Introduction
Breast cancer has ranked as the second most common
and the fifth most lethal malignancy in the world, with ~1.7
million women diagnosed with this disease in 2012 (1,2). The
heterogeneity of breast tumors and their distinct prognosis require
systemic therapeutic strategies and targeted treatment for
different patients (3), making
breast cancer one of the most challenging solid tumors to
treat.
Radiofrequency ablation (RFA) is a less invasive
approach for the percutaneous treatment compared with traditional
surgical resection; RFA utilizes radiofrequency electrode and image
guidance to heat and coagulate specific tissues (4). It has been widely used in the treatment
of hyperplasia and gastroenterological malignancies (5). The application of RFA guided by
ultrasound for the therapy of breast cancer has drawn great
attention of clinicians in the last two decades, and some important
outcomes have been discovered using RFA (6,7).
However, the viable tumor cells located at the clefts of ablation
zones can lead to the re-progression of tumor tissues (5). Accumulating evidence has demonstrated
that the combination of RFA and chemical agents, including
paclitaxel, vinorelbine and cisplatin, increases the therapeutic
efficacy and patient survival (8).
However, side effects, such as nausea and neurotoxicity, induced by
these agents during the treatment are sometimes unbearable
(9).
Formosanin C (FC) is a diosgenin isolated from
Rhizoma paridis and is a crucial immunomodulatory agent
(10). Previous research has
demonstrated the antitumor effect of FC in the progression of
hepatocarcinoma, as well as lung adenocarcinoma (11,12). It
has been reported that FC functions as an inhibitor of matrix
metalloproteinases to induce the apoptosis of tumor cells, and thus
suppresses the proliferation and metastasis of multiple types of
cancer such as liver cancer and lung cancer (13). The FC can modulate the formation of
granulocyte/macrophage colonies and regulate the activation of
natural killer T cells (10,11).
Tumor-infiltrating lymphocytes (TILs) have been
demonstrated to participate in the response mediation of breast
carcinoma to chemotherapy (14). The
CD8+ cytotoxic T cells in the TILs are essential for the
apoptosis of cancer cells and further tumor destruction (15).
The present study aimed to investigate the role of
FC as an adjuvant drug in the anti-breast tumor function of
ultrasound-guided RFA. We hypothesized that FC enhanced the
curative effects of ultrasound-guided RFA against breast cancer by
regulating adaptive immune responses. The current study may provide
a potential therapeutic strategy for breast cancer in the
clinic.
Materials and methods
Regents
FC used in the present study was purchased from
Selleck Chemicals. Ethanol (Thermo Fisher Scientific, Inc.) was
used to dissolve FC, and FC solution was stored as −20°C for
further experiments.
Animals
Adult BALB/c nude mice (female, 4–6 weeks; n=55)
weighing 18–22 g were obtained from Charles River Laboratories,
Inc. All mice used in the present study were kept in a
virus/antigen-free system with permanent humidity and constant
temperature (humidity, 40–70%; temperature, 20–26°C; 12 h
light/dark cycle) and free access to food and water. Animal studies
were approved by the Ethics Committee of Liaocheng People's
Hospital (Liaocheng, China). MDA-MB-231 cells were purchased from
Hunan Fenghui Biotechnology Co., Ltd., and 1×106 cells
dissolved in PBS buffer (Thermo Fisher Scientific, Inc.) were
injected subcutaneously into the axilla of each nude mouse. When
the tumors became palpable (after 1 week), the tumorigenic nude
mice were divided into five treatment groups (n=8/group; the
remaining 7 mice were not used as the tumor did not grow in time):
Group I, control; Group II, 10 mg/kg FC; Group III, 20 mg/kg FC;
Group IV, RFA; Group V, RFA+10 mg/kg FC; and Group VI, RFA+20 mg/kg
FC. Isoflurane (2%) with oxygen was applied to induce the inhalant
anesthesia of the mice, and RFA was only performed in the mice of
Groups IV, V and VI. The same surgical procedures without
radiofrequency heating were performed in the mice in Groups I, II
and III. FC was injected intraperitoneally at the second day and
the same volume of ethanol was used as the negative control. The
tumor volumes of nude mice were monitored at days 0, 3, 6, 9, 12,
15, 18 and 21, and the animal health and behavior were monitored
every three days. The total duration of animal experiments was 21
days. No mice died during the experiment. The maximum tumor
diameter was 17 mm (not exceeding 20 mm), and then mice were
sacrificed by cervical dislocation. The death of the mice was
confirmed by the stop of the mouse thorax and cardiac arrest. The
doses of FC administration were selected according to a previous
study (16).
