|
1
|
Hwang SY, Park S and Kwon Y: Recent
therapeutic trends and promising targets in triple negative breast
cancer. Pharmacol Ther. 199:30–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. Feb 4–2021.(Epub ahead
of print). doi: 10.3322/caac.21660. View Article : Google Scholar : PubMed/NCBI
|
|
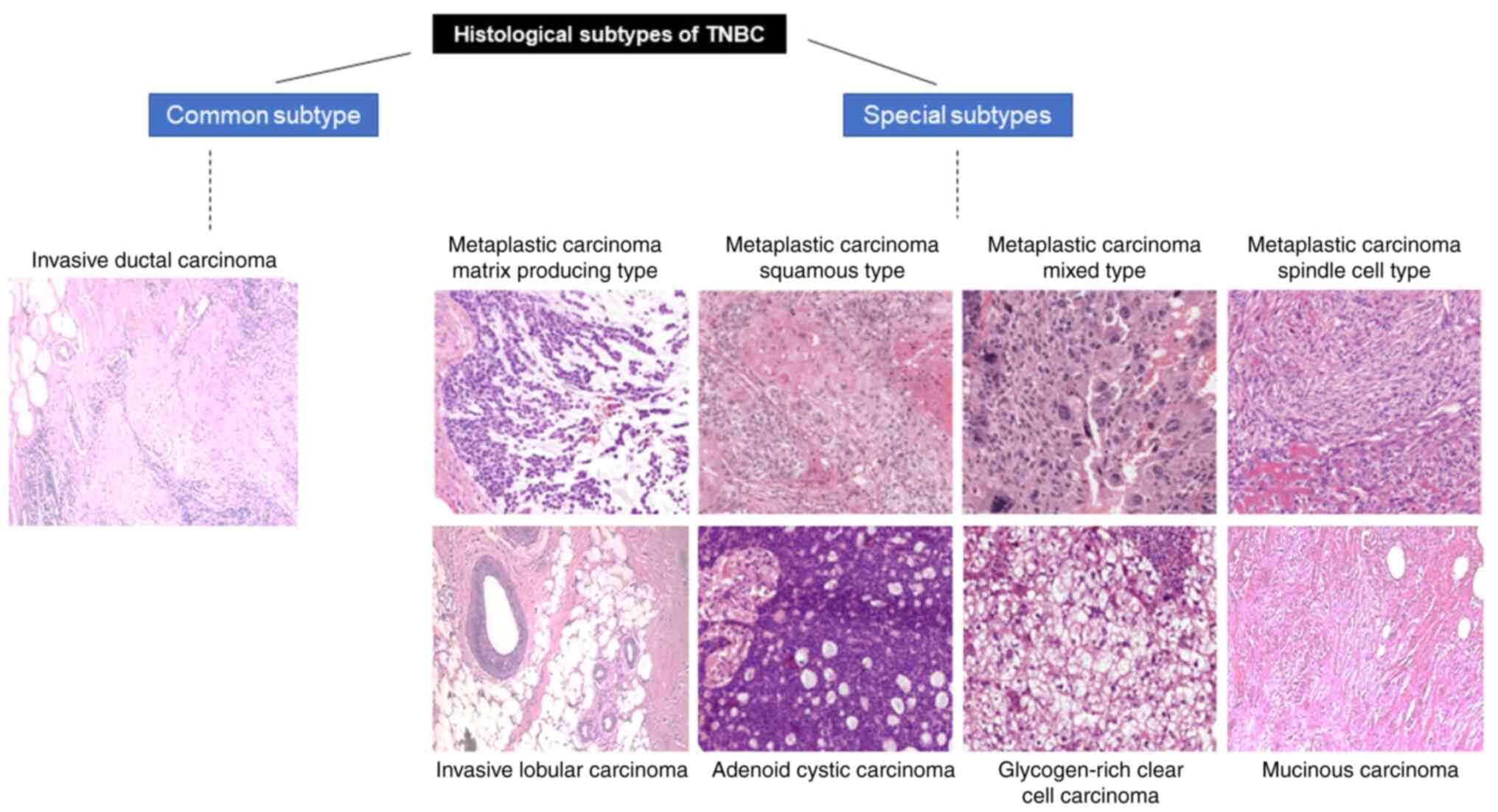
3
|
Perue CM, Sorlie T, Elsen MB, van de Rijn
M, Jeffrey S and Rees C: Molecular portraits of human breast
tumors. Nature. 406:747–52. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Penault-Llorca F and Viale G: Pathological
and molecular diagnosis of triple-negative breast cancer: A
clinical perspective. Ann Oncol. 23:vi19–vi22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yeh IT and Mies C: Application of
immunohistochemistry to breast lesions. Arch Pathol Lab Med.
132:349–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fedele M, Cerchia L and Chiappetta G: The
epithelial-to-mesenchymal transition in breast cancer: Focus on
basal-like carcinomas. Cancers. 9:1342017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dias K, Dvorkin-Gheva A, Hallett RM, Wu Y,
Hassell J, Pond GR, Levine M, Whelan T and Bane AL: Claudin-low
breast cancer; clinical & pathological characteristics. PLoS
One. 12:e01686692017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
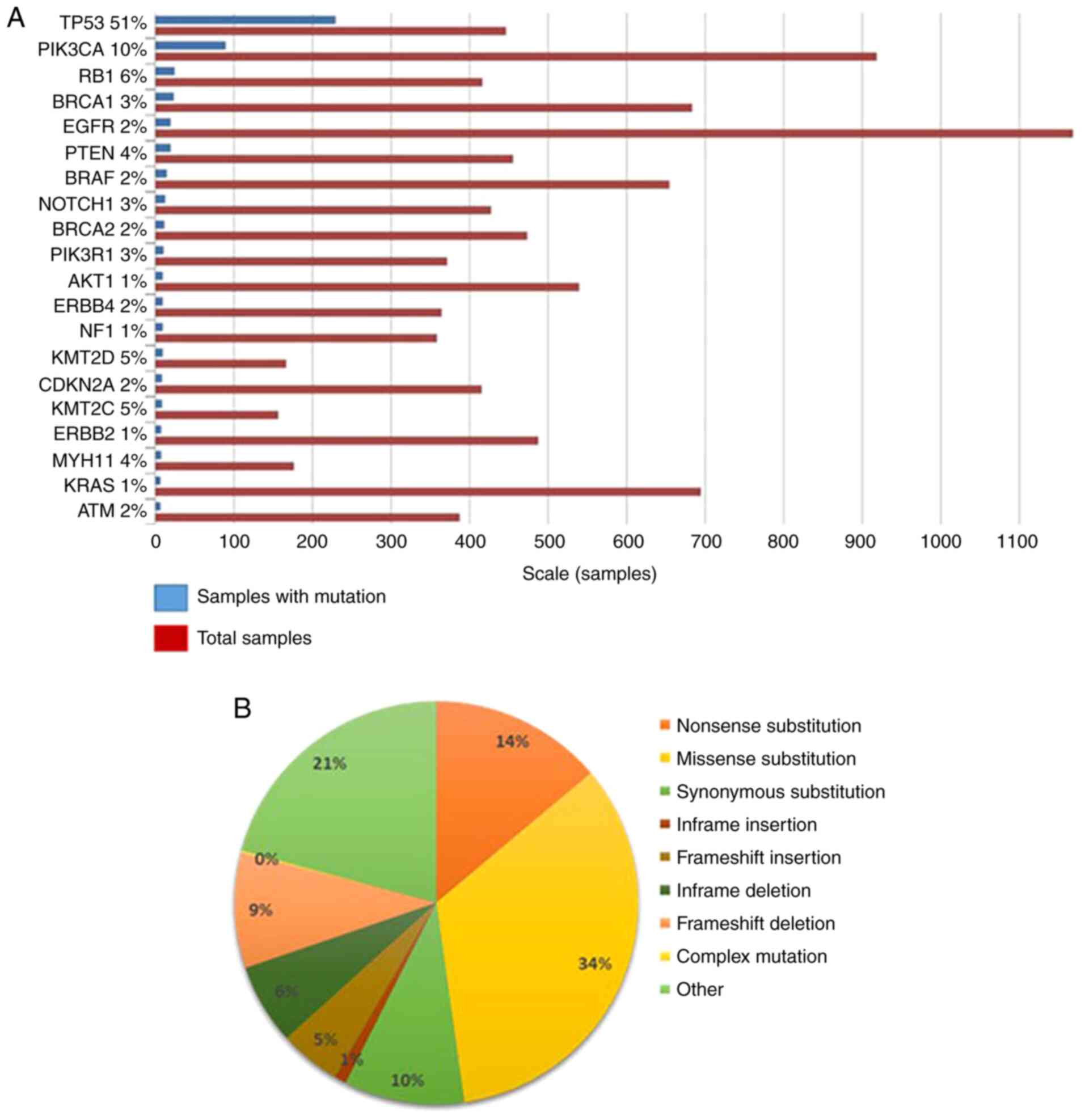
Spigel DR and Burstein HJ: HER2
overexpressing metastatic breast cancer. Curr Treat Options Oncol.
