Introduction
Hepatocellular carcinoma (HCC) is a common cause of
cancer-associated death in Taiwan. Generally, HCC is most prevalent
in Asia (~75% of cases were reported in Asia in 2015) compared with
the rest of the world (1); however,
HCC has become a common disease worldwide and is currently the
ninth leading cause of cancer-associated death in the United States
(2). Unlike other cancer types, the
prognosis of HCC remains poor, although new diagnosis and treatment
strategies have been developed. Late diagnosis is the main
contributor to poor prognosis, and it strongly impacts overall HCC
outcomes. Due to late diagnosis, only 30% of patients with HCC are
eligible for surgery, and the remaining patients are usually given
other treatments, including sorafenib, embolization and
radiotherapy (RT).
The Sorafenib HCC Assessment Randomized Protocol
trial conducted in 2008 showed that overall survival was prolonged
by only 3 months when using sorafenib as a single agent to treat
advanced HCC (3). Hence, sorafenib
has been combined with conventional treatments, such as
radiofrequency ablation (RFA) (4)
and transarterial chemoembolization (TACE) (5), to enhance treatment outcomes and reduce
the incidence of sorafenib-induced side effects. However, combining
sorafenib with radiotherapy (RT) is controversial because both
treatments have potential toxicities, including skin reactions and
thrombosis (6–9).
The liver is a radiosensitive organ (10), and HCC tumors are relatively
resistant to RT (11). Thus, RT is
not the primary treatment for HCC. Hsieh et al (6) was the first group to report that
improved HCC tumor control was observed in patients after treatment
with a combination of sorafenib and RT, but severe skin reactions
occurred. Since then, improved treatment outcomes resulting from
the combination of sorafenib and RT have also been reported by
other groups. However, severe side effects, such as hand-foot
syndrome and gastrointestinal bleeding, have also been observed in
patients receiving the combination treatment (7,8). When a
large portion of the liver needs to be irradiated, the combination
of sorafenib and RT is not recommended because of its severe
toxicity (9). Radioembolization is a
minimally invasive procedure that merges the advantages of
embolization and RT for HCC treatment (12,13).
Radioembolization mostly uses β-emitters, which have relatively
short penetration ranges, and minimizes the volume of liver
irradiated, therefore decreasing the likelihood of RT-induced
toxicities (14,15). A combination of sorafenib and
radioembolization has been tested in patients; however, conflicting
results have been found. Improved outcomes were observed by Mahvash
et al (16), but Ricke et
al (17) reported that the
combination treatment did not improve outcomes and suggested that a
improved trial design would be needed.
Yu et al (18)
proposed that sorafenib enhances outcomes by inhibiting MAPK, NF-κB
and VEGF pathways, suggesting that sorafenib should be given after
RT. Our group previously demonstrated that sorafenib suppresses the
ERK/NF-κB pathway and ameliorates the therapeutic efficacy of RT in
oral cancer (19) and HCC (20) in orthotopic and subcutaneous models,
respectively. As aforementioned, the volume of liver irradiated is
strongly associated with radiation-induced toxicity and might
influence treatment outcomes. Though our previous study did not
observe severe toxicity caused by the combination of sorafenib and
RT in the subcutaneous HCC model, it is important to understand
whether this combination strategy works in an orthotopic HCC model.
Therefore, the present study aimed to evaluate the effectiveness
and safety of the combination of sorafenib with RT in an orthotopic
HCC model and to study the possible underlying mechanisms. These
results may improve our understanding of whether the combination of
sorafenib and RT would be feasible and safe for HCC treatment in
human clinical settings.
Materials and methods
Cell lines
The human HCC cell line
Huh7/NF-κB-tk-luc2/rfp used in this study was established
previously (21).
Huh7/NF-κB-tk-luc2/rfp cells were maintained in DMEM
supplemented with 10% FBS and 1% PS (both HyClone; Cyvita)
supplemented with 500 µg/ml of G418 to maintain tk and
luc2 expression.
Orthotopic HCC/NF-κB-tk-luc2/rfp
tumor-bearing model
In total, 40 7- to 8-week old male nude mice
(average weight, 25 g) were used to generate the orthotopic HCC
mouse model. All the mice had free access to food and water during
the whole experimental period with a 12/12 h light/dark cycle.
Briefly, mice were anesthetized with 1.5–2% isoflurane, and a 1- to
1.5-cm incision was made below the ensiform process after the
surgical area was cleaned with iodine-alcohol. In total, 50 µl of
5×105 Huh7/NF-κB-tk-luc2/rfp cells mixed with
Matrigel (cat no. 354263; Corning, Inc.) was slowly injected into
the top lobe of the liver that was pushed out through the incision.
Then the wound was sutured, and the mice were monitored and sent
back to their cages until they recovered from anesthesia. Humane
endpoints were set as follows: 20% Body weight loss, loss of
mobility and activity. However, no mice reached these humane
endpoints during the study.
Bioluminescent imaging (BLI) was used to track in
vivo NF-κB activity. BLI was conducted at Taiwan Mouse Clinic.
Body weights were also monitored to assess the general toxicity
caused by treatments. The tumor-bearing mice were randomly divided
into four groups (n=5/group): Control, sorafenib (10 mg/kg/per day,
peroral), radiotherapy (single dose, 6 Gy) and combination
treatment. The detailed experimental design is shown in Fig. 1. The animal experiment was performed
twice. The mice were euthanized on day 29 using carbon dioxide (20%
volume displacement per minute), and their deaths were verified by
checking eye color and reflex action. Then cervical dislocation was
performed to ensure mice would not recover from CO2
inhalation. All the animal experiments and procedures were approved
by The Institutional Animal Care and Use Committee of National
Yang-Ming University (Taipei, Taiwan; approval no. 1001238).
BLI
BLI was used to evaluate in vivo NF-κB
activity on the designated days (days-6, 1, 8, 15, and 22).
Briefly, mice were anesthetized with 1.5–2% isoflurane and injected
with 150 mg/kg D-luciferin intraperitoneally. After 10 min, images
were acquired over 5 min, and the photons emitted from tumors were
detected using an in vivo Imaging system (Xenogen IVIS 50;
Caliper Life Sciences). The images were analyzed using Living
Imaging software 4.3.1 (PerkinElmer, Inc.). BLI signals emitted
from the tumors were quantified and plotted against the days after
the first treatment (three mice showing high BLI signals on day −6
were excluded).