Flow cytometry
The TILs of each mouse were collected and made to
single-cell suspension with cell staining buffer targeting IFNγ,
TNFα, CD8, CD45 and CD107a (Thermo Fisher Scientific, Inc.)
respectively. Fc receptor blockers (Thermo Fisher Scientific, Inc.)
were used to block non-specific effects on staining results.
Fluorescently labeled antibodies (Abcam; CD8 (APC), cat. no.
ab237368, 1:500; CD45 (FITC), cat. no. ab210225, 1:100; CD107a
(Alexa Fluor® 488), cat. no. ab187591, 1:50; IFNγ (PE),
cat. no. ab95673, 1:50; TNFα (Alexa Fluor 488), cat. no. ab237353,
1:50] were then incubated with the cell suspension at 4°C
overnight, and 0.1% PBS buffer was used to wash the cells. A flow
cytometer (Thermo 100022777; Thermo Fisher Scientific, Inc.) was
used, and the results were analyzed by FlowJo software (Version
7.6.1; Treestar, Inc.). For intracellular cytokine staining,
harvested cells were stimulated with PMA (10 ng/ml) and ionomycin
(1 mg/ml) for 4 h and incubated for the last 1 h with brefeldin A
(10 mg/ml) at 37°C. IFNγ- and TNFα-producing cells were examined by
flow cytometry.
Immunonephelometric assay
The blood samples of mice were collected immediately
after sacrifice and were centrifuged to obtain the serum (4°C,
4,000 × g, 15 min). All serum samples were kept at −80°C for
further experiments. Levels of immunoglobins, including IgG (cat.
no. SNM259), IgM (cat. no. SNM260) and IgA (cat. no. SNM258), in
mice serum were determined using an Immunoglobulin Assay kit
(Beijing Biolab Technology Co., Ltd.) according to the
manufacturer's protocol.
Statistical analysis
All experiments were repeated independently at least
three times for the accuracy of the data, which were represented as
the mean ± SD. GraphPad Prism v7.0 software (GraphPad Software,
Inc.) was used to analyze the raw data and construct curves and
histogram. One- or two-way ANOVA analysis, followed by Tukey's
post-hoc test, was performed to analyze the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
FC enhances the therapeutic efficacy
of RFA in breast cancer
The molecular structure of FC used in the present
study is shown in Fig. 1A; FC is a
type of steroidal saponin with four sugars isolated from Rhizoma
paridis. To demonstrate that the combined treatment of RFA and
FC was able to improve the curative efficacy and prognosis, nude
mice were used to establish the animal model. As shown in Fig. 1B, RFA procedure alone significantly
decreased the volume of breast tumors compared with that in the
control group. However, the tumor volume still increased over time
even though the nude mice received RFA treatment. On the other
hand, the combination of RFA and 10 or 20 mg/kg FC treatment
markedly inhibited the increase in tumor volume. Similarly, the
administration of FC in combination with RFA significantly
inhibited the tumor weight compared with RFA alone (Fig. 1C).
 | Figure 1.FC enhances the therapeutic efficacy
of RFA in breast cancer. (A) Chemical structure of FC. (B) Tumor
growth curves of control, 10 mg/kg FC, 20 mg/kg FC, RFA, RFA+10
mg/kg FC and RFA+20 mg/kg FC groups. (C) At the end of the
experiment, the tumors of all nude mice from control, 10 mg/kg FC,
20 mg/kg FC, RFA, RFA+10 mg/kg FC and RFA+20 mg/kg FC groups were
excised and weighed. Error bars indicate SEM; P-values were
calculated using ANOVA with Tukey's test. *P<0.05; **P<0.01.
