Introduction
Breast cancer is among the most frequently diagnosed
cancers globally and is the most common cancer in women with an
estimated 1.67 million new cases of breast cancer being diagnosed
in 2012, constituting 25% of all cancers (1). Despite the notable progress that has
been made in the treatment of breast cancer over past decades, it
remains the leading cause of cancer-associated mortality among
women worldwide, resulting in 14% of cancer-associated mortalities
(2,3). Survival rates for patients with
metastatic breast cancer remain poor (4), and the metastatic spread and invasion
of cancer cells are responsible for treatment failure in breast
cancer (5).
The metastatic process involves the detachment of
cancer cells and their invasion into adjacent normal tissues,
penetration of blood vessels and passive transport to distant
sites, implantation and the proliferation of metastatic colonies
(6). The proteolytic degradation of
parts of the extracellular matrix (ECM), including type IV
collagen, laminin, heparin sulfate proteoglycan, nidogens and
fibronectin, is required for metastasis to occur (6,7).
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent endopeptidases that have key functions in remodeling
the ECM during development, inflammation and wound repair
processes. The degradation of ECM components by MMPs serves a
crucial role in the migration and invasion of cancer cells
(8,9). Among MMPs, MMP-9 (also known as
gelatinase-B) is a key enzyme for the degradation of type IV
collagen, which is the main collagen component of the basement
membrane. Elevated expression of MMP-9 has been shown to be
critical in the invasive process in a number of tumors,
particularly breast tumors (7,9,10). Therefore, study of MMP-9 inhibition
and the underlying molecular mechanisms has been an important
strategy in the search for treatments for potentially invasive
tumors, including breast tumors.
The MMP-9 promoter contains various functional
regulatory motifs that have the ability to bind with transcription
factors, including activator protein-1 (AP-1; at positions −533 and
−79 in the MMP-9 promoter), nuclear factor-κB (NF-κB; at −600 bp)
and stimulatory protein-1 (Sp1; at −558) (11,12).
Through the binding of transcription factors to the specified
functional elements in the MMP-9 genes, the expression of MMP-9 is
controlled by a variety of stimulatory factors, including growth
factors, inflammatory cytokines, tumor necrosis factor (TNF)-α,
phorbol ester, epidermal growth factor and
12-O-tetradecanoylphorbol-13-acetate (TPA) (12–16).
Cytokines and TPA treatment induce the expression of MMP-9 via the
activation of transcription factors, including NF-κB and AP-1
(7,17). Furthermore, phosphoinositide 3-kinase
(PI3K) and mitogen-activated protein kinases (MAPKs) regulate the
predominant cascade participating in MMP-9 expression (11). MAPK signaling involves NF-κB
inhibitor (IκB) kinase, p38 MAPK, extracellular signal-regulated
kinase (ERK) or c-Jun N-terminal kinase (JNK), according to the
type of cell in which the signaling occurs (7,12,18). The
MAPK signaling pathway is involved in the activation of
transcription factors such as NF-κB and AP-1, which are known
regulators of the MMP-9 promoter (7,19).
Triptolide (Fig. 1A)
is a biologically active diterpenoid triepoxide that has been
isolated from the traditional Chinese herb, the thunder god vine
Tripterygium wilfordii Hook F (20). This natural product has been
demonstrated to have anti-inflammatory, immunosuppressant and
antitumor effects in vivo and in vitro (21,22).
Previous studies have attributed the antitumor effects of
triptolide to its ability to inhibit the proliferation of tumor
cells and induce their apoptosis (23,24).
However, the potential inhibitory effect of triptolide on MMP-9 has
not yet been evaluated.
Therefore, the present study investigated the
effects of triptolide on TPA-induced MMP-9 expression in MCF-7
human breast cancer cells. The molecular mechanisms underlying the
inhibition of MMP-9 expression by triptolide were also
investigated.
Materials and methods
Reagents
Triptolide, TPA and β-actin (cat. no. A3688)
antibodies were purchased from Sigma-Aldrich (Merck KGaA).
Inhibitors of AP-1 (SR 11302) and NF-κB (Bay 11-7092) were
purchased from Santa Cruz Biotechnology, Inc. The MAPK inhibitors
SB203580 (p38 inhibitor), SP600125 (JNK inhibitor) and PD98059 (ERK
inhibitor) were acquired from Merck Millipore. Rabbit antibodies
against phosphorylated (p-)c-Fos (cat. no. 5348), p-IκB kinase α/β
(p-IκKα/β; cat. no. 2697), stress activated protein kinase
(SAPK)/JNK (cat. no. 9258), p-SAPK/JNK (cat. no. 4668), p38 MAPK
(cat. no. 8690), p-p38 MAPK (cat. no. 4511), p44/42 MAPK (ERK1/2;
cat. no. 4695), p-p44/42 MAPK (p-ERK1/2; cat. no. 4370) and c-Fos
(cat. no. 2250) were purchased from Cell Signaling Technology, Inc.