3:163–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
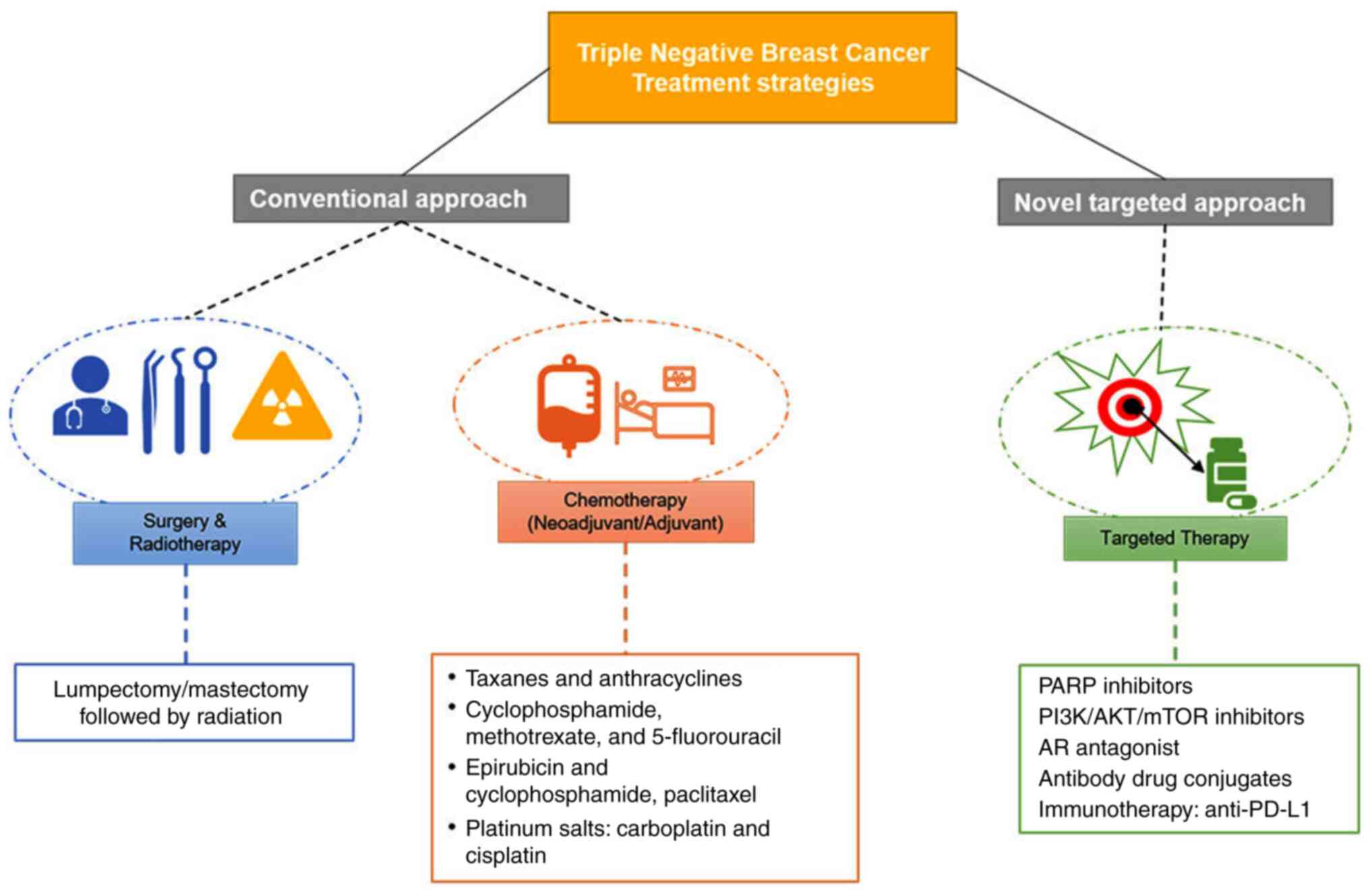
|
10
|
Hu Z, Fan C, Oh DS, Marron JS, He X,
Qaqish BF, Livasy C, Carey LA, Reynolds E, Dressler L, et al: The
molecular portraits of breast tumors are conserved across
microarray platforms. BMC Genomics. 7:962006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, Xu M, Sun Y, Chen J, Chen C, Qian
C, Chen Y, Cao L, Xu Q, Du X and Yang W: Gene expression profiling
for diagnosis of triple-negative breast cancer: A multicenter,
retrospective cohort study. Front Oncol. 9:3542019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slamon D, Eiermann W, Robert N, Pienkowski
T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et
al: Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J
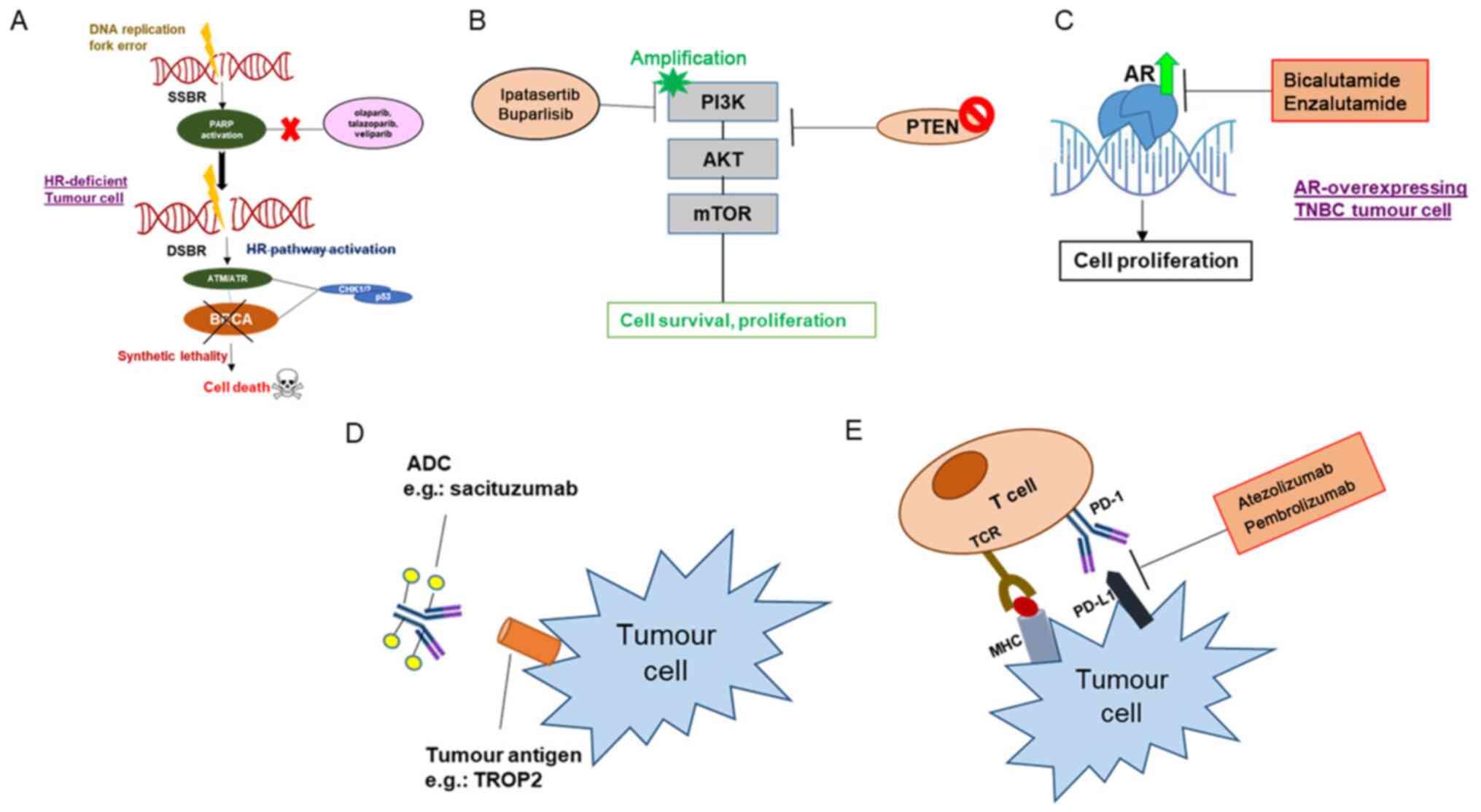
Med. 365:1273–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marotti JD, de Abreu FB, Wells WA and
Tsongalis GJ: Triple-negative breast cancer: Next-generation
sequencing for target identification. Am J Pathol. 187:2133–2138.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reis-Filho JS and Tutt ANJ: Triple
negative tumours: A critical review. Histopathology. 52:108–118.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaplan HG, Malmgren JA and Atwood M: T1N0
triple negative breast cancer: Risk of recurrence and adjuvant
chemotherapy. Breast J. 15:454–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang-Qing Y, Jie L, Shi-Qi Z, Kun Z,
Zi-Qian G, Ran X, Hui-Meng L, Ren-Bin Z, Gang Z, Da-Chuan Y and
Chen-Yan Z: Recent treatment progress of triple negative breast
cancer. Prog Biophys Mol Biol. 151:40–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weigelt B and Reis-Filho JS: Histological
and molecular types of breast cancer: Is there a unifying taxonomy?
Nat Rev Clin Oncol. 6:7182009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shah SP, Roth A, Goya R, Oloumi A, Ha G,
Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, et al: The clonal
and mutational evolution spectrum of primary triple-negative breast
cancers. Nature. 486:395–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malhotra GK, Zhao X, Band H and Band V:
Histological, molecular and functional subtypes of breast cancers.
Cancer Biol Ther. 10:955–960. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balkenhol MC, Vreuls W, Wauters CA, Mol
SJ, van der Laak JA and Bult P: Histological subtypes in triple
negative breast cancer are associated with specific information on
survival. Ann Diagn Pathol. 46:1514902020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romero P, Benhamo V, Deniziaut G, Fuhrmann
L, Berger F, Manié E, Bhalshankar J, Vacher S, Laurent C, Marangoni
E, et al: Medullary breast carcinoma, a triple-negative breast
cancer associated with BCLG overexpression. Am J Pathol.
188:2378–2391. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huober J, Gelber S, Thurlimann B,
Goldhirsch A, Coates AS, Viale G, Öhlschlegel C, Price KN, Gelber
RD, Regan MM and Thürlimann B: Prognosis of medullary breast
cancer: Analyses of 13 International Breast Cancer Study Group