FC, formosanin C; RFA, radiofrequency ablation. |
Combination of FC and RFA improves the
immune function of nude mice
Previous studies have revealed the influence of FC
on the immune responses of mice to tumor cells (13,17).
Thus, the present study investigated the immunoglobin levels in the
serum of mice. Similar alterations in IgG, IgM and IgA levels under
the influence of RFA and FC treatment were observed, as shown in
Fig. 2A-C. Compared with that in
mice in the control group, the immunoglobin levels of mice in the
RFA and FC groups were significantly increased, suggesting the
antitumor functions of RFA and FC alone. Furthermore, the
immunoglobin levels of mice in the RFA + FC group were
significantly higher than those in the other three groups. These
findings indicated that the combination of RFA and FC significantly
increased the immune function of nude mice by upregulating the
immunoglobin levels.
Combination of FC and RFA increases
the proportion of CD8+ T cells in TILs
CD45+ and CD8+ T cells serve a
crucial role in the cell-mediated immunity against cancer, and the
proportion of CD45+ and CD8+ T cells in TILs
generally determines the progression and prognosis of certain
tumors (18). The present study
investigated the influence of RFA and FC on the proportion of
T-cell groups in TILs. As shown in Fig.
3A, either RFA or FC treatment alone increased the proportion
of CD45+ and CD8+ T cells in TILs compared
with the control group, and the combination of RFA and FC further
increased their proportion. With the combined treatment of RFA and
FC, the proportion of CD45+ CD8− T cells
increased by 3-fold compared with the control group (Fig. 3B), and the proportion of
CD45+ CD8+ T cells increased by >5-fold
(Fig. 3C).
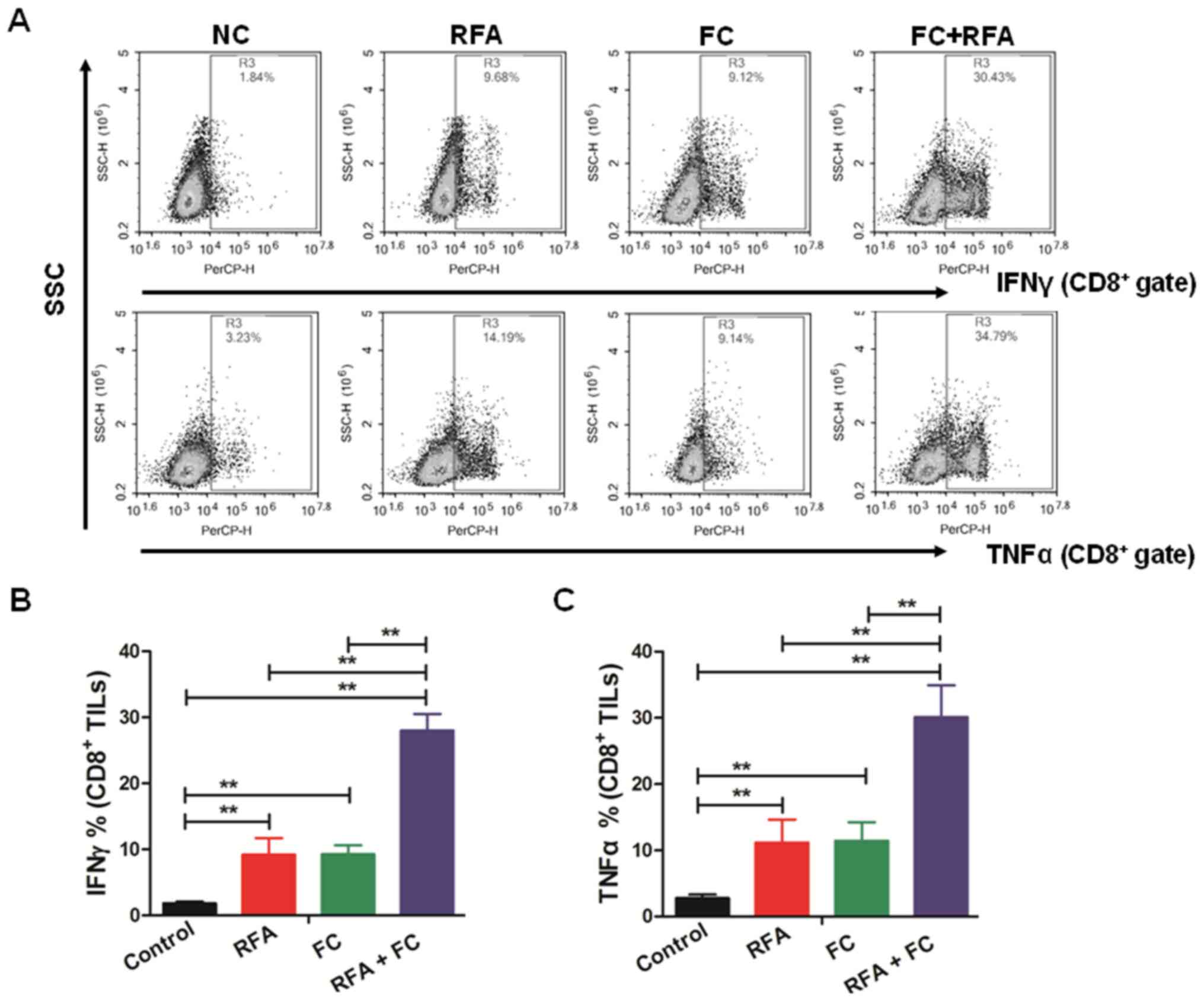
Combination of FC and RFA increases
the percentage of IFNγ+ and TNFα+
CD8+ TILs
IFNγ is a type of interferon secreted by T cells,
and TNFα is a type of tumor necrosis factor secreted by monocytes
and macrophages. They are both antitumor factors that participate
in tumor destruction (19). The
present study identified the percentage alteration of
IFNγ+ and TNFα+ CD8+ TILs. As
shown in Fig. 4A-C, the combination
treatment of RFA and FC significantly increased the percentages of
both IFNγ+ and TNFα+ CD8+ TILs
compared with RFA alone, suggesting that this combined therapeutic
