon polyps in ApcMin mice treated
with 4-HC or Veh for 12 weeks. Images were depicted macroscopically
(scale bar, 10 mm; top images) and under a stereoscopic dissection
microscope (scale bar, 1 mm; bottom images). ApcMin mice
treated with 10 mg/kg/day 4-HC (n=12) and Veh (n=11) for 12 weeks
and (B) colon polyp number, (C) DSI/PSI polyp number, (D) colon
polyp size, and (E) DSI/PSI polyp size were analyzed. Polyp size is
the tumor surface area observed under the microscope. (F)
Representative images of polyps in the small intestine of
ApcMin mice under a stereoscopic dissection microscope.
Scale bar, 1 mm. Representative images of adenoma burden in the (G)
colon and (H) DSI from mice treated with Veh or 4-HC. Green
triangles indicate microscopic intestinal adenomas. Scale bar, 2
mm. Data are presented as the mean ± SEM. *P<0.05, **P<0.01.
4-HC, 4′-hydroxychalcone; ApcMin, adenomatous polyposis coli
multiple intestinal neoplasia mouse model; DSI, distal small
intestine; ns, not significant; PSI, proximal small intestine; Veh,
vehicle. |
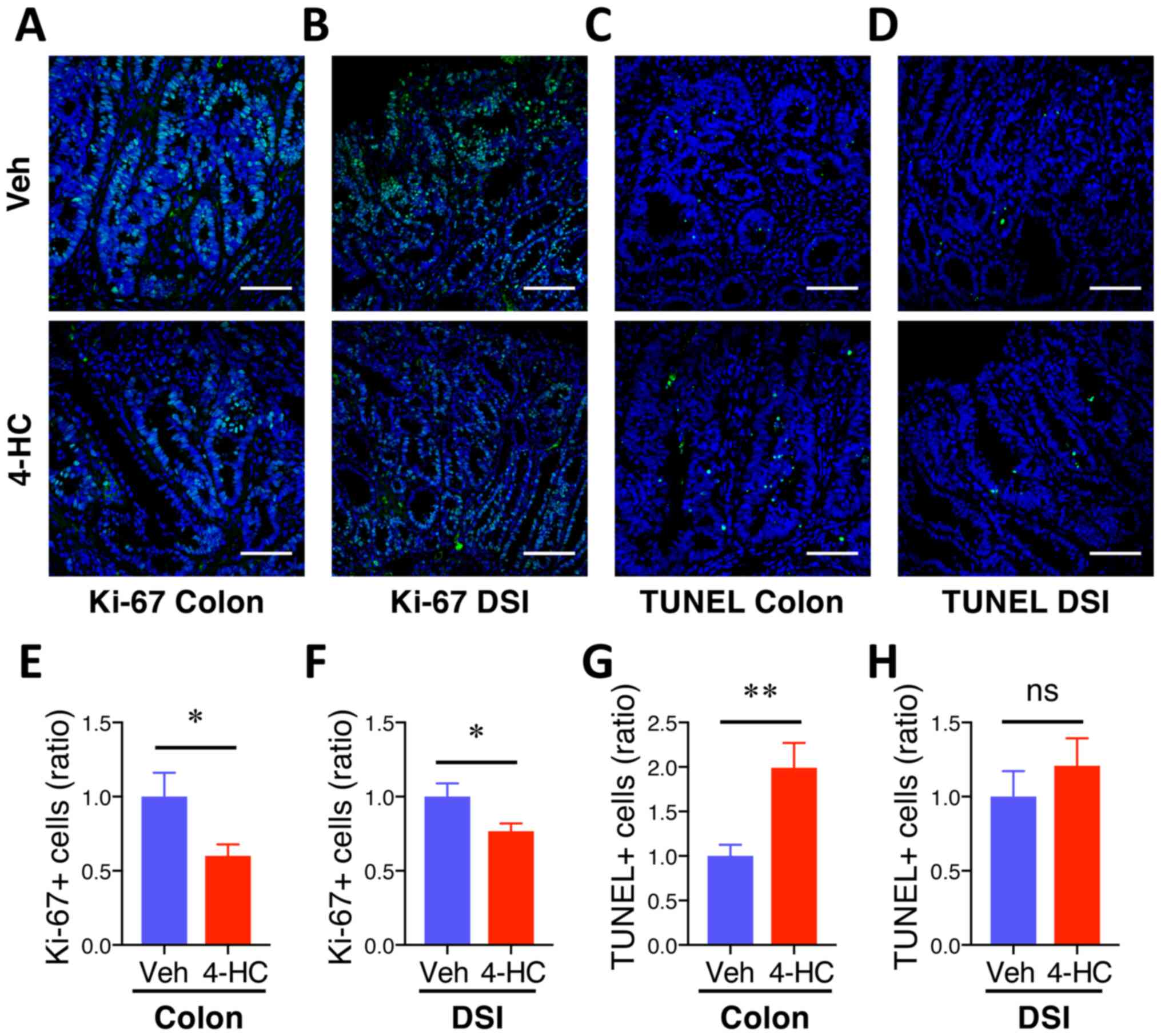
4-HC treatment prevents proliferation
and induces apoptosis during intestinal adenoma formation
To assess whether 4-HC efficacy is associated with
antiproliferative and proapoptotic properties, Ki-67 and TUNEL
immunofluorescence staining were performed on colon and DSI
adenomas. Qualitative microscopic examination of Ki-67-stained
sections showed a decrease in Ki-67-positive cells in both colon
and DSI adenomas from mice treated with 4-HC (Fig. 3A and B) compared with the vehicle
control. The quantification of Ki-67 immunofluorescence staining
showed 40% (P=0.03) and 23% (P=0.03) decreases in Ki-67 positive
cells from polyps from the colon and DSI, respectively, compared
with the vehicle control (Fig. 3E and
F). Fig. 3C, D, G and H
summarizes the effects of 4-HC on adenoma cell apoptosis.
Qualitative microscopic examination of TUNEL-stained sections
showed an increase in TUNEL-positive cells selectively in colon
adenomas from mice treated with 4-HC compared with the vehicle. The
quantification of TUNEL staining showed a 99% increase in
TUNEL-positive cells in colon polyps with 4-HC treatment compared