(IBCSG) trials. Ann Oncol. 23:2843–2851. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geyer FC, Weigelt B, Natrajan R, Lambros
MB, de Biase D, Vatcheva R, Savage K, Mackay A, Ashworth A and
Reis-Filho JS: Molecular analysis reveals a genetic basis for the
phenotypic diversity of metaplastic breast carcinomas. J Pathol.
220:562–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hennessy BT, Gonzalez-Angulo AM,
Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J,
Sahin A, Agarwal R, Joy C, et al: Characterization of a naturally
occurring breast cancer subset enriched in
epithelial-to-mesenchymal transition and stem cell characteristics.
Cancer Res. 69:4116–4124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayes MJ, Thomas D, Emmons A, Giordano TJ
and Kleer CG: Genetic changes of Wnt pathway genes are common
events in metaplastic carcinomas of the breast. Clin Cancer Res.
14:4038–4044. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thomas DN, Asarian A and Xiao P: Adenoid
cystic carcinoma of the breast. J Surg Case Rep. 2019:rjy3552019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ichikawa K, Mizukami Y, Takayama T,
Takemura A, Miyati T and Taniya T: A case of adenoid cystic
carcinoma of the breast. J Med Ultrasonics. 34:193–196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun JY, Wu SG, Chen SY, Li FY, Lin HX,
Chen YX and He ZY: Adjuvant radiation therapy and survival for
adenoid cystic carcinoma of the breast. Breast. 31:214–218. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aktepe F, Sarsenov D and Özmen V:
Secretory carcinoma of the breast. J Breast Health. 12:1742016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Wu N, Li F, Li L, Wei L and Liu J:
Clinicopathologic and molecular characteristics of 44 patients with
pure secretory breast carcinoma. Cancer Biol Med. 16:1392019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pareja F, Geyer FC, Marchiò C, Burke KA,
Weigelt B and Reis-Filho JS: Triple-negative breast cancer: The
importance of molecular and histologic subtyping, and recognition
of low-grade variants. NPJ Breast Cancer. 2:160362016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuroda H, Sakamoto G, Ohnisi K and Itoyama
S: Clinical and pathological features of glycogen-rich clear cell
carcinoma of the breast. Breast Cancer. 12:189–195. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Geyer FC, Pareja F, Weigelt B, Rakha E,
Ellis IO, Schnitt SJ and Reis-Filho JS: The spectrum of
triple-negative breast disease: High-and low-grade lesions. Am J
Pathol. 187:2139–2151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Degnim AC, Brahmbhatt RD, Radisky DC,
Hoskin TL, Stallings-Mann M, Laudenschlager M, Mansfield A, Frost
MH, Murphy L, Knutson K and Visscher DW: Immune cell quantitation
in normal breast tissue lobules with and without lobulitis. Breast
Cancer Res Treat. 144:539–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aaltomaa S, Lipponen P, Eskelinen M, Kosma
VM, Marin S, Alhava E and Syrjänen K: Lymphocyte infiltrates as a
prognostic variable in female breast cancer. Eur J Cancer.