strategy significantly improved the immune response of nude mice to
the orthotic tumor.
CD107a is currently recognized as a marker protein
for degranulation of cytotoxic T cells (20). Therefore, the present study
investigated the influence of RFA and FC on the CD107a-expressing
CD8+ TILs. As shown in Fig.
5A and B, the combined therapy of RFA and FC significantly
increased CD107a-expressing CD8+ TILs compared with RFA
alone, suggesting that the tumor destruction induced by cytotoxic T
cells was elevated with the combined treatment of RFA and FC.
Discussion
The global incidence of breast cancer has been on
the rise since the late 1970s, and ~1/8 women in the United States
develops breast cancer in their life time (21). Thus, breast cancer has become a major
public health issue in modern society (22). Breast cancer screening improves the
proportion of early diagnosis, and new therapeutic strategies
increase the curative efficacy, both of which contribute to the
decreased mortality and increased prognosis of patients with breast
carcinoma (23). As a result, the
global breast cancer mortality rate has shown a downward trend
since the 1990s (21). In the last
two decades, the management of breast cancer has entered the era of
comprehensive treatment, forming a treatment model that focuses
both on local breast cancer treatment and systemic treatment.
However, surgery, radiotherapy, chemotherapy, endocrine therapy,
bio-targeted therapy and drug-assisted therapy performed in the
treatment of breast tumor can lead to unbearable side effects that
significantly impact the quality of life of patients (24). Additionally, the recurrence of breast
tumor is a problem that cannot be ignored in the clinic, which
greatly impacts the prognosis of patients (25). The present study reported a new
potential therapeutic strategy for breast carcinoma, which may
improve the treatment efficacy and inhibit the relapse of breast
tumor at the same time. The combination of RFA and FC treatment
significantly inhibited the proliferation of remnant tumor compared
with RFA alone or FC alone. Notably, it was revealed that FC
treatment induced significant upregulation of immune functions in
nude mice by improving their immunoglobin levels. RFA treatment
also seemed to significantly upregulate immunoglobulin levels. The
adaptive immune response was markedly activated by the combination
of RFA and FC.