with those that received control treatment (P<0.01; Fig. 3G). No significant difference in the
percentage of TUNEL-positive cells was identified in DSI polyps
between the treatment groups (P>0.05; Fig. 3H).
β-catenin and related gene expression
levels are selectively suppressed by 4-HC in colon adenomas
The aforementioned data demonstrated the
antiproliferative and apoptosis-promoting role of 4-HC in
spontaneous intestinal tumorigenesis, which is associated with the
properties of the β-catenin signaling pathway in human CRC
progression (31). Furthermore,
chalcone and a number of its analogues have been described to
possess the ability to inhibit β-catenin signaling pathways
(25,32–35). To
examine this hypothesis, β-catenin target gene expression levels
were analyzed in adenomas by RT-qPCR. A marked suppression of
c-Myc, Axin2 and CD44 gene expression was observed in
colon adenomas treated with 10 mg/kg/day 4-HC compared with those
treated with the vehicle (Fig. 4A).
Notably, the suppressive effect of 4-HC was demonstrated to occur
selectively in adenomas from the colon, but not in adenomas from
the DSI (Fig. 4B). This effect was
confirmed by immunofluorescence staining of β-catenin. Microscopic
examination of β-catenin staining images depicted a decrease in the
accumulation of cellular β-catenin in the colon adenomas of
4-HC-treated mice (Fig. 4C). The
quantification of β-catenin staining showed a significant 52%
reduction in colon adenomas (P<0.01; Fig. 4D), whereas no significant difference
was observed in the DSI adenomas (data not shown).
Discussion
To the best of our knowledge, the present study
demonstrated the chemopreventive effects of 4-HC on spontaneous
intestinal adenoma formation in ApcMin mice for the first
time, which is a model that mimics numerous gene regulatory changes
present in sporadic human CRC (8).
Examination of intestinal adenoma number and size under a
dissection microscope revealed that 10 mg/kg/day 4-HC led to a
significant suppression of both colon and small intestinal adenoma
formation. Treatment with 4-HC decreased both the number and size
of adenomas, particularly those from the colon, suggesting its
ability to affect tumor initiation and also tumor progression.