Electrophoretic mobility shift assay
(EMSA)
Mice were sacrificed on day 29, and cytosolic and
nuclear proteins were extracted from the tumors using a Nuclear
Extraction kit (EMD Millipore). A LightShift Chemiluminescent EMSA
kit (Thermo Fisher Scientific, Inc.) was utilized to assess
NF-κB/DNA binding activity. The procedures were conducted according
to the manufacturer's instructions. Nuclear proteins isolated from
tumors were mixed with biotinylated DNA probes at room temperature
for 20 min. The protein (20 µg)/DNA probe mixtures were separated
on a 5% polyacrylamide gel and transferred to nylon membranes. UV
cross-linking was performed for permanent DNA fixation. ECL
substrate provided with the EMSA kit was added to enhance signals
after streptavidin-horseradish peroxidase incubation for 5 min at
room temperature, and the signals were detected using X-ray film
(Fujifilm Corporation). ImageJ 1.52 a (National Institutes of
Health) was used for signal quantification. The following DNA
sequences were synthesized for EMSA analysis:
AGTTGAGGGGACTTTCCCAGGC (Sense) and GCCTGGGAAAGTCCCCTCAACT
(antisense) (20).
Western blotting
For in vitro analysis, cells treated with 6
Gy X-ray, 10 µM sorafenib or combination treatment were harvested
and lysed with NP-40 lysis buffer (50 mM Tris, pH 7.4, 250 mM NaCl,
1% NP-40). For ex vivo examination, mice were sacrificed on
day 29. Proteins were extracted from tumors using tissue protein
extraction reagent (Thermo Fisher Scientific, Inc.) to evaluate
protein expression changes caused by the treatments. Protein
concentrations were determined using a Bradford assay. In total, 30
µg cell or tissue lysates were separated using 8–15% SDS-PAGE,
transferred to PVDF membranes, and incubated with specific primary
antibodies overnight at 4°C after 1-h blocking with 5% non-fat milk
at room temperature. Primary antibodies used in this study included
anti-matrix metalloproteinase (MMP)-9 (cat. no. #13667),
anti-cyclooxygenase (COX)-2 (cat. no. #12282), anti-cyclin D1 (cat.
no. #55506), anti-cellular FLICE-like inhibitory protein (c-FLIP;
cat. no. #8510), anti-caspase-8 (cat. no. #4790), anti-cleaved
caspase-3 (cat. no. #9664), anti-Bcl-2 (cat. no. #4223),
anti-Bcl-xL (cat. no. #2764), anti-Mcl-1 (cat. no. #5453),
anti-Bcl-2-like protein 11 (Bim; cat. no. #2933), anti-Bak (cat.
no. #12105), anti-BH3-interacting domain death agonist (BID; cat.
no. #2002) and anti-β-actin (cat. no. #3700). All primary
antibodies were purchased from Cell Signaling Technology, Inc., and
diluted to 1:1,000 in 1X TBST (TBS containing 0.1% Tween-20) before
incubation with membranes, except for anti-β-actin that was diluted
to 1:5,000. Membranes were washed with 1X TBST and incubated with
anti-rabbit and anti-mouse IgG horseradish peroxidase
(HRP)-conjugated secondary antibodies (cat nos. 5220-0337 and
5220-0339, respectively) with 1:10,000 dilution (SeraCare) at room
temperature for 1 h. Finally, the proteins of interest were
detected using an Enhanced Chemiluminescent system (MilliporeSigma)
and the LAS-4000 imaging system (Fujifilm, Corporation). The band
intensities were quantified using ImageJ, and β-actin served as an
internal control.
Statistics
All the in vitro experiments were repeated
three times, and the animal studies were repeated twice. GraphPad
Prism 8 (GraphPad Software) was used to generate plots and perform
statistical analyses. All the results are presented as mean ±
standard error or the mean. One-way ANOVA and Tukey's post hoc
tests were performed to compare difference between groups for
western blotting. Two-way ANOVAs and Tukey's post hoc tests were
performed to compare differences between groups for the animal
studies, and the two variables were treatments and time.
Results
In vivo BLI shows that combination of
sorafenib and RT results in the most significant NF-κB suppression
in orthotopic HCC tumors
The NF-κB/tk-luc2 construct contains an
NF-κB-responsive element to drive the downstream reporter genes,
tk and luc2, which allows determination of NF-κB
activity using imaging. We previously established a stable clone
named Huh7/ NF-κB/tk-luc2 (21). Using these cells, the present study
monitored NF-κB activity in HCC tumors using in vivo BLI on
the same cohorts of mice over time. Except for three, the mice had
minimal BLI signals on day 6 (Fig.
2). All the images shown in Fig.
2 have the same scale bar, which helps observe longitudinal
signal changes. Fig. 2 shows that
the BLI signals increased over time in the control, RT and
sorafenib groups. Notably, the BLI signal did not change
significantly in the combination group.
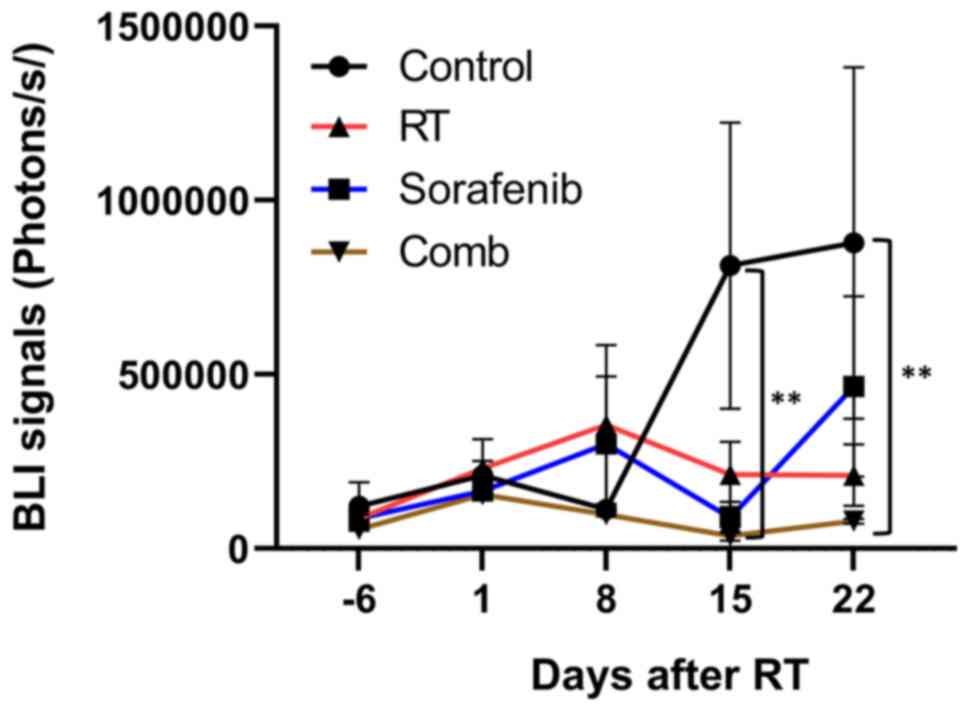
Fig. 3 shows
quantified BLI results of all groups. On day 15, significant
differences were first detected in both the sorafenib and
combination groups compared with the control group (P<0.01).