Tumor RFA utilizes electrode needles inserted into
the tumor to produce a radiofrequency current, which causes
high-speed particle movement and friction inside the tumor tissues
(26). The heat generated by the
electrode needles results in high temperature, which conducts
outward and induces the coagulation and shrinkage of solid tumors
(27). RFA is an improved, minimally
invasive method for treating tumors compared with other surgical
procedures. RFA is able to ablate tumors of 5–7 cm in size and is
particularly suitable for solid tumors of the liver and lung, with
limited side effects and higher curative efficacy compared with
traditional chemotherapies (28). In
the last decade, the application of RFA for the treatment of breast
carcinoma and related diseases has drawn great attention from
clinicians. For instance, previous research has demonstrated that
RFA can be used to treat liver metastasis from breast cancer, and
the outcomes of RFA treatment are similar to hepatic resection,
which is the traditional surgical procedure for this disease, with
similar median survival times (41 vs. 37 months) and 3-year overall
survival rates (55.4 vs. 52.6%) (29). However, the re-proliferation of the
breast cancer cells located at the margins or clefts of overlapping
ablation zones represent a big problem for RFA treatment of breast
tumor (30). A possible way to solve
this problem may be to improve the completeness of RFA treatment
and enlarge the heating zones of these electrode needles, but the
corresponding over-destruction of adjacent normal tissues can be
unbearable for patients (31).
Previous studies have revealed that the combination
of RFA and other chemical agents can effectively improve the
treatment outcomes for a variety of tumors (32). For instance, Ahmed and Goldberg
(33) demonstrated that liposomal
doxorubicin combined with RFA treatment markedly improved the drug
concentration in metastatic liver tumor tissues and improved the
therapeutic outcomes in vivo. Wu et al (34) revealed that the combination of
thermosensitive liposomal vinorelbine treatment and RFA procedure
increased the end-point survival of mice with liver tumor.
Similarly, the present study revealed that the combination of RFA
procedure and FC significantly improved the treatment efficacy in a
breast cancer mouse model. The combined therapy effectively
inhibited the increase of the volume of orthotopic breast tumors.
Another study has demonstrated that intratumorally injected
paclitaxel improves the treatment outcomes of RFA in breast cancer
and inhibits the toxicity of paclitaxel to normal tissues, compared
with oral or intravenous administration of the agent (34). Thus, a more detailed and accurate
mode of drug administration to improve chemotherapy efficacy and
decrease the toxicity for the current combined therapy of RFA and
FC should be further investigated in the future (34).
FC is a diosgenin isolated from Rhizoma
paridis and is an important active ingredient of some
traditional Chinese medicine with antitumor, anti-inflammatory and
anti-venom functions (17). In
recent years, the antitumor function of FC has drawn great
attention, and accumulating evidence has demonstrated that FC can
mediate the body immune responses against tumor cells and thus
inhibit the progression of liver and lung cancer (13,17,35). For
instance, the synergistic antitumor function of FC and polyphyllin
I in liver cancer has been revealed (10). It has been reported that FC can
inhibit the pulmonary metastasis of lung carcinoma by suppressing
matrix metalloproteinases (10). In
the present study, it was reported that FC treatment alone
inhibited the growth of the orthotopic breast tumors in nude mice
and 20 mg/kg FC administration significantly suppressed the
increase of tumor volume in nude mice. The combination of FC and
RFA treatment dramatically improved the adaptive immune response of
nude mice and altered the proportion of different cells in TILs,
thus inhibiting the relapse of orthotopic tumor.
CD8 co-receptors are mainly expressed on the surface
of cytotoxic T cells, natural killer cells, cortical thymocytes and
dendritic cells (36).