28:859–864. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsumoto H, Koo S, Dent R, Tan PH and
Iqbal J: Role of inflammatory infiltrates in triple negative breast
cancer. J Clin Pathol. 68:506–510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fridman WH, Galon J, Pagès F, Tartour E,
Sautès-Fridman C and Kroemer G: Prognostic and predictive impact of
intra-and peritumoral immune infiltrates. Cancer Res. 71:5601–5605.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karn T, Jiang T, Hatzis C, Sänger N,
El-Balat A, Holtrich U, Becker S, Bianchini G and Pusztai L:
Abstract S1-07: Immune sculpting of the triple negative breast
cancer genome. Cancer Res. 772017.doi:
10.1158/1538-7445.SABCS16-S1-07.
|
|
39
|
Bottai G, Raschioni C, Losurdo A, Di
Tommaso L, Tinterri C, Torrisi R, Reis-Filho JS, Roncalli M,
Sotiriou C, Santoro A, et al: An immune stratification reveals a
subset of PD-1/LAG-3 double-positive triple-negative breast
cancers. Breast Cancer Res. 18:1212016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gruosso T, Gigoux M, Bertos N, Manem VSK,
Guiot MC, Buisseret L, Salgado R, Van den Eyden G, Haibe-Kains B
and Park M: Distinct immune microenvironments stratify
triple-negative breast cancer and predict outcome. Ann Oncol.
28:i162017. View Article : Google Scholar
|
|
41
|
Abramson VG, Lehmann BD, Ballinger TJ and
Pietenpol JA: Subtyping of triple-negative breast cancer:
Implications for therapy. Cancer. 121:8–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lehmann BD and Pietenpol JA:
Identification and use of biomarkers in treatment strategies for
triple-negative breast cancer subtypes. J Pathol. 232:142–150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Burstein MD, Tsimelzon A, Poage GM,
Covington KR, Contreras A, Fuqua SA, Savage MI, Osborne CK,
Hilsenbeck SG, Chang JC, et al: Comprehensive genomic analysis
identifies novel subtypes and targets of triple-negative breast
cancer. Clin Cancer Res. 21:1688–1698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ahn SG, Kim SJ, Kim C and Jeong J:
Molecular classification of triple-negative breast cancer. J Breast
Cancer. 19:223–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu YR, Jiang YZ, Xu XE, Yu KD, Jin X, Hu
X, Zuo WJ, Hao S, Wu J, Liu GY, et al: Comprehensive transcriptome
analysis identifies novel molecular subtypes and subtype-specific
RNAs of triple-negative breast cancer. Breast Cancer Res.
18:332016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yin L, Duan JJ, Bian XW and Yu S:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22:612020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al: The genomic and transcriptomic architecture of 2,000 breast
tumours reveals novel subgroups. Nature. 486:346–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Venkitaraman AR: Linking the cellular
functions of BRCA genes to cancer pathogenesis and treatment. Ann
Rev Pathol. 4:461–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Atchley DP, Albarracin CT, Lopez A, Valero
V, Amos CI, Gonzalez-Angulo AM, Hortobagyi GN and Arun BK: Clinical
and pathologic characteristics of patients with BRCA-positive and
BRCA-negative breast cancer. J Clin Oncol. 26:4282–4288. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Foulkes WD, Stefansson IM, Chappuis PO,
Bégin LR, Goffin JR, Wong N, Trudel M and Akslen LA: Germline BRCA1
mutations and a basal epithelial phenotype in breast cancer. J Natl
Cancer Inst. 95:1482–1485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Antoniou A, Pharoah PD, Narod S, Risch HA,
Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, et
al: Average risks of breast and ovarian cancer associated with
BRCA1 or BRCA2 mutations detected in case series unselected for
family history: A combined analysis of 22 studies. Am J Hum Genet.
72:1117–1130. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
53
|
Turner N, Tutt A and Ashworth A: Hallmarks
of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 4:814–819. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lord CJ and Ashworth A: BRCAness
revisited. Nat Rev Cancer. 16:110–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bertucci F, Ng CK, Patsouris A, Droin N,
Piscuoglio S, Carbuccia N, Soria JC, Dien AT, Adnani Y, Kamal M, et
al: Genomic characterization of metastatic breast cancers. Nature.
569:560–564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jiang YZ, Ma D, Suo C, Shi J, Xue M, Hu X,
Xiao Y, Yu KD, Liu YR, Yu Y, et al: Genomic and transcriptomic
landscape of triple-negative breast cancers: Subtypes and treatment
strategies. Cancer Cell. 35:428–440.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nik-Zainal S, Davies H, Staaf J,
Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB,
Martin S, Wedge DC, et al: Landscape of somatic mutations in 560
breast cancer whole-genome sequences. Nature. 534:47–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhao Y, Sheng M, Zheng L, Xiong D, Yang K
and Luo Y: Application of circulating tumor DNA in breast cancer.
Breast J. 26:1797–1800. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lustberg MB, Stover DG and Chalmers JJ:
Implementing liquid biopsies in clinical trials: State of affairs,
opportunities and challenges. Cancer J. 24:61–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Thompson AM and Moulder-Thompson SL:
Neoadjuvant treatment of breast cancer. Ann Oncol. 23 (Suppl
10):x231–x236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Stover DG, Parsons HA, Ha G, Freeman SS,
Barry WT, Guo H, Choudhury AD, Gydush G, Reed SC, Rhoades J, et al:
Association of cell-free DNA tumor fraction and somatic copy number
alterations with survival in metastatic triple-negative breast
cancer. J Clin Oncol. 36:543–553. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bidard FC, Peeters DJ, Fehm T, Nolé F,
Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz
JA, Stebbing J, et al: Clinical validity of circulating tumour
cells in patients with metastatic breast cancer: A pooled analysis
of individual patient data. Lancet Oncol. 15:406–414. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cristofanilli M, Pierga JY, Reuben J,
Rademaker A, Davis AA, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado
R, Mavroudis D, et al: The clinical use of circulating tumor cells
(CTCs) enumeration for staging of metastatic breast cancer (MBC):
International expert consensus paper. Crit Rev Oncol Hematol.
134:39–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Riva F, Bidard FC, Houy A, Saliou A, Madic
J, Rampanou A, Hego C, Milder M, Cottu P, Sablin MP, et al:
Patient-specific circulating tumor DNA detection during neoadjuvant
chemotherapy in triple-negative breast cancer. Clin Chem.