Moreover, a significant difference between the control and
combination groups (P<0.01) was still observed on day 22.
It is worth noting that the average BLI signal of RT
group was comparable to that of the sorafenib group until day 15,
but an increased signal was observed in the RT group on day 22. The
trends in BLI signal changes were similar between the sorafenib and
combination groups until day 22 after RT, and the average BLI
signal was slightly higher in the sorafenib group compared with in
the combination group.
Combination of sorafenib and RT
suppresses NF-κB/DNA binding ability and represses NF-κB regulated
proteins in orthotopic HCC tumors
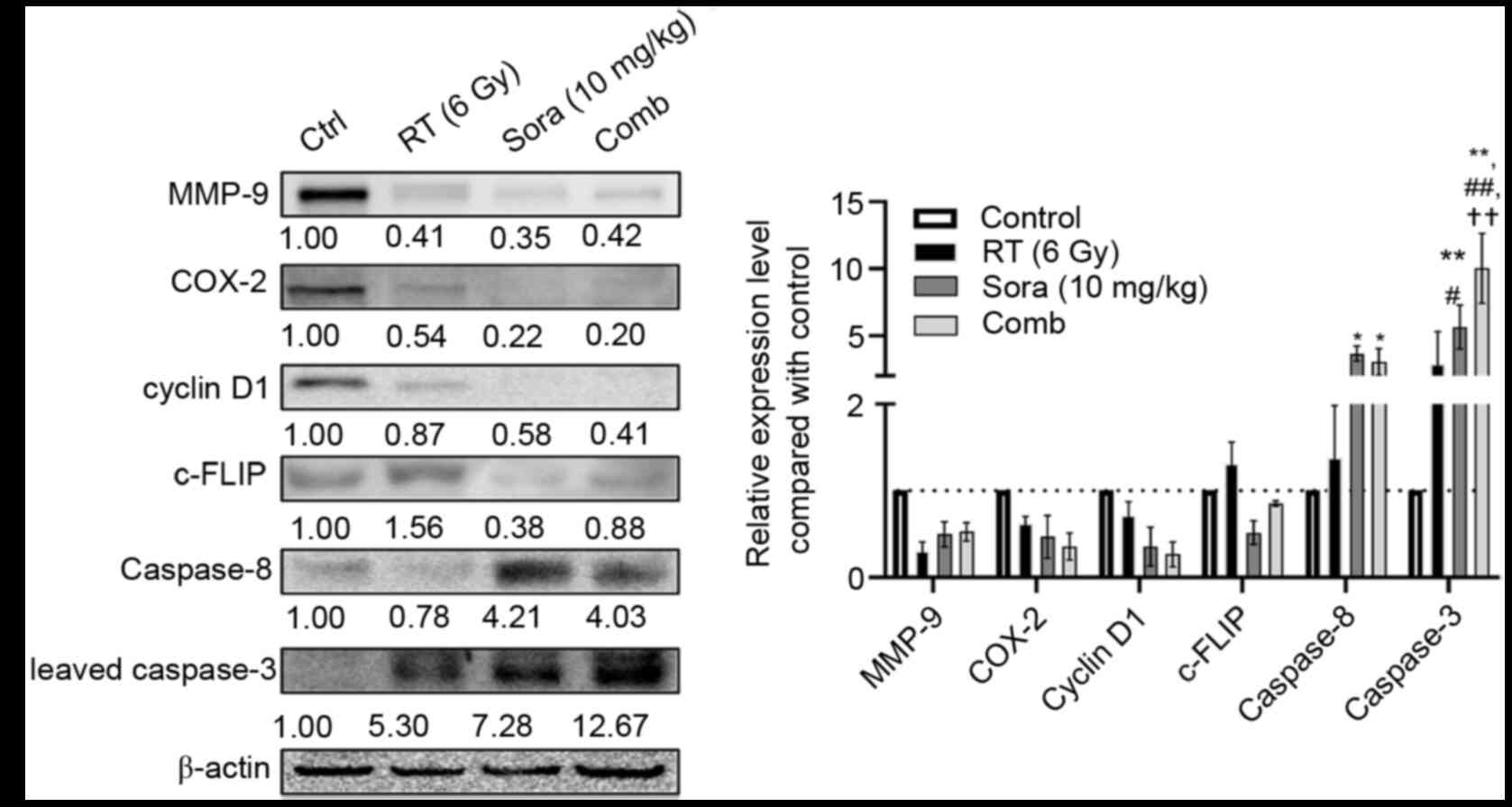
As shown in Fig. 4,
RT did not decrease NF-κB/DNA binding ability, which was suppressed
by sorafenib. Also, combination treatment reduced NF-κB/DNA binding
ability in orthotopic HCC tumors. These results are consistent with
the aforementioned in vivo BLI observations.
The EMSA results indicated that combination
treatment strongly reduced NF-κB/DNA binding ability in orthotopic
HCC tumors. NF-κB is a signaling transduction hub and regulates
several pathways associated with metastasis, inflammatory tumor
microenvironments, proliferation and anti-apoptosis (22). Therefore, the expression of the
following proteins was examined: MMP-9, COX-2, Cyclin D1 and
c-FLIP. Fig. 5 shows that MMP-9,
COX-2 and cyclin D1 were suppressed by RT, sorafenib and
combination treatment. Notably, c-FLIP expression was decreased by
sorafenib but elevated after RT, and the combination treatment
resulted in slight c-FLIP suppression compared with the control
group. Lastly, the levels of caspase-8 and cleaved caspase-3 were
examined. RT failed to increase caspase-8 and cleaved caspase-3
levels, unlike sorafenib and combination treatment significantly
increased cleaved caspase-3 expression in orthotopic HCC tumors
(P<0.01 compared with control; P<0.05, P<0.01 compared
with RT; P<0.01 compared with sorafenib) (Fig. 5).