CD8+ T cells serve an important role in tumor
destruction (15,37). CD45 co-receptors are expressed on all
leukocytes and are known as common leukocyte antigens, and they are
key molecules for signal transduction on the cell membrane. CD45
serves a crucial role in lymphocyte development, maturation,
functional regulation and signal transmission. According to the
type of CD45 molecules expressed by T cells, human T cells can be
divided into CD45RA+ initial T cells and
CD45RO+ memory T cells (38). CD107a co-receptors participate in the
antitumor, antiviral infection and immune regulatory functions of
natural killer cells (39,40). In the present study, it was revealed
that the combination of RFA and FC increased the proportion of
CD8+, CD45+ and CD107a+ T cells in
the TILs of orthotopic breast tumors in nude mice, suggesting that
this therapy significantly improved the T-cell-related tumor
destruction in the current mouse model.
In conclusion, the present study reported the
synergistic antitumor function of RFA procedure and FC
administration for breast cancer in vivo. It was identified
that RFA combined with FC improved the proportion of
IFNγ+ and TNFα+ CD8+ T cells and
CD107a+ CD8+ T cells in TILs of orthotopic
breast tumors in nude mice, and thus increased the immune responses
caused by surgery and chemotherapy. As a result, FC promoted the
curative efficacy of ultrasound-guided RFA in breast tumor by
regulating adaptive immune responses. The current study may provide
a potential therapeutic strategy for breast cancer in the
clinic.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no.
bs2015sw016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization: ZC and JL; performed experiments
and analyzed data: ZC, JL, QC, FL and GZ; funding acquisition: ZC;
project administration: ZC and JL; supervision: ZC and JL;
validation: ZC, JL, QC, FL and GZ; writing-original draft: ZC, JL,
QC, FL and GZ; writing-review & editing: ZC, JL, QC, FL and GZ.
All authors have read and approved the manuscript. All authors
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Animal studies were approved by the Ethics Committee
of Liaocheng People's Hospital (Liaocheng, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Webster S, Lawn S, Chan R and Koczwara B:
The role of comorbidity assessment in guiding treatment
decision-making for women with early breast cancer: A systematic
literature review. Support Care Cancer. 28:1041–1050. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wanchai A and Armer JM: A systematic
review association of reflexology in managing symptoms and side
effects of breast cancer treatment. Complement Ther Clin Pract.
38:1010742020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y and Chen Z: Mutation detection and
molecular targeted tumor therapies. STEMedicine. 1:e112020.
View Article : Google Scholar
|
|
4
|
Sun Y, Ji S, Ji H, Liu L and Li C:
Clinical efficacy analysis of transcatheter arterial
chemoembolization (TACE) combined with radiofrequency ablation
(RFA) in primary liver cancer and recurrent liver cancer. J BUON.
24:1402–1407. 2019.PubMed/NCBI
|
|
5
|
Zimmermann M, Pedersoli F, Schulze-Hagen
M, Lurje G, Isfort P, Kuhl C and Bruners P: Salvage RFA in patients
with intrahepatic recurrence after major hepatic surgery for
colorectal cancer liver metastases: Mid-term outcome. Eur Radiol.
30:1221–1227. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Veltri A, Gazzera C, Barrera M, Busso M,
Solitro F, Filippini C and Garetto I: Radiofrequency thermal
ablation (RFA) of hepatic metastases (METS) from breast cancer
(BC): An adjunctive tool in the multimodal treatment of advanced
disease. Radiol Med. 119:327–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carrafiello G, Fontana F, Cotta E, Petullà
M, Brunese L, Mangini M and Fugazzola C: Ultrasound-guided thermal
radiofrequency ablation (RFA) as an adjunct to systemic
chemotherapy for breast cancer liver metastases. Radiol Med.
116:1059–1066. 2011.(In Italian). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueki A, Okuma T, Hamamoto S, Kageyama K,
Murai K and Miki Y: Combination therapy involving radiofrequency
ablation and targeted chemotherapy with bevacizumab plus paclitaxel
and cisplatin in a rabbit VX2 lung tumor model. BMC Res Notes.
11:2512018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA;
Gynecologic Oncology Group, : Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu RT, Chiang HC, Fu WC, Chien KY, Chung
YM and Horng LY: Formosanin-C, an immunomodulator with antitumor
activity. Int J Immunopharmacol. 12:777–786. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Man S, Li J, Chai H, Fan W, Liu Z
and Gao W: The antitumor effect of formosanin C on HepG2 cell as
revealed by 1H-NMR based metabolic profiling. Chem Biol Interact.