Rabbit antibodies against NF-κB p65 (cat. no. ab16502), NF-κB
p105/p50 (cat. no. ab32360) and MMP-9 (cat. no. ab76003) were
purchased from Abcam. Rabbit antibodies against IκBα (cat. no.
sc-371) were purchased from Santa Cruz Biotechnology, Inc. Mouse
antibodies against p-IκBα (cat. no. 9246) were purchased from Cell
Signaling Technology, Inc. Mouse antibodies against proliferating
cell nuclear antigen (PCNA; cat. no. sc-7907), IκKα (cat. no.
sc-71333) and IκKβ (cat. no. sc-56918) were purchased from Santa
Cruz Biotechnology. The secondary antibodies anti-rabbit IgG
HRP-linked antibody (1:1,000 dilution; cat. no. 7074) and
anti-mouse IgG HRP-linked antibody (1:1,000 dilution; cat. no.
7076) were purchased from Cell Signaling Technology, Inc.
Cell culture
The MCF-7 human breast cancer cell line was acquired
from the American Type Culture Collection. The cells were cultured
in high glucose-containing Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 1%
antibiotic (10,000 U/ml penicillin and 10,000 µg/ml streptomycin)
and 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) in a 5% CO2 incubator at 37°C.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay. MCF-7 cells (2×104 cells/well) were seeded in a
96-well plate and incubated at 37°C for 24 h to allow attachment.
Cells were either untreated or treated with 1, 5, 10, 25, 50 or 100
nM triptolide at 37°C for 24 h and then washed with
phosphate-buffered saline (PBS; Gibco; Thermo Fisher Scientific,
Inc.). The MTT assay was then performed using 0.5 mg/ml MTT
(Sigma-Aldrich; Merck KGaA). Following the addition of MTT, the
cells were incubated at 37°C for 30 min. Dimethyl sulfoxide was
added to dissolve the formazan crystals, and the absorbance at 570
nm was determined using a microplate reader (Bio-Rad Laboratories,
Inc.).
Isolation of nuclear and cytoplasmic
extracts
Cells were pretreated with 5 or 10 nM triptolide and
then treated with 100 nM TPA at 37°C for 3 h, then washed with PBS
and pelleted. Nuclear and cytoplasmic extracts were obtained from
the pelleted cells using NE-PER® Cytoplasmic and Nuclear
Extraction Reagents, respectively (Pierce; Thermo Fisher
Scientific, Inc.).
Western blot analysis
Cells were pretreated with inhibitors of JNK
(SP600125; 10 and 20 µM), p38 (SB203580; 10 and 20 µM), ERK
(PD98059; 10 and 20 µM), NF-κB (Bay 11-7092; 2.5 and 5 µM) and AP-1
(SR 11302; 2.5 and 5 µM) at 37°C for 1 h, and then treated with TPA
at 37°C for 24 h. Proteins were extracted from cells by lysis using
M-PER Mammalian Protein Extraction Reagent (Pierce; Thermo Fisher
Scientific, Inc.) and a proteinase inhibitor. The protein
concentration was determined using a Protein Assay Dye Reagent
Concentrate (cat. no. 5000006) from Bio-Rad Laboratories, Inc. Cell
lysates (10 µg protein/lane) were separated by 10% SDS-PAGE and
transferred to Hybond™ polyvinylidene fluoride membranes (Cytiva).
Each membrane was blocked at 4°C for 2 h with skimmed milk or
bovine serum albumin (5% in PBS; purchased from MP Biomedicals,
LLC) and then incubated overnight at 4°C with the aforementioned
primary antibodies (diluted 1:1,000 in 5% skimmed milk/1X TBS
buffer). The corresponding HRP-conjugated anti-IgG antibody
(1:1,000 dilution) was used as the secondary antibody and was
incubated with the membrane for 1 h at 4°C. The immunoreactive
signals were visualized using an electrochemiluminescent HRP
substrate peroxide solution and luminol reagent (Merck Millipore;
cat. no. WBKLS0500). Protein levels were measured using an imaging
system (Las-4000; FujiFilm Corporation) and an image analyzer
software (Multi Gauge v3.0; FujiFilm Corporation). PCNA was used as
a loading control for the nucleus, and β-actin was used as an
internal control for the cytoplasm.