Although different biological changes are involved in these two
events, inhibition of tumor initiation and progression could both
be beneficial for the treatment of CRC. Generally, intestinal
polyps are often found in middle-aged patients during colonoscopy
examinations, and their progression to cancerous lesions occurs
within 10–15 years (36). Therefore,
agents with the ability to inhibit tumor initiation and progression
have a wider window of time to intervene with cancer development
and could be used effectively even years after cancer initiation
(37).
Although the association between flavonoid intake
and CRC risk remains to be fully elucidated, mechanistic studies
have revealed the anti-CRC properties of various flavonoids, such
as anthocyanidins, apigenin and quercetin. Anthocyanidins have been
reported to decrease CRC risk (38,39),
largely due to their ability to negatively regulate inflammatory
signaling pathways, including NF-κB, MAPK, JNK and STAT. Apigenin
can induce G2/M cell cycle arrest in multiple colon
cancer cell lines with decreased expression of cyclin B1 proteins
and the cyclin-dependent kinase p34 (40), and possesses proapoptotic features
that are associated with its pro-oxidative effect, leading to the
increased production of reactive oxygen species and oxidative
stress (41). A chalcone derivative,
L2H17, was previously reported to have cytotoxic effects on colon
cancer cell lines through various biological processes, including
induction of G0/G1 cell cycle arrest and
apoptosis, attenuation of cell migration and invasion, and
inactivation of the NF-κB signaling pathway (42). L2H17 also possesses in vivo
antitumor activity, as determined using a xenograft mouse model of
colon cancer (42).
Wnt/β-catenin signaling pathways are abnormally
activated in the early stages of CRC (43). A crucial and heavily studied Wnt
pathway is canonical Wnt signaling, which functions by regulating
the transcriptional coactivator β-catenin and promotes the
expression of key developmental genes (44). For example, c-Myc, which is a
key component of the Wnt signaling pathway and an important
transcription factor, is often constitutively expressed in CRC and
leads to increased expression of numerous genes that are involved
in cell proliferation, differentiation and apoptosis, including
c-MYC, CDKN1A, LGR5, CD44, AXIN2 and CCND1 (45–47).
Several studies have demonstrated the chemopreventive effect of
flavonoids that act as inhibitors of components in the
Wnt/β-catenin signaling pathways. For example, apigenin can inhibit
Wnt signaling in CRC cells in vitro, potentially through
lysosomal degradation of β-catenin and downregulation of Wnt target
genes such as cyclin D1 and c-Myc (48). Quercetin has been reported to disrupt
the TCF/β-catenin interaction by suppressing the binding of the Tcf
complexes to its specific DNA-binding sites (49). Chalcone lonchocarpin is reported to
be a potent inhibitor of the Wnt/β-catenin pathway that acts
downstream to stabilize β-catenin expression and impair
TCF-mediated transcription (34).
Cardamonin, a natural chalcone, was recently described to increase
5-fluorouracil chemosensitivity in gastric cancer cells (BGC-823
cells) by targeting Wnt/β-catenin signaling pathways and blocking
β-catenin/TCF4 complex formation (33).
To the best of our knowledge, the present study is
the first to identify 4-HC as a negative modulator of the
Wnt/β-catenin signaling pathway exclusively in colon adenoma
formation in ApcMin mice. Treatment with 4-HC attenuated
β-catenin expression at the post-transcriptional level and also
affected the expression of downstream genes, including c-Myc,
Axin2 and CD44. Further studies are required to
ascertain why 4-HC inhibits the Wnt/β-catenin pathway selectively
in adenomas from the colon, but not in those from the small
intestine. Interactions between dietary flavonoids and intestinal
microbiota have been proven to be important in the metabolism of
dietary flavonoids, and to influence their efficacy on human
disease, which may contribute to the phenotypical difference
between small intestine and colon adenoma formation (50,51).
There are a number of limitations to the present
study. The experimental design in the present study is not optimal.