220:193–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiang HC, Wang JJ and Wu RT:
Immunomodulating effects of the hydrolysis products of formosanin C
and beta-ecdysone from Paris formosana Hayata. Anticancer Res.
12:1475–1478. 1992.PubMed/NCBI
|
|
13
|
Man S, Gao W, Zhang Y, Liu Z, Yan L, Huang
L and Liu C: Formosanin C-inhibited pulmonary metastasis through
repression of matrix metalloproteinases on mouse lung
adenocarcinoma. Cancer Biol Ther. 11:592–598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savas P and Loi S: Metastatic breast
cancer: TIL it is too late. Clin Cancer Res. 26:526–528. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harao M, Forget MA, Roszik J, Gao H,
Babiera GV, Krishnamurthy S, Chacon JA, Li S, Mittendorf EA,
DeSnyder SM, et al: 4-1BB-Enhanced Expansion of CD8+ TIL
from triple-negative breast cancer unveils mutation-specific
CD8+ T cells. Cancer Immunol Res. 5:439–445. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao X, Yang M, Xiao J, Zou J, Huang Q,
Yang K, Zhang B, Yang F, Liu S, Wang H and Bai P: Paris Saponin II
suppresses the growth of human ovarian cancer xenografts via
modulating VEGF-mediated angiogenesis and tumor cell migration.
Cancer Chemother Pharmacol. 73:807–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JC, Su CL, Chen LL and Won SJ:
Formosanin C-induced apoptosis requires activation of caspase-2 and
change of mitochondrial membrane potential. Cancer Sci.
100:503–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dwerryhouse SJ, Soon Lee C, King J,
Magarey C, Schwartz P and Morris DL: Cimetidine does not influence
TIL in breast cancer. Int J Surg Investig. 1:191–194.
1999.PubMed/NCBI
|
|
19
|
Shen J, Xiao Z, Zhao Q, Li M, Wu X, Zhang
L, Hu W and Cho CH: Anti-cancer therapy with TNFα and IFNγ: A
comprehensive review. Cell Prolif. 51:e124412018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alter G, Malenfant JM and Altfeld M:
CD107a as a functional marker for the identification of natural
killer cell activity. J Immunol Methods. 294:15–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iacoviello L, Bonaccio M, de Gaetano G and
Donati MB: Epidemiology of breast cancer, a paradigm of the ‘common
soil’ hypothesis. Semin Cancer Biol. Feb 20–2020.(Epub ahead of
print). PubMed/NCBI
|
|
22
|
Malmgren JA, Calip GS, Atwood MK, Mayer M
and Kaplan HG: Metastatic breast cancer survival improvement
restricted by regional disparity: Surveillance, epidemiology, and
end results and institutional analysis: 1990 to 2011. Cancer.
126:390–399. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin M, Verschraegen C, Vincent VH, Patel
SM, George T and Truica CI: Impact of lack of surgery on outcomes
in elderly women with nonmetastatic breast cancer-A surveillance,
epidemiology, and end results 18 population based study. Medicine
(Baltimore). 99:e187452020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee J and Lee MG: Effects of exercise
interventions on breast cancer patients during adjuvant therapy: A
systematic review and meta-analysis of randomized controlled
trials. Cancer Nurs. 43:115–125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banys-Paluchowski M, Gruber IV, Hartkopf
A, Paluchowski P, Krawczyk N, Marx M, Brucker S and Hahn M:
Axillary ultrasound for prediction of response to neoadjuvant
therapy in the context of surgical strategies to axillary
dissection in primary breast cancer: A systematic review of the
current literature. Arch Gynecol Obstet. 301:341–353. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Napoletano C, Taurino F, Biffoni M, De
Majo A, Coscarella G, Bellati F, Rahimi H, Pauselli S, Pellicciotta
I, Burchell JM, et al: RFA strongly modulates the immune system and
anti-tumor immune responses in metastatic liver patients. Int J
Oncol. 32:481–490. 2008.PubMed/NCBI
|
|
27
|
Singh S, Bhowmik A and Repaka R: Thermal