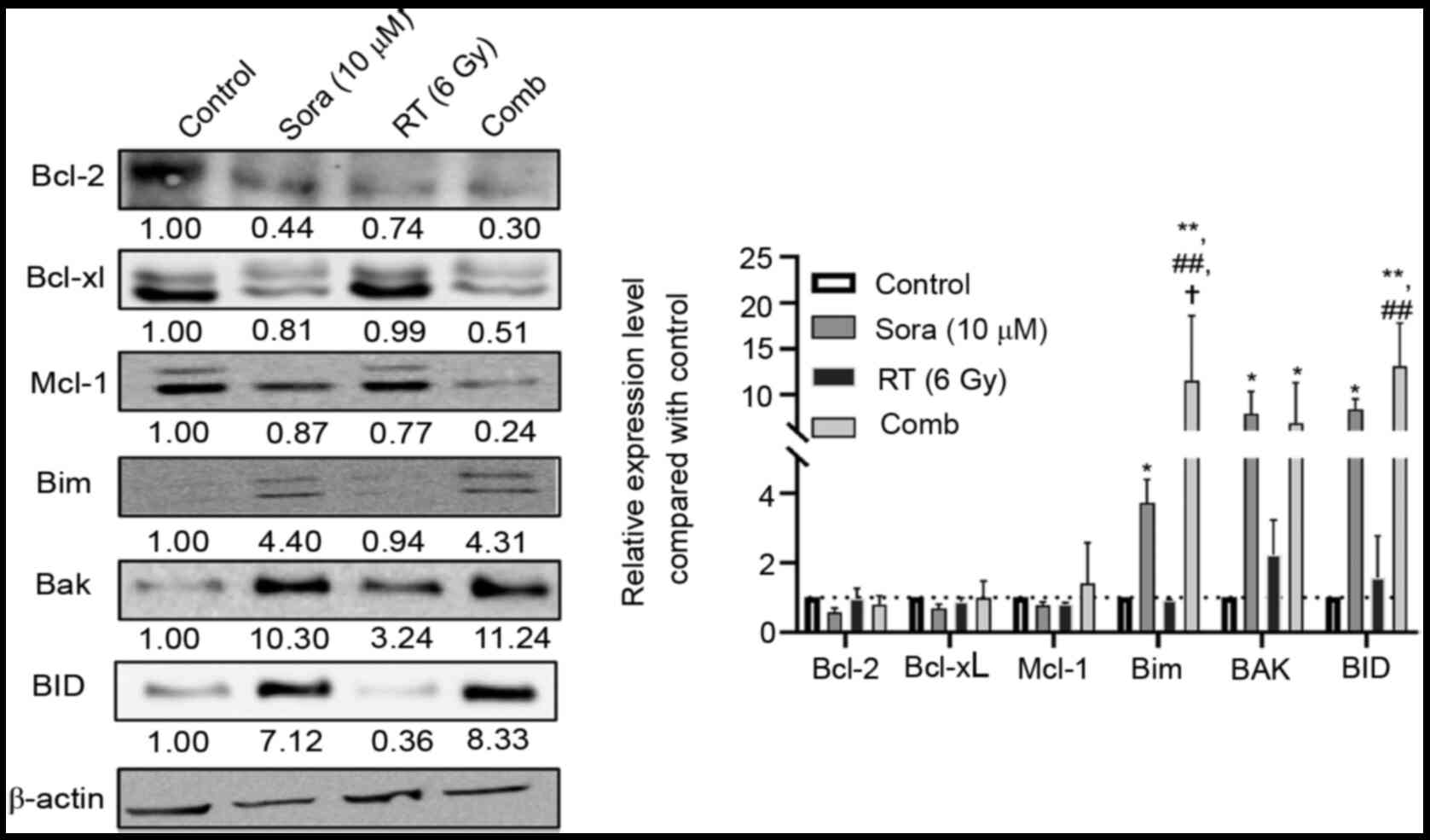
Cells treated with 6 Gy RT, 10 µM sorafenib or
combination treatment were analyzed using western blotting to
determine how these treatments influenced Bcl-2 family proteins and
subsequent signaling pathways. As shown in Fig. 6, Bcl-2 and pro-survival Bcl-xL and
Mcl-1 were slightly decreased in all treatment groups. However,
sorafenib and combination treatment significantly elevated the
expression of pro-apoptotic Bim, BAK and BID compared with control
group (P<0.05 and P<0.01 compared with control; P<0.01
compared with RT; P<0.05 compared with sorafenib).
Combination treatment not only
suppresses NF-κB/DNA binding activity but also inhibits the growth
of orthotopic HCC tumors
Fig. 7 shows
representative tumors removed from control mice and those that
received different treatments. The images show that all treatments
shrank the orthotopic HCC tumors, and the combination treatment
resulted in the most notable tumor inhibition. The largest tumor
diameters found in each group were also compared (Table I). Visible HCC tumors were still
found in more than half of the mice after treatment; however, the
largest tumor diameters detected in the treatment groups were
smaller compared with those in the control group. Moreover, there
were fewer total tumors found in the combination group compared
with in the other three groups. All 10 mice in the control group
had visible liver tumors (establishment rate=100%). Both sorafenib
and combination treatment reduced the numbers of mice with visible
liver tumors to six out of nine (establishment rate=67%). Fig. 8 shows transient but significant
decreases in body weight were detected in the combination group on
day 2 post RT. The body weight decrease was within ±20% of the mean
body weight and recovered at later time points.
 | Table I.Rates of orthotopic HCC
establishment, the total tumor numbers and the largest tumor
diameter detected in each group. |
Table I.
Rates of orthotopic HCC
establishment, the total tumor numbers and the largest tumor
diameter detected in each group.
| Treatment
group | HCC establishing
rate, %a | Largest tumor
diameter, mmb | Total tumor
numberc |
|---|
| Control | 100 | 24 | 33 |
| RT | 90 | 10 | 16 |
| Sorafenib | 70 | 10 | 18 |
| Combination | 67 | 7 | 16 |
Discussion
HCC is mainly treated by surgical removal; however,
only small numbers of patients can have surgery because of tumor
location and numbers (23). Patients
with unresectable HCC may undergo RFA, TACE, chemotherapy and RT,
but none of these treatments are efficient for advanced HCC
(24,25). Sorafenib is a targeted therapy for
advanced HCC, and it blocks signaling pathways initiated by
different receptor tyrosine kinases (RTKs), such as VEGF and
platelet-derived growth factor (26). RT is not the first-line treatment for
HCC because HCC is relatively resistant to RT compared with other
cancer types, such as lymphoma and head and neck cancer (27). HCC cells that survive RT exhibit more
aggressive behaviors, including proliferation, anti-apoptosis and
metastasis (28). Proteins
associated with these aggressive behaviors, such as cyclin D1,
Bcl-2 and MMP-9, are regulated by a key transcriptional factor,
NF-κB. NF-κB activity modulates the balance between RT-induced
apoptosis and radioresistance that influences the efficacy of RT in
certain types of cancer, including oropharyngeal cancer, HCC and
lung cancer (29–32).