Using other drugs as positive controls may improve the ability to
demonstrate chemopreventive effects of 4-HC. Furthermore,
additional studies are needed to investigate the dose-dependent
effect of 4-HC in intestinal tumorigenesis. Such studies are
currently under investigation in our laboratory, awaiting results.
Last, the assessment of toxicity in the present study relies on the
change of body weight. However, the result would be biased if there
is a significant difference in the food consumption, which is
lacking in the present study. We will include this in future
studies. Overall, the results of the present study provided
compelling evidence that 4-HC may prevent the development of small
intestinal and colon adenomas in ApcMin mice by inhibiting
cancer cell proliferation (Ki-67), increasing cancer cell apoptosis
(TUNEL), and in colon adenomas, by attenuating β-catenin activation
and limiting downstream gene expression. 4-HC treatment may
therefore be a promising natural preventive agent against
intestinal tumorigenesis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by Shanghai Science and
Technology Committee (grant no. 19441905700) and Shanghai Tongji
Hospital [grant no. ITJ(ZD)1802].
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
QC, QH and BG designed and supervised the project.
QC, JL, JZ and SM performed the experiments. QC, JL, JZ and SM
performed the data analysis and figure preparation. QC drafted the
manuscript. QH and BG edited the manuscript and oversaw the project
and made intellectual efforts in paper revision. All authors have
read and approved the final manuscript.
Ethics approval and consent for
participate
All animal experiments were approved by and
performed in accordance with the recommendations of the Animal
Investigation Committee of the West China Second University
Hospital, Sichuan University (Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hofseth LJ, Hebert JR, Chanda A, Chen H,
Love BL, Pena MM, Murphy EA, Sajish M, Sheth A, Buckhaults PJ and
Berger FG: Early-onset colorectal cancer: Initial clues and current
views. Nat Rev Gastroenterol Hepatol. 17:352–364. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grady WM and Carethers JM: Genomic and
epigenetic instability in colorectal cancer pathogenesis.
Gastroenterology. 135:1079–1099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubinfeld B, Souza B, Albert I, Müller O,
Chamberlain SH, Masiarz FR, Munemitsu S and Polakis P: Association
of the APC gene product with beta-catenin. Science. 262:1731–1734.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powell SM, Zilz N, Beazer-Barclay Y, Bryan
TM, Hamilton SR, Thibodeau SN, Vogelstein B and Kinzler KW: APC
mutations occur early during colorectal tumorigenesis. Nature.
359:235–237. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roose J and Clevers H: TCF transcription
factors: Molecular switches in carcinogenesis. Biochim Biophys
Acta. 1424:M23–M37. 1999.PubMed/NCBI
|
|
7
|
Stamos JL and Weis WI: The β-catenin
destruction complex. Cold Spring Harb Perspect Biol. 5:a0078982013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moser AR, Luongo C, Gould KA, McNeley MK,
Shoemaker AR and Dove WF: ApcMin: A mouse model for intestinal and
mammary tumorigenesis. Eur J Cancer. 31A:1061–1064. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Corpet DE and Pierre F: Point: From animal
models to prevention of colon cancer. Systematic review of
chemoprevention in min mice and choice of the mod'el system. Cancer
Epidemiol Biomarkers Prev. 12:391–400. 2003.PubMed/NCBI
|
|
10
|
Aune D, Giovannucci E, Boffetta P, Fadnes
LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ and Tonstad
S: Fruit and vegetable intake and the risk of cardiovascular
disease, total cancer and all-cause mortality-a systematic review
and dose-response meta-analysis of prospective studies. Int J
Epidemiol. 46:1029–1056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo WP, Fang YJ, Lu MS, Zhong X, Then YM
and Zhang CX: High consumption of vegetable and fruit colour groups
is inversely associated with the risk of colorectal cancer: A
case-control study. Br J Nutr. 113:1129–1138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Keefe SJ: Diet, microorganisms and their
metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol.
13:691–706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang K, Lamprecht SA, Liu Y, Shinozaki H,
Fan K, Leung D, Newmark H, Steele VE, Kelloff GJ and Lipkin M:
Chemoprevention studies of the flavonoids quercetin and rutin in
normal and azoxymethane-treated mouse colon. Carcinogenesis.