Zymography assay
Conditioned medium was collected from the cells,
mixed with sample buffer (0.5M Tris-HCI pH 6.8 2.5 ml, Glycerol 2
ml, 10% SDS 4 ml, 0.1% bromopherol blue 0.5 ml and D.W 1 ml) and
separated by PAGE containing gelatin (0.1%). The gel was washed for
30 min with Triton X-100 solution (2.5%) at room temperature and
then incubated for 16 h in developing buffer (5 mM
CaCl2, 0.02% Brij and 50 mM Tris-HCl, pH 7.5) at 37°C.
Afterwards, the gel was stained for 30 min at room temperature with
0.25% Coomassie brilliant blue (40% methanol and 7% acetic acid)
and the staining was measured using an image analyzer (FujiFilm
Corporation). Densitometric analysis was performed using Multi
Gauge image analysis software (Multi Gauge v3.0; FujiFilm
Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RNA was isolated from cells using RNAiso Plus
reagent (Takara Bio, Inc.; cat. no. 9108) and purified using a
FastPure RNA kit (Takara Bio, Inc.; cat. no. 9767). cDNA was
synthesized from the RNA using a PrimeScript RT reagent kit (Takara
Bio, Inc.; cat. no. RR037A) with heating at 37°C for 15 min and
85°C for 5 sec. The mRNA levels of MMP-9 and GAPDH were analyzed by
qPCR using an ABI PRISM™ 7900 Sequence Detection system and Power
SYBR® Green PCR Master mix (both Applied Biosystems;
Thermo Fisher Scientific, Inc.; cat. no. 330521). The primers used
were as follows: MMP-9 (NM 004994) sense,
5′-CCTGGAGACCTGAGAACCAATCT-3′ and antisense,
5′-CCACCCGAGTGTAACCATAGC-3′; and GAPDH (NM002046) sense,
5′-ATGGAAATCCCATCACCATCTT-3′ and antisense,
5′-CGCCCCACTTGATTTTGG-3′. qPCR was conducted with 40 cycles of 50°C
for 2 min, 95°C for 10 min, 95°C for 15 sec and 60°C for 1 min. The
results for MMP-9 were normalized to those of GAPDH. Relative
quantitation was conducted using the comparative Cq
(2−∆∆Cq) method (25).
Electrophoretic mobility shift assay
(EMSA)
Nuclear extracts were prepared using the
aforementioned protocol. Oligonucleotides containing AP-1
(5′-CGCTTGATGAGTCAGCCGGAA-3′; Promega Corporation; cat. no. E3201)
or NF-κB (5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′; Promega Corporation;
cat. no. E3291) binding sites were produced by Promega Corporation
and used as probes. Complementary strands were labeled with
[α-32P]dCTP (Amersham; Cytiva). The labeled
oligonucleotides (10,000 cpm), 10 µg nuclear extracts and binding
buffer (10 mM Tris-HCl, pH 7.6, 500 mM KCl, 10 mM EDTA, 50%
glycerol, 100 ng poly [dI·dC], 1 mM DTT) were incubated for 30 min
at room temperature. Reaction products were analyzed by
electrophoresis using 4% PAGE with 0.5X Tris-borate buffer. The
gels were then dried and analyzed by autoradiography. A 50-fold
excess of cold NF-κB or AP-1 oligonucleotide was used as a control
to confirm specific binding.
Luciferase assay
Cells (3×105 cells/well) were seeded onto
24-well plates and transfected with MMP-9, AP-1 or NF-κB reporter
plasmids (provided by Professor Kim Chul Ho, SungKyunKwan
University, Suwon, Korea) using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) as directed by
the manufacturer. The transfected cells were pretreated with 5 or
10 nM triptolide at 37°C for 1 h and then treated with 100 nM TPA
at 37°C for 3 h. Luciferase reporter assays were implemented using
a Dual Luciferase assay kit (Promega Corporation; cat. no. E1910)
as recommended by the manufacturer, and the results were collected
using a luminometer (Lumat LB 9507; Berthold Technologies GmbH
& Co.). The luciferase assay was performed by sequentially
measuring the firefly and Renilla luciferase activities,
with the results expressed as the ratio of firefly to
Renilla luciferase activity.
Invasion assay
An invasion assay was conducted using 24-well
chambers (8-µm pore size) in which the upper side of the Transwell
insert was coated at 37°C for 30 min with Matrigel (BD
Biosciences). Cells (3×105 cells/well) were added to the
upper chamber in serum-free DMEM, while the lower compartment
contained conditioned DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 1% antibiotic (10,000 U/ml penicillin and 10,000
µg/ml streptomycin) and 10% FBS. Then, 100 nM TPA with or without 5
or 10 nM triptolide was added to the upper chamber. After
incubation at 37°C for 24 h, the cells in the upper chamber were
removed using cotton swabs. The invaded cells on the bottom of the
filter were fixed with 3.7–4.0% formalin for 10 min at room
temperature and stained with crystal violet for 30 min at room
temperature. Invading cells were counted in five random regions of
the membrane using light microscopy.