analysis of induced damage to the healthy cell during RFA of breast
tumor. J Therm Biol. 58:80–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sommer CM, Pallwein-Prettner L, Vollherbst
DF, Seidel R, Rieder C, Radeleff BA, Kauczor HU, Wacker F, Richter
GM, Bücker A, et al: Transarterial embolization (TAE) as add-on to
percutaneous radiofrequency ablation (RFA) for the treatment of
renal tumors: Review of the literature, overview of
state-of-the-art embolization materials and further perspective of
advanced image-guided tumor ablation. Eur J Radiol. 86:143–162.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao YB, Zhang B and Wu YL: Radiofrequency
ablation versus hepatic resection for breast cancer liver
metastasis: A systematic review and meta-analysis. J Zhejiang Univ
Sci B. 19:829–843. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sofocleous CT, Nascimento RG, Petrovic LM,
Klimstra DS, Gonen M, Brown KT, Brody LA, Covey AM, Thornton RH,
Fong Y, et al: Histopathologic and immunohistochemical features of
tissue adherent to multitined electrodes after RF ablation of liver
malignancies can help predict local tumor progression: Initial
results. Radiology. 249:364–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ni JY, Liu SS, Xu LF, Sun HL and Chen YT:
Meta-analysis of radiofrequency ablation in combination with
transarterial chemoembolization for hepatocellular carcinoma. World
J Gastroenterol. 19:3872–3882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo W, He X, Li Z and Li Y: Combination of
Transarterial Chemoembolization (TACE) and Radiofrequency Ablation
(RFA) vs. Surgical Resection (SR) on survival outcome of early
hepatocellular carcinoma: A meta-analysis. Hepatogastroenterology.
62:710–714. 2015.PubMed/NCBI
|
|
33
|
Ahmed M and Goldberg SN: Combination
radiofrequency thermal ablation and adjuvant IV liposomal
doxorubicin increases tissue coagulation and intratumoural drug
accumulation. Int J Hyperthermia. 20:781–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu H, Fan ZP, Jiang AN, Di XS, He B, Wang
S, Goldberg SN, Ahmed M, Zhang Q and Yang W: Combination of
intratumoural micellar paclitaxel with radiofrequency ablation:
Efficacy and toxicity in rodents. Eur Radiol. 29:6202–6210. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu J, Man S, Liu Z, Ma L and Gao W: A
synergistic antitumor effect of polyphyllin I and formosanin C on
hepatocarcinoma cells. Bioorg Med Chem Lett. 26:4970–4975. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He QF, Xu Y, Li J, Huang ZM, Li XH and
Wang X: CD8+ T-cell exhaustion in cancer: Mechanisms and new area
for cancer immunotherapy. Brief Funct Genomics. 18:99–106. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hammerl D, Massink MPG, Smid M, van
Deurzen CHM, Meijers-Heijboer HEJ, Waisfisz Q, Debets R and Martens
JWM: Clonality, antigen recognition, and suppression of CD8(+) T
cells differentially affect prognosis of breast cancer subtypes.
Clin Cancer Res. 26:505–517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang MQ, Hou M, Lin DP and Wang YG:
Proportion and role of CD45+ erythroid progenitor cells in patients
with tongue cancer metastasis. Zhonghua Kou Qiang Yi Xue Za Zhi.
54:445–449. 2019.(In Chinese). PubMed/NCBI
|
|
39
|
Kitahara T, Haraguchi N, Takahashi H,
Nishimura J, Hata T, Takemasa I, Mizushima T, Yamamoto H, Doki Y
and Mori M: Identification and characterization of CD107a as a
marker of low reactive oxygen species in chemoresistant cells in
colorectal cancer. Ann Surg Oncol. 24:1110–1119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kittaneh M, Badve S, Caldera H, Coleman R,
Goetz MP, Mahtani R, Mamounas E, Kalinsky K, Lower E, Pegram M, et
al: Case-based review and clinical guidance on the use of genomic
assays for early-stage breast cancer: Breast Cancer therapy expert
group (BCTEG). Clin Breast Cancer. 20:183–193. 2020. View Article : Google Scholar : PubMed/NCBI
|