Our previous study showed that pretreatment with
sorafenib combined with RT led to improved tumor inhibition in
subcutaneous HCC tumor-bearing mice through inhibition of NF-κB
activity (20). The present study
aimed to understand whether this combination treatment is also
feasible and safe for application in an orthotopic HCC model
because the liver is a relatively radiosensitive organ (6). Huh7/NF-κB-tk-luc2/rfp cells were
used to generate an orthotopic HCC model. As the NF-κB-responsive
element controls both reporter genes, tk and luc2,
molecular imaging could be used to monitor in vivo NF-κB
activity longitudinally with the same cohorts of mice (21).
In vivo BLI revealed changes in NF-κB
activity over time. BLI signals were similar among all groups until
day 15. Unlike the results obtained from previous subcutaneous HCC
models (20), RT and sorafenib
slightly increased NF-κB activity in orthotopic HCC tumors over
time. Notably, NF-κB activity decreased over time in the
combination group.
Then, nuclear proteins were extracted and EMSA was
used to determine NF-κB activity; the results were consistent with
the BLI findings. The combination treatment suppressed NF-κB/DNA
binding activity, which was also repressed in the sorafenib and RT
groups. Similar patterns, NF-κB-driven BLI signals and EMSA results
have been observed in our previous studies (20,21). It
has been reported that the NF-κB-driven reporter assay is more
sensitive compared with EMSA because the use of a specific
promoter/responsive element-driven reporter system could further
enhance signals via both transcription and translation (33,34).
Additionally, luciferase is not expressed by mammalian cells; thus,
BLI should have minimal background signals and should detect small
differences between groups (35).
NF-κB is a signaling hub controlling multiple
proteins such as VEGF and XIAP and promoting tumor progression
(22). The present study extracted
proteins from tumors and used western blotting to examine the
expression of MMP-9, cyclin D1, and COX-2, which are associated
with invasiveness (36),
proliferation (36) and inflammation
(37). Although the expression of
these proteins was found to be decreased in all treatment groups,
they were reduced very little in the RT group compared with the
other groups. Our previous studies (20,21)
indicated that sorafenib slows HCC growth through ERK/NF-κB
inhibition. Therefore, NF-κB activity was also examined, and the
result was consistent with those for the three NF-κB downstream
proteins aforementioned. Sorafenib and combination treatment
markedly suppressed cyclin D1 expression in tumors.
Both anti-proliferation and increased apoptosis can
lead to tumor suppression (38).
Therefore, apoptosis-related proteins, including c-FLIP, caspase-8
and cleaved caspase-3, were further examined. Cleaved caspase-3
expression was increased in all treatment groups. It is worth
noting that RT caused increased c-FLIP and decreased caspase-8
expression compared with the control group; however, c-FLIP was
decreased and caspase-8 was increased in the sorafenib and
combination groups. The apoptotic pathway can be further divided
into extrinsic and intrinsic pathways, and caspase-8 is present in
both pathways. c-FLIP is known as a master anti-apoptosis regulator
(39) and prevents activation of
caspase-8 and its downstream caspase cascades. Stagni et al
(40) proposed that ATM activation
may modulate c-FLIP expression in lymphoid cells. The ATM
serine/threonine kinase is activated by DNA damage resulting from
chemotherapy or ionizing radiation, then initiates DNA repair or
apoptotic processes. Ivanov et al (41) showed that pretreatment with an ATM
inhibitor, KU-55933, decreased radiation-induced c-FLIP, p53 and
NF-κB activation in melanoma cells. c-FLIP-silencing can also
enhance TNF-related apoptosis-inducing ligand (TRAIL)-mediated cell
killing by restoring apoptosis in cervical, ovarian and breast
cancer cells (42,43).
It is known that RT-induced apoptosis occurs mainly
through the intrinsic pathway (44),
and this could partially explain the present western blotting
results. RT increased cleaved caspase-3 and c-FLIP expression, and
reduced caspase-8 expression compared with the control group. These
results implied that the development of radioresistance is not only
caused by NF-κB but also c-FLIP in orthotopic HCC tumors.
McLaughlin et al (45)
reported that c-FLIP expression negatively modulates
radiosensitivity in non-small cell lung cancer by overexpressing
and silencing c-FLIP. c-FLIP is also one of the proteins downstream
of NF-κB (46).
NF-κB and c-FLIP influence Bcl-2 family protein
expression through different mechanisms. NF-κB transcriptionally
promotes Bcl-2 expression (47), and
c-FLIP prevents caspase-8-mediated BID cleavage and intrinsic
apoptosis (48). Bcl-2 family
proteins can trigger both pro-apoptotic and pro-survival pathways
(49). All treatments slightly
suppressed the expression of Bcl-2 and pro-survival Bcl-2 family
proteins including Bcl-xL and Mcl-1 in the present study. In
contrast, Bcl-2-related pro-apoptotic Bim, Bak and BID were
markedly increased after sorafenib and combination treatment. These
results indicated that sorafenib and combination treatment promoted
cell death mainly by enhancing pro-apoptotic signaling in HCC
cells. Several studies have shown that the combination of sorafenib
with Mcl-1 (50,51), Bcl-2 (52) or Bcl-xL (53) inhibitors synergistically enhance its
ability to kill different types of cancer cells.
Bidirectional regulation between NF-κB and c-FLIP
has been reported (39). c-FLIP
upregulates NF-κB expression (54)
and enhances its nuclear translocation (55). However, the present EMSA results
showed that RT did not change NF-κB/DNA binding activity, even when
c-FLIP expression was increased in orthotopic HCC tumors. These
results suggested that other transcription factors are involved.
Accumulating evidence demonstrates that STAT3 inhibition could
suppress STAT3 and reverse TRAIL resistance in multiple cancer
types, such as lung cancer, renal carcinoma and HCC (56,57).