63:691–699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Radovich M, Jiang G, Chitambar C, Nanda R,
Falkson C, Lynce FC, Gallagher C, Isaacs C, Blaya M, Paplomata E,
et al: Abstract GS5-02: Detection of circulating tumor DNA (ctDNA)
after neoadjuvant chemotherapy is significantly associated with
disease recurrence in early-stage triple-negative breast cancer
(TNBC): Preplanned correlative results from clinical trial
BRE12-158. Cancer Res. 802020.doi:
10.1158/1538-7445.SABCS19-GS5-02.
|
|
67
|
Becker S: A historic and scientific review
of breast cancer: The next global healthcare challenge. Int J
Gynecol Obstet. 131 (Suppl 1):S36–S39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Blum JL, Flynn PJ, Yothers G, Asmar L,
Geyer CE Jr, Jacobs SA, Robert NJ, Hopkins JO, O'Shaughnessy JA,
Dang CT, et al: Anthracyclines in early breast cancer: The ABC
Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG
Oncology). J Clin Oncol. 35:2647–2655. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mansel RE, Fodstad O and Jiang WG:
Metastasis of breast cancer. Springer; 2007, View Article : Google Scholar
|
|
70
|
Mosca L, Ilari A, Fazi F, Assaraf YG and
Colotti G: Taxanes in cancer treatment: Activity, chemoresistance
and its overcoming. Drug Resist Updat. 54:1007422021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bachegowda LS, Makower DF and Sparano JA:
Taxanes: Impact on breast cancer therapy. Anticancer Drugs.
25:512–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Park JH, Ahn JH and Kim SB: How shall we
treat early triple-negative breast cancer (TNBC): From the current
standard to upcoming immuno-molecular strategies. ESMO Open.
3:e0003572018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Greene J and Hennessy B: The role of
anthracyclines in the treatment of early breast cancer. J Oncol
Pharm Pract. 21:201–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Park JS, Jeung HC, Rha SY, Ahn JB, Kang B,
Chon HJ, Hong MH, Lim S, Yang WI, Nam CM and Chung HC: Phase II
gemcitabine and capecitabine combination therapy in recurrent or
metastatic breast cancer patients pretreated with anthracycline and
taxane. Cancer Chemother Pharmacol. 74:799–808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Karachaliou N, Ziras N, Syrigos K,
Tryfonidis K, Papadimitraki E, Kontopodis E, Bozionelou V, Kalykaki
A, Georgoulias V and Mavroudis D: A multicenter phase II trial of
docetaxel and capecitabine as salvage treatment in
anthracycline-and taxane-pretreated patients with metastatic breast
cancer. Cancer Chemother Pharmacol. 70:169–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Anton A, Lluch A, Casado A, Provencio M,
Muñoz M, Lao J, Bermejo B, Paules AB, Gayo J and Martin M: Phase I
study of oral vinorelbine and capecitabine in patients with
metastatic breast cancer. Anticancer Res. 30:2255–2261.
2010.PubMed/NCBI
|
|
80
|
Kennedy RD, Quinn JE, Mullan PB, Johnston
PG and Harkin DP: The role of BRCA1 in the cellular response to
chemotherapy. J Natl Cancer Inst. 96:1659–1668. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Huang L, Liu Q, Chen S and Shao Z:
Cisplatin versus carboplatin in combination with paclitaxel as
neoadjuvant regimen for triple negative breast cancer. Onco Targets
Ther. 10:5739–5744. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Isakoff SJ, Mayer EL, He L, Traina TA,
Carey LA, Krag KJ, Rugo HS, Liu MC, Stearns V, Come SE, et al:
TBCRC009: A multicenter phase II clinical trial of platinum
monotherapy with biomarker assessment in metastatic triple-negative
breast cancer. J Clin Oncol. 33:1902–1909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Byrski T, Dent R, Blecharz P,
Foszczynska-Kloda M, Gronwald J, Huzarski T, Cybulski C, Marczyk E,
Chrzan R, Eisen A, et al: Results of a phase II open-label,
non-randomized trial of cisplatin chemotherapy in patients with
BRCA1-positive metastatic breast cancer. Breast Cancer Res.
14:R1102012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Isakoff SJ: Triple negative breast cancer:
Role of specific chemotherapy agents. Cancer J. 16:53–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kim GM, Jeung HC, Jung KH, Kim HJ, Lee KH,
Park KH, Lee JE, Anh MS, Kohn S, Lee SS, et al: PEARLY: A
randomized, multicenter, open-label, phase III trial comparing
anthracyclines followed by taxane versus anthracyclines followed by
taxane plus carboplatin as (neo) adjuvant therapy in patients with
early triple-negative breast cancer. J Clin Oncol. 35
(15_suppl):TPS587. 2017. View Article : Google Scholar
|
|
86
|
Chen XS, Nie XQ, Chen CM, Wu JY, Wu J, Lu
JS, Shao ZM, Shen ZZ and Shen KW: Weekly paclitaxel plus
carboplatin is an effective nonanthracycline-containing regimen as
neoadjuvant chemotherapy for breast cancer. Ann Oncol. 21:961–967.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sawyers C: Targeted cancer therapy.
Nature. 432:294–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dancey JE and Chen HX: Strategies for
optimizing combinations of molecularly targeted anticancer agents.
Nat Rev Drug Dis. 5:649–659. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jhan JR and Andrechek ER: Triple-negative
breast cancer and the potential for targeted therapy.
Pharmacogenomics. 18:1595–1609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Audebert M, Salles B and Calsou P:
Involvement of poly (ADP-ribose) polymerase-1 and XRCC1/DNA ligase
III in an alternative route for DNA double-strand breaks rejoining.
J Biol Chem. 279:55117–55126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shall S and de Murcia G: Poly(ADP-ribose)
polymerase-1: What have we learned from the deficient mouse model?
Mutat Res. 460:1–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Turner N, Tutt A and Ashworth A: Targeting
the DNA repair defect of BRCA tumours. Curr Opin Pharmacol.
5:388–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Turner NC, Lord CJ, Iorns E, Brough R,
Swift S, Elliott R, Rayter S, Tutt AN and Ashworth A: A synthetic
lethal siRNA screen identifying genes mediating sensitivity to a
PARP inhibitor. EMBO J. 27:1368–1377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Calabrese CR, Almassy R, Barton S, Batey
MA, Calvert AH, Canan-Koch S, Durkacz BW, Hostomsky Z, Kumpf RA,
Kyle S, et al: Anticancer chemosensitization and radiosensitization
by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J
Natl Cancer Inst. 96:56–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Robson ME, Im SA, Senkus E, Xu B, Domchek
SM, Masuda N, Delaloge S, Li W, Tung NM, Armstrong A, et al:
OlympiAD: Phase III trial of olaparib monotherapy versus
chemotherapy for patients (pts) with HER2-negative metastatic
breast cancer (mBC) and a germline BRCA mutation (gBRCAm). J Clin
Oncol. 352017.PubMed/NCBI
|
|
98
|
Robson ME, Tung N, Conte P, Im SA, Senkus
E, Xu B, Masuda N, Delaloge S, Li W, Armstrong A, et al: OlympiAD
final overall survival and tolerability results: Olaparib versus
chemotherapy treatment of physician's choice in patients with a
germline BRCA mutation and HER2-negative metastatic breast cancer.