21:1655–1660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du WJ, Yang XL, Song ZJ, Wang JY, Zhang
WJ, He X, Zhang RQ, Zhang CF, Li F, Yu CH, et al: Antitumor
Activity of total flavonoids from daphne genkwa in colorectal
cancer. Phytother Res. 30:323–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang H, Lei L, Zhou Y, Ye F and Zhao G:
Dietary flavonoids and the risk of colorectal cancer: An updated
Meta-analysis of epidemiological studies. Nutrients. 10:9502018.
View Article : Google Scholar
|
|
16
|
Cho YA, Lee J, Oh JH, Chang HJ, Sohn DK,
Shin A and Kim J: Dietary flavonoids, CYP1A1 genetic variants, and
the risk of colorectal cancer in a korean population. Sci Rep.
7:1282017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zamora-Ros R, Not C, Guinó E,
Luján-Barroso L, García RM, Biondo S, Salazar R and Moreno V:
Association between habitual dietary flavonoid and lignan intake
and colorectal cancer in a Spanish case-control study (the
Bellvitge Colorectal Cancer Study). Cancer Causes Control.
24:549–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsieh CT, Hsieh TJ, El-Shazly M, Chuang
DW, Tsai YH, Yen CT, Wu SF, Wu YC and Chang F: Synthesis of
chalcone derivatives as potential anti-diabetic agents. Bioorg Med
Chem Lett. 22:3912–3915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mirossay L, Varinska L and Mojzis J:
Antiangiogenic effect of flavonoids and chalcones: An update. Int J
Mol Sci. 19:272017. View Article : Google Scholar
|
|
20
|
Fernandes I, Pérez-Gregorio R, Soares S,
Mateus N and de Freitas V: Wine flavonoids in health and disease
prevention. Molecules. 22:2922017. View Article : Google Scholar
|
|
21
|
Kar Mahapatra D, Asati V and Bharti SK: An
updated patent review of therapeutic applications of chalcone
derivatives (2014-present). Expert Opin Ther Pat. 29:385–406. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahapatra DK, Bharti SK and Asati V:
Anti-cancer chalcones: Structural and molecular target
perspectives. Eur J Med Chem. 98:69–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karthikeyan C, Moorthy NS, Ramasamy S,
Vanam U, Manivannan E, Karunagaran D and Trivedi P: Advances in
chalcones with anticancer activities. Recent Pat Anticancer Drug
Discov. 10:97–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HG, Oh HJ, Ko JH, Song HS, Lee YG,
Kang SC, Lee DY and Baek NI: Lanceoleins A-G, hydroxychalcones,
from the flowers of Coreopsis lanceolata and their chemopreventive
effects against human colon cancer cells. Bioorg Chem. 85:274–281.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin S, Son Y, Liu KH, Kang W and Oh S:
Cytotoxic activity of broussochalcone a against colon and liver
cancer cells by promoting destruction complex-independent β-catenin
degradation. Food Chem Toxicol. 131:1105502019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kello M, Drutovic D, Pilatova MB,
Tischlerova V, Perjesi P and Mojzis J: Chalcone derivatives cause
accumulation of colon cancer cells in the G2/M phase and induce
apoptosis. Life Sci. 150:32–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Loa J, Chow P and Zhang K: Studies of
structure-activity relationship on plant polyphenol-induced
suppression of human liver cancer cells. Cancer Chemother
Pharmacol. 63:1007–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qu Q, Dai B, Yang B, Li X, Liu Y and Zhang
F: 4-Hydroxychalcone attenuates hyperaldosteronism, inflammation,
and renal injury in cryptochrome-null mice. Biomed Res Int.
2014:6034152014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Polakis P, Hart M and Rubinfeld B: Defects
in the regulation of beta-catenin in colorectal cancer. Adv Exp Med
Biol. 470:23–32. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fonseca BF, Predes D, Cerqueira DM, Reis
AH, Amado NG, Cayres MC, Kuster RM, Oliveira FL, Mendes FA and
Abreu JG: Derricin and derricidin inhibit Wnt/β-catenin signaling
and suppress colon cancer cell growth in vitro. PLoS One.