Sorafenib inhibits tumor growth and metastasis by targeting RTKs
and blocking the STAT3 pathway in HCC (58,59).
Additionally, sorafenib has been shown to enhance radiation-induced
apoptosis (60) and reverses TRAIL
resistance (61) in HCC by targeting
STAT3. There remain some interesting avenues for future research,
for example the changes and interactions between STAT3 and NF-κB
after sorafenib combined with radiotherapy, the roles of immune
cells in sorafenib combined with radiotherapy and the possibilities
of combining sorafenib, radiotherapy and immunotherapy in HCC.
Although the changes in NF-κB activity in tumors were evaluated
using EMSA, the NF-κB protein level was not determined in the
current study. The changes in NF-κB-regulated Bcl-2 family proteins
by treatments were studied with Huh7 cells rather than HCC tumors.
The lack of in vivo experiments to confirm these findings is
also a limitation of the present study.
Decreased cyclin D1 and elevated cleaved caspase-3
levels from tumors harvested in the present study were detected
using western blotting, suggesting tumor reduction may have
resulted from impaired cell proliferation and enhanced apoptosis,
respectively. Moreover, all treatment groups showed smaller and
fewer tumors compared with the control group. Again, the smaller
tumor sizes and fewer metastatic lesions are consistent with the
results obtained by western blotting. All treatments decreased
cyclin D1 and MMP-9 expression and increased cleaved caspase-3
expression in orthotopic HCC tumors.
As the liver is a radiosensitive organ, potential
toxicity limits RT applications in HCC treatment. Although changes
in body weight were not observed in the subcutaneous HCC models
(18), transient but significant
decreases in body weight were detected in the combination group in
the current study. However, the body weight decrease was within
±20% of the mean body weight and recovered at later time points.
The reduction in body weight was due to RT-induced toxicity rather
than cachexia. Although only the liver was exposed to RT during
irradiation, intestinal damage may have occurred contributing to
weight loss. It was speculated that the toxicity resulting from
combination treatment would be acceptable. The transient toxicity
could be further decreased if the irradiation dose could be
delivered more precisely and exclusively to tumors. Particle
therapies like proton therapy and carbon ion therapy could be ideal
candidates. Both protons and carbon ions have narrow Bragg peak,
which means that irradiation doses can be delivered at specific
depths and reduce the doses received by surrounding normal tissues
(62,63).
To the best of our knowledge, the present study is
the first showing that the combination of sorafenib and RT could
suppress tumor growth using an orthotopic HCC model. In summary,
pretreatment with sorafenib plus RT led to the best tumor
inhibition with acceptable general toxicity. The sorafenib and RT
combination acts through NF-κB inhibition and likely STAT3
suppression as well. Moreover, it would be worth investigating how
the combination treatment modulates tumor metastasis and influences
the tumor microenvironment in the future as both NF-κB and STAT3
are critical signaling hubs that regulate cancer progression
(64–66).
Acknowledgments
Not applicable.
Funding
The study was supported by grants from The Ministry
of Science and Technology (grant no. NSC101-2314-B-010-045-MY3) and
The Chung Shan Medical University Hospital (grant no.
CSH-2017-C008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HYC carried out experimental work, data analysis,
manuscript preparation and editing. YST assisted with data
analysis, manuscript preparation and editing. KCS assisted with
western blotting experiments. WCL assisted with experimental
design, data analysis and manuscript editing. JJH designed and
supervised the experiments, edited the manuscript and acquired
funding. HYC and JJH confirmed the authenticity of all raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All the animal experiments and procedures were
approved by The Institutional Animal Care and Use Committee of
National Yang-Ming University (Taipei, Taiwan; approval no.
1001238).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McGlynn KA, Petrick JL and London WT:
Global epidemiology of hepatocellular carcinoma: An emphasis on
demographic and regional variability. Clin Liver Dis. 19:223–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golabi P, Fazel S, Otgonsuren M, Sayiner
M, Locklear CT and Younossi ZM: Mortality assessment of patients
with hepatocellular carcinoma according to underlying disease and
treatment modalities. Medicine (Baltimore). 96:e5904. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Sun J and Yang X: Radiofrequency
ablation-combined multimodel therapies for hepatocellular
carcinoma: Current status. Cancer Lett. 370:78–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chao Y, Chung YH, Han G, Yoon JH, Yang J,
Wang J, Shao GL, Kim BI and Lee TY: The combination of
transcatheter arterial chemoembolization and sorafenib is well
tolerated and effective in Asian patients with hepatocellular
carcinoma: Final results of the START trial. Int J Cancer.
136:1458–1467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsieh CH, Jeng KS, Lin CC, Chen CK, Liu
CY, Lin CP, Tai HC, Wang CH, Shueng PW and Chen YJ: Combination of
sorafenib and intensity modulated radiotherapy for unresectable
hepatocellular carcinoma. Clin Drug Investig. 29:65–71. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cha J, Seong J, Lee IJ, Kim JW and Han KH:
Feasibility of Sorafenib combined with local radiotherapy in
advanced hepatocellular carcinoma. Yonsei Med J. 54:1178–1185.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen SW, Lin LC, Kuo YC, Liang JA, Kuo CC
and Chiou JF: Phase 2 study of combined sorafenib and radiation
therapy in patients with advanced hepatocellular carcinoma. Int J
Radiat Oncol Biol Phys. 88:1041–1047. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brade AM, Ng S, Brierley J, Kim J,
Dinniwell R, Ringash J, Wong RR, Cho C, Knox J and Dawson LA: Phase
1 trial of sorafenib and stereotactic body radiation therapy for
hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 94:580–587.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stryker JA: Science to practice: Why is
the liver a radiosensitive organ? Radiology. 242:1–2. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalogeridi MA, Zygogianni A, Kyrgias G,
Kouvaris J, Chatziioannou S, Kelekis N and Kouloulias V: Role of
radiotherapy in the management of hepatocellular carcinoma: A
systematic review. World J Hepatol. 7:101–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ricke J, Bulla K, Kolligs F,
Peck-Radosavljevic M, Reimer P, Sangro B, Schott E, Schütte K,
Verslype C, Walecki J, et al: Safety and toxicity of
radioembolization plus Sorafenib in advanced hepatocellular
carcinoma: Analysis of the European multicentre trial SORAMIC.