Ann Oncol. 30:558–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Litton JK, Rugo HS, Ettl J, Hurvitz SA,
Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin
M, et al: Talazoparib in patients with advanced breast cancer and a
germline BRCA mutation. N Engl J Med. 379:753–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Poggio F, Bruzzone M, Ceppi M, Conte B,
Martel S, Maurer C, Tagliamento M, Viglietti G, Del Mastro L, de
Azambuja E and Lambertini M: Single-agent PARP inhibitors for the
treatment of patients with BRCA-mutated HER2-negative metastatic
breast cancer: A systematic review and meta-analysis. ESMO Open.
3:e0003612018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Miller K, Tong Y, Jones DR, Walsh T, Danso
MA and Ma CX; MCSSSM, : Cisplatin with or without rucaparib after
preoperative chemotherapy in patients with triple negative breast
cancer: Final efficacy results of Hoosier Oncology Group BRE09-146.
J Clin Oncol. 33:10822015. View Article : Google Scholar
|
|
102
|
Isakoff SJ, Puhalla S, Domchek SM,
Friedlander M, Kaufman B, Robson M, Telli ML, Diéras V, Han HS,
Garber JE, et al: A randomized phase II study of veliparib with
temozolomide or carboplatin/paclitaxel versus placebo with
carboplatin/paclitaxel in BRCA1/2 metastatic breast cancer: Design
and rationale. Future Oncol. 13:307–320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zimmer AS, Gillard M, Lipkowitz S and Lee
JM: Update on PARP inhibitors in breast cancer. Curr Treat Options
Oncol. 19:212018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Rugo HS, Olopade OI, DeMichele A, Yau C,
van't Veer LJ, Buxton MB, Hogarth M, Hylton NM, Paoloni M,
Perlmutter J, et al: Adaptive randomization of
veliparib-carboplatin treatment in breast cancer. N Engl J Med.
375:23–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Domchek SM, Postel-Vinay S, Im SA, Hee
Park Y, Delord JP, Italiano A, Alexandre J, You B, Bastian S, Krebs
MG, et al: Abstract PD5-04: An open-label, phase II basket study of
olaparib and durvalumab (MEDIOLA): Updated results in patients with
germline BRCA-mutated (gBRCAm) metastatic breast cancer (MBC).
Cancer Res. 792019.doi: 10.1158/1538-7445.SABCS18-PD5-04.
|
|
106
|
Tutt A, Kaufman B, Gelber RD, McFadden E,
Goessl C, Viale G, Geyer G, Zardavas D, Arahmani A, Fumagalli D, et
al: OlympiA: A randomized phase III trial of olaparib as adjuvant
therapy in patients with high-risk HER2-negative breast cancer (BC)
and a germline BRCA1/2 mutation (gBRCAm). Ann Oncol. 28:V672017.
View Article : Google Scholar
|
|
107
|
Earl HM, Vallier AL, Qian W, Grybowicz L,
Thomas S, Mahmud S, Harvey C, McAdam K, Hughes-Davies L, Roylance
R, et al: PARTNER: Randomised, phase II/III trial to evaluate the
safety and efficacy of the addition of olaparib to platinum-based
neoadjuvant chemotherapy in triple negative and/or germline BRCA
mutated breast cancer patients. J Clin Oncol. 35:TPS5912017.
View Article : Google Scholar
|
|
108
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Delaloge S and DeForceville L: Targeting
PI3K/AKT pathway in triple-negative breast cancer. Lancet Oncol.
18:1293–1294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Katso R, Okkenhaug K, Ahmadi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: Implications for development, immunity, homeostasis, and
cancer. Annu Rev Cell Dev Biol. 17:615–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kim SB, Dent R, Im SA, Espié M, Blau S,
Tan AR, Isakoff SJ, Oliveira M, Saura C, Wongchenko MJ, et al:
Ipatasertib plus paclitaxel versus placebo plus paclitaxel as
first-line therapy for metastatic triple-negative breast cancer
(LOTUS): A multicentre, randomised, double-blind,
placebo-controlled, phase 2 trial. Lancet Oncol. 18:1360–1372.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Martín M, Chan A, Dirix L, O'Shaughnessy
J, Hegg R, Manikhas A, Shtivelband M, Krivorotko P, Batista López
N, Campone M, et al: A randomized adaptive phase II/III study of
buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel
for the treatment of HER2-advanced breast cancer (BELLE-4). Ann
Oncol. 28:313–320. 2017. View Article : Google Scholar
|
|
114
|
Schmid P, Cortes J, Robson ME, Iwata H,
Hegg R, Nechaeva M, Xu B, Verma S, Haddad V, Imedio R, et al: A
phase III trial of capivasertib and paclitaxel in first-line
treatment of patients with metastatic triple-negative breast cancer
(CAPItello290). J Clin Oncol. 38:TPS11092020. View Article : Google Scholar
|
|
115
|
Schmid P, Abraham J, Chan S, Wheatley D,
Brunt M, Nemsadze G, Baird R, Park YH, Hall P, Perren T, et al:
AZD5363 plus paclitaxel versus placebo plus paclitaxel as
first-line therapy for metastatic triple-negative breast cancer
(PAKT): A randomised, double-blind, placebo-controlled, phase II
trial. J Clin Oncol. 36:10072018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gonzalez-Angulo AM, Green MC, Murray JL,
Palla SL, Koenig KH, Valero Brewster NK; SLJKDJ, ; et al: Open
label, randomized clinical trial of standard neoadjuvant
chemotherapy with paclitaxel followed by FEC (T-FEC) versus the
combination of paclitaxel and RAD001 followed by FEC (TR-FEC) in
women with triple receptor-negative breast cancer (TNBC). J Clin
Oncol. 29:10162011. View Article : Google Scholar
|
|
117
|
Basho RK, Gilcrease M, Murthy RK, Helgason
T, Karp DD, Meric-Bernstam F, Hess KR, Herbrich SM, Valero V,
Albarracin C, et al: Targeting the PI3K/AKT/mTOR pathway for the
treatment of mesenchymal triple-negative breast cancer: Evidence
from a phase 1 trial of mTOR inhibition in combination with
liposomal doxorubicin and bevacizumab. JAMA Oncol. 3:509–515. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kim Y, Jae E and Yoon M: Influence of
androgen receptor expression on the survival outcomes in breast
cancer: A meta-analysis. J Breast Cancer. 18:134–142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Gucalp A, Tolaney S, Isakoff SJ, Ingle JN,
Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, et al:
Phase II trial of bicalutamide in patients with androgen
receptor-positive, estrogen receptor-negative metastatic breast
cancer. Clin Cancer Res. 19:5505–5512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Traina TA, Miller K, Yardley DA, Eakle J,
Schwartzberg LS, O'Shaughnessy J, Gradishar W, Schmid P, Winer E,
Kelly C, et al: Enzalutamide for the treatment of androgen
receptor-expressing triple-negative breast cancer. J Clin Oncol.