10:e01209192015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hou G, Yuan X, Li Y, Hou G and Liu X:
Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance
of gastric cancer cells through targeting Wnt/β-catenin signal
pathway. Invest New Drugs. 38:329–339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Predes D, Oliveira LFS, Ferreira LSS, Maia
LA, Delou JMA, Faletti A, Oliveira I, Amado NG, Reis AH, Fraga CAM,
et al: The chalcone lonchocarpin inhibits wnt/beta-catenin
signaling and suppresses colorectal cancer proliferation. Cancers
(Basel). 11:19682019. View Article : Google Scholar
|
|
35
|
Yin L, Niu C, Liao LX, Dou J, Habasi M and
Aisa HA: An isoxazole chalcone derivative enhances melanogenesis in
B16 melanoma cells via the Akt/GSK3β/β-catenin signaling pathways.
Molecules. 22:20772017. View Article : Google Scholar
|
|
36
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawamori T, Lubet R, Steele VE, Kelloff
GJ, Kaskey RB, Rao CV and Reddy BS: Chemopreventive effect of
curcumin, a naturally occurring anti-inflammatory agent, during the
promotion/progression stages of colon cancer. Cancer Res.
59:597–601. 1999.PubMed/NCBI
|
|
38
|
Charepalli V, Reddivari L, Vadde R, Walia
S, Radhakrishnan S and Vanamala JK: Eugenia jambolana (Java Plum)
fruit extract exhibits anti-cancer activity against early stage
human HCT-116 colon cancer cells and colon cancer stem cells.
Cancers (Basel). 8:292016. View Article : Google Scholar
|
|
39
|
Mazewski C, Liang K and Gonzalez de Mejia
E: Comparison of the effect of chemical composition of
anthocyanin-rich plant extracts on colon cancer cell proliferation
and their potential mechanism of action using in vitro, in silico,
and biochemical assays. Food Chem. 242:378–388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang W, Heideman L, Chung CS, Pelling JC,
Koehler KJ and Birt DF: Cell-cycle arrest at G2/M and growth
inhibition by apigenin in human colon carcinoma cell lines. Mol
Carcinog. 28:102–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Banerjee K and Mandal M: Oxidative stress
triggered by naturally occurring flavone apigenin results in
senescence and chemotherapeutic effect in human colorectal cancer
cells. Redox Biol. 5:153–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu S, Chen M, Chen W, Hui J, Ji J, Hu S,
Zhou J, Wang Y and Liang G: Chemopreventive effect of chalcone
derivative, L2H17, in colon cancer development. BMC Cancer.
15:8702015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kaemmerer E, Jeon MK, Berndt A, Liedtke C
and Gassler N: Targeting wnt signaling via notch in intestinal
carcinogenesis. Cancers (Basel). 11:5552019. View Article : Google Scholar
|
|
45
|
Krausova M and Korinek V: Wnt signaling in
adult intestinal stem cells and cancer. Cell Signal. 26:570–579.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wielenga VJ, Smits R, Korinek V, Smit L,
Kielman M, Fodde R, Clevers H and Pals ST: Expression of CD44 in
Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J
Pathol. 154:515–523. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin CM, Chen HH, Lin CA, Wu HC, Sheu JJ
and Chen HJ: Apigenin-induced lysosomal degradation of β-catenin in
Wnt/β-catenin signaling. Sci Rep. 7:3722017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park CH, Chang JY, Hahm ER, Park S, Kim HK
and Yang CH: Quercetin, a potent inhibitor against beta-catenin/Tcf
signaling in SW480 colon cancer cells. Biochem Biophys Res Commun.
328:227–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cassidy A and Minihane AM: The role of
metabolism (and the microbiome) in defining the clinical efficacy
of dietary flavonoids. Am J Clin Nutr. 105:10–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Murota K, Nakamura Y and Uehara M:
Flavonoid metabolism: The interaction of metabolites and gut
microbiota. Biosci Biotechnol Biochem. 82:600–610. 2018. View Article : Google Scholar : PubMed/NCBI
|