Liver Int. 35:620–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riaz A, Gabr A, Abouchaleh N, Ali R, Al
Asadi A, Mora R, Kulik L, Desai K, Thornburg B, Mouli S, et al:
Radioembolization for hepatocellular carcinoma: Statistical
confirmation of improved survival in responders by landmark
analyses. Hepatology. 67:873–883. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guha C and Kavanagh BD: Hepatic radiation
toxicity: Avoidance and amelioration. Semin Radiat Oncol.
21:256–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seidensticker R, Seidensticker M, Damm R,
Mohnike K, Schütte K, Malfertheiner P, Van Buskirk M, Pech M,
Amthauer H and Ricke J: Hepatic toxicity after radioembolization of
the liver using (90)Y-microspheres: Sequential lobar versus whole
liver approach. Cardiovasc Intervent Radiol. 35:1109–1118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mahvash A, Murthy R, Odisio BC, Raghav KP,
Girard L, Cheung S, Nguyen V, Ensor J, Gadani S, Elsayes KM, et al:
Yttrium-90 resin microspheres as an adjunct to sorafenib in
patients with unresectable hepatocellular carcinoma. J Hepatocell
Carcinoma. 3:1–7. 2016.PubMed/NCBI
|
|
17
|
Ricke J, Klumpen HJ, Amthauer H,
Bargellini I, Bartenstein P, de Toni EN, Gasbarrini A, Pech M,
Peck-Radosavljevic M, Popovič P, et al: Impact of combined
selective internal radiation therapy and sorafenib on survival in
advanced hepatocellular carcinoma. J Hepatol. 71:1164–1174. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu W, Gu K, Yu Z, Yuan D, He M, Ma N, Lai
S, Zhao J, Ren Z, Zhang X, et al: Sorafenib potentiates irradiation
effect in hepatocellular carcinoma in vitro and in vivo. Cancer
Lett. 329:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu FT, Chang B, Chen JC, Chiang IT, Liu
YC, Kwang WK and Hwang JJ: Synergistic effect of sorafenib and
radiation on human oral carcinoma in vivo. Sci Rep. 5:153912015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen JCH, Chuang HY, Hsu FT, Chen YC,
Chien YC and Hwang JJ: Sorafenib pretreatment enhances radiotherapy
through targeting MEK/ERK/NF-κB pathway in human hepatocellular
carcinoma-bearing mouse model. Oncotarget. 7:85450–85463. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang WH, Chiang IT, Liu YC, Hsu FT, Chen
HW, Chen CL, Lee YJ, Lin WJ and Hwang JJ: Simultaneous imaging of
temporal changes of NF-κB activity and viable tumor cells in
Huh7/NF-κB-tk-luc2/rfp tumor-bearing mice. In Vivo. 27:339–350.
2013.PubMed/NCBI
|
|
22
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zamora-Valdes D, Taner T and Nagorney DM:
Surgical treatment of hepatocellular carcinoma. Cancer Control.
24:10732748177292582017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eggert T and Greten TF: Current standard
and future perspectives in non-surgical therapy for hepatocellular
carcinoma. Digestion. 96:1–4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang A, Yang XR, Chung WY, Dennison AR
and Zhou J: Targeted therapy for hepatocellular carcinoma. Signal
Transduct Target Ther. 5:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayabuchi N: Radiocurable tumors and
non-radiocurable tumors. Japan Med Assoc J. 47:79–83. 2004.
|
|
28
|
Piao LS, Hur W, Kim TK, Hong SW, Kim SW,
Choi JE, Sung PS, Song MJ, Lee BC, Hwang D and Yoon SK:
CD133+ liver cancer stem cells modulate radioresistance
in human hepatocellular carcinoma. Cancer Lett. 315:129–137. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li F and Sethi G: Targeting transcription
factor NF-kappaB to overcome chemoresistance and radioresistance in
cancer therapy. Biochim Biophys Acta Rev Cancer. 1805:167–180.
2010. View Article : Google Scholar
|
|
30
|
Qiao Q, Sun C, Han C, Han N, Zhang M and
Li G: Endoplasmic reticulum stress pathway PERK-eIF2α confers
radioresistance in oropharyngeal carcinoma by activating NF-κB.
Cancer Sci. 108:1421–1431. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren K, Li Z, Li Y, Zhang W and Han X:
Sulforaphene enhances radiosensitivity of hepatocellular carcinoma
through suppression of the NF-κB pathway. J Biochem Mol Toxicol.
31:e219172017. View Article : Google Scholar
|
|
32
|
Ji K, Sun X, Liu Y, Du L, Wang Y, He N,
Wang J, Xu C and Liu Q: Regulation of apoptosis and radiation
sensitization in lung cancer cells via the Sirt1/NF-κB/smac
pathway. Cell Physiol Biochem. 48:304–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smartt HJ, Elder DJ, Hicks DJ, Williams NA
and Paraskeva C: Increased NF-kappaB DNA binding but not
transcriptional activity during apoptosis induced by the
COX-2-selective inhibitor NS-398 in colorectal carcinoma cells. Br
J Cancer. 89:1358–1365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vanden Berghe W, Dijsselbloem N, Vermeulen
L, Ndlovu MN, Boone E and Haegeman G: Attenuation of mitogen- and
stress-activated protein kinase-1-driven nuclear factor-kappaB gene
expression by soy isoflavones does not require estrogenic activity.
Cancer Res. 66:4852–4862. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Close DM, Xu T, Sayler GS and Ripp S: In
vivo bioluminescent imaging (BLI): Noninvasive visualization and
interrogation of biological processes in living animals. Sensors
(Basel). 11:180–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kunnumakkara AB, Diagaradjane P, Anand P,
Harikumar KB, Deorukhkar A, Gelovani J, Guha S, Krishnan S and
Aggarwal BB: Curcumin sensitizes human colorectal cancer to
capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and
CXCR4 expression in an orthotopic mouse model. Int J Cancer.
125:2187–2197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bachmeier BE, Killian PH and Melchart D:
The role of curcumin in prevention and management of metastatic
disease. Int J Mol Sci. 19:17162018. View Article : Google Scholar
|
|
38
|
Lai ZC, Wei X, Shimizu T, Ramos E,
Rohrbaugh M, Nikolaidis N, Ho LL and Li Y: Control of cell
proliferation and apoptosis by mob as tumor suppressor, Mats. Cell.