36:884–890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Traina TA, Miller K, Yardley DA,
O'Shaughnessy J, Cortes J, Kelly AACM, Trudeau ME, Schmid P, Gianni
L, García-Estevez A, et al: Results from a phase 2 study of
enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in
advanced AR+ triple-negative breast cancer (TNBC). J Clin Oncol.
33:10032015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lehmann BD, Bauer JA, Schafer JM,
Pendleton CS, Tang L, Johnson KC, Chen X, Balko JM, Gómez H,
Arteaga CL, et al: PIK3CA mutations in androgen receptor-positive
triple negative breast cancer confer sensitivity to the combination
of PI3K and androgen receptor inhibitors. Breast Cancer Res.
16:4062014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lehmann BD, Abramson VG, Sanders ME, Mayer
EL, Haddad TC, Nanda R, Van Poznak C, Storniolo AM, Nangia JR,
Gonzalez-Ericsson PI, et al: TBCRC 032 IB/II multicenter study:
Molecular insights to AR antagonist and PI3K inhibitor efficacy in
patients with AR+ metastatic triple-negative breast cancer. Clin
Cancer Res. 26:2111–2123. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Panowski S, Bhakta S, Raab H, Polakis P
and Junutula JR: Site-specific antibody drug conjugates for cancer
therapy. MAbs. 6:34–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Nejadmoghaddam MR, Minai-Tehrani A,
Ghahremanzadeh R, Mahmoudi M, Dinarvand R and Zarnani AH:
Antibody-drug conjugates: Possibilities and challenges. Avicenna J
Med Biotechnol. 11:3–23. 2019.PubMed/NCBI
|
|
126
|
Goldenberg DM, Stein R and Sharkey RM: The
emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel
cancer target. Oncotarget. 9:28989–29006. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bardia A, Mayer IA, Vahdat LT, Tolaney SM,
Isakoff SJ, Diamond JR, O'Shaughnessy J, Moroose RL, Santin AD,
Abramson VG, et al: Sacituzumab govitecan-hziy in refractory
metastatic triple-negative breast cancer. N Engl J Med.
380:741–751. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liu Y, Lian W, Zhao X, Diao Y, Xu J, Xiao
L, Qing Y, Xue T and Wang J: SKB264 ADC: A first-in-human study of
SKB264 in patients with locally advanced unresectable/metastatic
solid tumors who are refractory to available standard therapies. J
Clin Oncol. 38((15_suppl)): TPS36592020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lyons TG: Targeted therapies for
triple-negative breast cancer. Curr Treat Options Oncol. 20:822019.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Giltnane JM and Balko JM: Rationale for
targeting the Ras/MAPK pathway in triple-negative breast cancer.
Dis Med. 17:275–283. 2014.PubMed/NCBI
|
|
131
|
Romanelli A, Clark A, Assayag F,
Chateau-Joubert S, Poupon MF, Servely JL, Fontaine JJ, Liu X,
Spooner E, Goodstal S, et al: Inhibiting aurora kinases reduces
tumor growth and suppresses tumor recurrence after chemotherapy in
patient-derived triple-negative breast cancer xenografts. Mol
Cancer Ther. 11:2693–2703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Finn RS: Östrogenrezeptor-positiver
Brustkrebs: Erfolgreiche Palbociblib-Letrozol-Kombination. Breast
Cancer. 375:1925–1936. 2016.PubMed/NCBI
|
|
133
|
Lucantoni F, Lindner AU, O'Donovan N,
Düssmann H and Prehn JH: Systems modeling accurately predicts
responses to genotoxic agents and their synergism with BCL-2
inhibitors in triple negative breast cancer cells. Cell Death Dis.
9:422018. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Inao T, Iida Y, Moritani T, Okimoto T,
Tanino R, Kotani H and Harada M: Bcl-2 inhibition sensitizes
triple-negative human breast cancer cells to doxorubicin.
Oncotarget. 9:25545–25556. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Marra A, Viale G and Curigliano G: Recent
advances in triple negative breast cancer: The immunotherapy era.
BMC Med. 17:902019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Weber S, Traunecker A, Oliveri F, Gerhard
W and Karjalainen K: Specific low-affinity recognition of major
histocompatibility complex plus peptide by soluble T-cell receptor.
Nature. 356:793–796. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Peggs KS, Quezada SA and Allison JP:
Cancer immunotherapy: Co-stimulatory agonists and co-inhibitory
antagonists. Clin Exp Immunol. 157:9–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Planes-Laine G, Rochigneux P, Bertucci F,
Chrétien A-S, Viens P, Sabatier R and Gonçalves A: PD-1/PD-L1
targeting in breast cancer: The first clinical evidences are
emerging. A literature review. Cancers (Basel). 11:10332019.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Mahoney KM, Rennert PD and Freeman GJ:
Combination cancer immunotherapy and new immunomodulatory targets.
Nat Rev Drug Dis. 14:561–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Sabatier R, Finetti P, Mamessier E,
Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D and
Bertucci F: Prognostic and predictive value of PDL1 expression in
breast cancer. Oncotarget. 6:5449–5464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Mittendorf EA, Philips AV, Meric-Bernstam
F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM,
Akcakanat A, et al: PD-L1 expression in triple-negative breast
cancer. Cancer Immunol Res. 2:361–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Schmidt M, Böhm D, Von Törne C, Steiner E,
Puhl A, Pilch H, Lehr HA, Hengstler JG, Kölbl H and Gehrmann M: The
humoral immune system has a key prognostic impact in node-negative
breast cancer. Cancer Res. 68:5405–5413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Aguiar PN Jr, Santoro IL, Tadokoro H, de
Lima Lopes G, Filardi BA, Oliveira P, Mountzios G and de Mello RA:
The role of PD-L1 expression as a predictive biomarker in advanced
non-small-cell lung cancer: A network meta-analysis. Immunotherapy.
8:479–488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Beckers RK, Selinger CI, Vilain R, Madore
J, Wilmott JS, Harvey K, Holliday A, Cooper CL, Robbins E, Gillett
D, et al: Programmed death ligand 1 expression in triple-negative
breast cancer is associated with tumour-infiltrating lymphocytes
and improved outcome. Histopathology. 69:25–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Vagia E, Mahalingam D and Cristofanilli M:
The landscape of targeted therapies in TNBC. Cancers (Basel.
12:9162020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Dieras V, Henschel V, Molinero L, Chui SY, et
al: IMpassion130: Updated overall survival (OS) from a global,
randomized, double-blind, placebo-controlled, Phase III study of
atezolizumab (atezo)+ nab-paclitaxel (nP) in previously untreated
locally advanced or metastatic triple-negative breast cancer
(mTNBC). J Clin Oncol. 37 (Suppl 15):S10032019. View Article : Google Scholar
|
|
149
|
Cortés J, Lipatov O, Im SA, Gonçalves A,
Lee KS, Schmid P, Tamura K, Testa L, Witzel I, Ohtani S, et al:
LBA21 KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus
single-agent chemotherapy (chemo) for metastatic triple negative
breast cancer (mTNBC). Ann Oncol. 30:v859–v860. 2019. View Article : Google Scholar
|
|
150
|
Cortes J, Guo Z, Karantza V and Aktan G:
Abstract CT069: KEYNOTE-355: Randomized, double-blind, phase III
study of pembrolizumab plus chemotherapy vs placebo plus
chemotherapy for previously untreated, locally recurrent,
inoperable or metastatic triple-negative breast cancer (mTNBC).