120:675–685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Safa AR: c-FLIP, a master anti-apoptotic
regulator. Exp Oncol. 34:176–184. 2012.PubMed/NCBI
|
|
40
|
Stagni V, di Bari MG, Cursi S, Condò I,
Cencioni MT, Testi R, Lerenthal Y, Cundari E and Barilà D: ATM
kinase activity modulates Fas sensitivity through the regulation of
FLIP in lymphoid cells. Blood. 111:829–837. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ivanov VN, Zhou H, Partridge MA and Hei
TK: Inhibition of ataxia telangiectasia mutated kinase activity
enhances TRAIL-mediated apoptosis in human melanoma cells. Cancer
Res. 69:3510–3519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim TE, Hong S, Song K, Park SH and Shin
YK: Sensitization of glycoengineered interferon-β1a-resistant
cancer cells by cFLIP inhibition for enhanced anti-cancer therapy.
Oncotarget. 8:13957–13970. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Poondla N, Chandrasekaran AP, Heese K, Kim
KS and Ramakrishna S: CRISPR-mediated upregulation of DR5 and
downregulation of cFLIP synergistically sensitize HeLa cells to
TRAIL-mediated apoptosis. Biochem Biophys Res Commun. 512:60–65.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maier P, Hartmann L, Wenz F and Herskind
C: Cellular pathways in response to ionizing radiation and their
targetability for tumor radiosensitization. Int J Mol Sci.
17:1022016. View Article : Google Scholar
|
|
45
|
McLaughlin KA, Nemeth Z, Bradley CA,
Humphreys L, Stasik I, Fenning C, Majkut J, Higgins C, Crawford N,
Holohan C, et al: FLIP: A targetable mediator of resistance to
radiation in non-small cell lung cancer. Mol Cancer Ther.
15:2432–2441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ranjan K and Pathak C: FADD regulates
NF-kappaB activation and promotes ubiquitination of cFLIPL to
induce apoptosis. Sci Rep. 6:227872016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Catz SD and Johnson JL: Transcriptional
regulation of bcl-2 by nuclear factor kappa B and its significance
in prostate cancer. Oncogene. 20:7342–7351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Engels IH, Stepczynska A, Stroh C, Lauber
K, Berg C, Schwenzer R, Wajant H, Jänicke RU, Porter AG, Belka C,
et al: Caspase-8/FLICE functions as an executioner caspase in
anticancer drug-induced apoptosis. Oncogene. 19:4563–4573. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Delbridge AR and Strasser A: The BCL-2
protein family, BH3-mimetics and cancer therapy. Cell Death Differ.
22:1071–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tong J, Wang P, Tan S, Chen D,
Nikolovska-Coleska Z, Zou F, Yu J and Zhang L: Mcl-1 degradation is
required for targeted therapeutics to eradicate colon cancer cells.
Cancer Res. 77:2512–2521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hsu C, Lin LI, Cheng YC, Feng ZR, Shao YY,
Cheng AL and Ou DL: Cyclin E1 Inhibition can overcome sorafenib
resistance in hepatocellular carcinoma cells through Mcl-1
suppression. Clin Cancer Res. 22:2555–2564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tutusaus A, Stefanovic M, Boix L, Cucarull
B, Zamora A, Blasco L, de Frutos PG, Reig M, Fernandez-Checa JC,
Marí M, et al: Antiapoptotic BCL-2 proteins determine
sorafenib/regorafenib resistance and BH3-mimetic efficacy in
hepatocellular carcinoma. Oncotarget. 9:16701–16717. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hikita H, Takehara T, Shimizu S, Kodama T,
Shigekawa M, Iwase K, Hosui A, Miyagi T, Tatsumi T, Ishida H, et
al: The Bcl-xL inhibitor, ABT-737, efficiently induces apoptosis
and suppresses growth of hepatoma cells in combination with
sorafenib. Hepatology. 52:1310–1321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ivanisenko NV, Buchbinder JH, Espe J,
Richter M, Bollmann M, Hillert LK, Ivanisenko VA and Lavrik IN:
Delineating the role of c-FLIP/NEMO interaction in the CD95 network
via rational design of molecular probes. BMC Genomics. 20 (Suppl
3):S2932019. View Article : Google Scholar
|
|
55
|
Jiang Z and Clemens PR: Cellular
caspase-8-like inhibitory protein (cFLIP) prevents inhibition of
muscle cell differentiation induced by cancer cells. FASEB J.
20:2570–2572. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guo ZL, Li JZ, Ma YY, Qian D, Zhong JY,
Jin MM, Huang P, Che LY, Pan B, Wang Y, et al: Shikonin sensitizes
A549 cells to TRAIL-induced apoptosis through the JNK, STAT3 and
AKT pathways. BMC Cell Biol. 19:292018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim S, Woo SM, Min KJ, Seo SU, Lee TJ,
Kubatka P, Kim DE and Kwon TK: WP1130 enhances TRAIL-induced
apoptosis through USP9X-dependent miR-708-mediated downregulation
of c-FLIP. Cancers (Basel). 11:3442019. View Article : Google Scholar
|
|
58
|
Gu FM, Li QL, Gao Q, Jiang JH, Huang XY,
Pan JF, Fan J and Zhou J: Sorafenib inhibits growth and metastasis
of hepatocellular carcinoma by blocking STAT3. World J
Gastroenterol. 17:3922–3932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tai WT, Cheng AL, Shiau CW, Huang HP,
Huang JW, Chen PJ and Chen KF: Signal transducer and activator of
transcription 3 is a major kinase-independent target of sorafenib
in hepatocellular carcinoma. J Hepatol. 55:1041–1048. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Huang CY, Lin CS, Tai WT, Hsieh CY, Shiau
CW, Cheng AL and Chen KF: Sorafenib enhances radiation-induced
apoptosis in hepatocellular carcinoma by inhibiting STAT3. Int J
Radiat Oncol Biol Phys. 86:456–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen KF, Tai WT, Liu TH, Huang HP, Lin YC,
Shiau CW, Li PK, Chen PJ and Cheng AL: Sorafenib overcomes TRAIL
resistance of hepatocellular carcinoma cells through the inhibition
of STAT3. Clin Cancer Res. 16:5189–5199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
La Tessa C, Berger T, Kaderka R, Schardt
D, Körner C, Ramm U, Licher J, Matsufuji N, Vallhagen Dahlgren C,
Lomax T, et al: Out-of-field dose studies with an anthropomorphic
phantom: Comparison of X-rays and particle therapy treatments.
Radiother Oncol. 105 1:133–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Loeffler JS and Durante M: Charged
particle therapy-optimization, challenges and future directions.
Nat Rev Clin Oncol. 10:411–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Archiv. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|