Cancer Res. 772017.doi: 10.1158/1538-7445.AM2017-CT069.
|
|
151
|
Schmid P, Salgado R, Park YH,
Muñoz-Couselo E, Kim SB, Sohn J, Im SA, Foukakis T, Kuemmel S, Dent
R, et al: Pembrolizumab plus chemotherapy as neoadjuvant treatment
of high-risk, early-stage triple-negative breast cancer: Results
from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann
Oncol. 31:569–581. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Barker AD, Sigman CC, Kelloff GJ, Hylton
NM, Berry DA and Esserman LJ: I-SPY 2: An adaptive breast cancer
trial design in the setting of neoadjuvant chemotherapy. Clin
Pharmacol Ther. 86:97–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Keenan TE and Tolaney SM: Role of
immunotherapy in Triple-negative breast cancer. J Natl Compr Canc
Netw. 18:479–489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Qattan A: Novel miRNA targets and
therapies in the triple-negative breast cancer microenvironment: An
emerging Hope for a challenging disease. Int J Mol Sci.
21:89052020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Si W, Shen J, Zheng H and Fan W: The role
and mechanisms of action of microRNAs in cancer drug resistance.
Clin Epigenetics. 11:252019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ding L, Gu H, Xiong X, Ao H, Cao J, Lin W,
Yu M, Lin J and Cui Q: MicroRNAs involved in carcinogenesis,
prognosis, therapeutic resistance, and applications in human
triple-negative breast cancer. Cells. 8:14922019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Lyng MB, Lænkholm AV, Søkilde R, Gravgaard
KH, Litman T and Ditzel HJ: Global microRNA expression profiling of
high-risk ER+ breast cancers from patients receiving adjuvant
tamoxifen mono-therapy: A DBCG study. PLoS One. 7:e361702012.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Gorur A, Bayraktar R, Ivan C, Mokhlis HA,
Bayraktar E, Kahraman N, Karakas D, Karamil S, Kabil NN,
Kanlikilicer P, et al: ncRNA therapy with miRNA-22-3p suppresses
the growth of triple-negative breast cancer. Mol Ther Nucleic
Acids. 23:930–943. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Pang Y, Liu J, Li X, Xiao G, Wang H, Yang
G, Li Y, Tang SC, Qin S, Du N, et al: MYC and DNMT 3A-mediated DNA
methylation represses micro RNA-200b in triple negative breast
cancer. J Cell Mol Med. 22:6262–6274. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Huang X, Taeb S, Jahangiri S, Emmenegger
U, Tran E, Bruce J, Mesci A, Korpela E, Vesprini D, Wong CS, et al:
miRNA-95 mediates radioresistance in tumors by targeting the
sphingolipid phosphatase SGPP1. Cancer Res. 73:6972–6986. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Tormo E, Ballester S, Adam-Artigues A,
Burgués O, Alonso E, Bermejo B, Menéndez S, Zazo S, Madoz-Gúrpide
J, Rovira A, et al: The miRNA-449 family mediates doxorubicin
resistance in triple-negative breast cancer by regulating cell
cycle factors. Sci Rep. 9:53162019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Naorem LD, Muthaiyan M and Venkatesan A:
Identification of dysregulated miRNAs in triple negative breast
cancer: A meta-analysis approach. J Cell Physiol. 234:11768–11779.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Malla RR, Kumari S, Gavara MM, Badana AK,
Gugalavath S, Kumar DKG and Rokkam P: A perspective on the
diagnostics, prognostics, and therapeutics of microRNAs of
triple-negative breast cancer. Biophys Rev. 11:227–234. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Liu Y, Cai Q, Bao PP, Su Y, Cai H, Wu J,
Ye F, Guo X, Zheng W, Zheng Y and Shu XO: Tumor tissue microRNA
expression in association with triple-negative breast cancer
outcomes. Breast Cancer Res Treat. 152:183–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Kahraman M, Röske A, Laufer T, Fehlmann T,
Backes C, Kern F, Kohlhaas J, Schrörs H, Saiz A, Zabler C, et al:
MicroRNA in diagnosis and therapy monitoring of early-stage
triple-negative breast cancer. Sci Rep. 8:115842018. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Mei J, Hao L, Wang H, Xu R, Liu Y, Zhu Y
and Liu C: Systematic characterization of non-coding RNAs in
triple-negative breast cancer. Cell Prolif. 53:e128012020.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Shu D, Li H, Shu Y, Xiong G, Carson WE
III, Haque F, Xu R and Guo P: Systemic delivery of anti-miRNA for
suppression of triple negative breast cancer utilizing RNA
nanotechnology. ACS Nano. 9:9731–9740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Yin H, Xiong G, Guo S, Xu C, Xu R, Guo P
and Shu D: Delivery of anti-miRNA for triple-negative breast cancer
therapy using RNA nanoparticles targeting stem cell marker CD133.
Mol Ther. 27:1252–1261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Lin A, Li C, Xing Z, Hu Q, Liang K, Han L,
Wang C, Hawke DH, Wang S, Zhang Y, et al: The LINK-A lncRNA
activates normoxic HIF1α signalling in triple-negative breast
cancer. Nat Cell Biol. 18:213–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Yang J, Meng X, Yu Y, Pan L, Zheng Q and
Lin W: LncRNA POU3F3 promotes proliferation and inhibits apoptosis
of cancer cells in triple-negative breast cancer by inactivating
caspase 9. Biosci Biotechnol Biochem. 83:1117–1123. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Jiang X, Zhou Y, Sun AJ and Xue JL: NEAT1
contributes to breast cancer progression through modulating miR-448
and ZEB1. J Cell Physiol. 233:8558–8566. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Ke H, Zhao L, Feng X, Xu H, Zou L, Yang Q,
Su X, Peng L and Jiao B: NEAT1 is required for survival of breast
cancer cells through FUS and miR-548. Gene Regul Syst Biol. 10
(Suppl 1):S11–S17. 2016.PubMed/NCBI
|
|
174
|
Wang LI, Liu D, Wu X, Zeng Y, Li L, Hou Y,
Li W and Liu Z: Long non-coding RNA (LncRNA) RMST in
triple-negative breast cancer (TNBC): Expression analysis and
biological roles research. J Cell Physiol. 233:6603–6612. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Xu Q, Deng F, Qin Y, Zhao Z, Wu Z, Xing Z,
Ji A and Wang QJ: Long non-coding RNA regulation of
epithelial-mesenchymal transition in cancer metastasis. Cell Death
Dis. 7:e22542016. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Vaidya AM, Sun Z, Ayat N, Schilb A, Liu X,
Jiang H, Sun D, Scheidt J, Qian V, He S, et al: Systemic delivery
of tumor-targeting siRNA nanoparticles against an oncogenic LncRNA
facilitates effective triple-negative breast cancer therapy.
Bioconjug Chem. 30:907–919. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Sha S, Yuan D, Liu Y, Han B and Zhong N:
Targeting long non-coding RNA DANCR inhibits triple negative breast
cancer progression. Biol Open. 6:1310–1316. 2017. View Article : Google Scholar : PubMed/NCBI
|