Statistical analysis
Data from three independent experiments are
presented as the mean ± standard error of the mean. Statistical
analyses were performed by analysis of variance and Tukey's tests
using OriginPro 8 (OriginLab Corporation). P<0.05 was considered
to indicate a statistically significant difference.
Results
Triptolide affects TPA-induced MMP-9
expression
The cytotoxicity of triptolide in MCF-7 cells was
investigated using an MTT assay. Cells were treated with triptolide
(0–100 nM) for 24 h and no cytotoxic effects were observed for
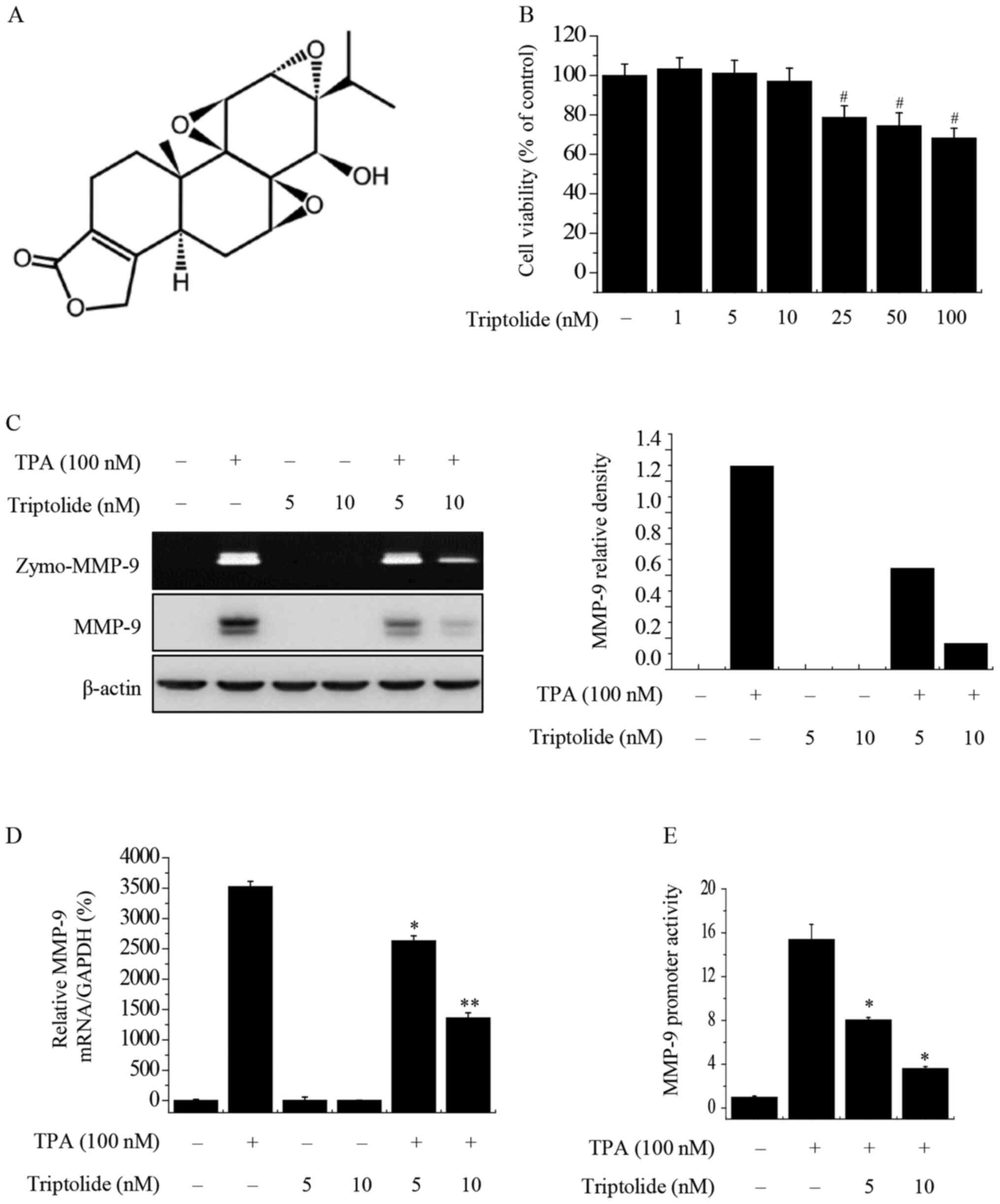
triptolide at concentrations from 0 to 10 nM (Fig. 1B). Therefore, non-toxic
concentrations (5 and 10 nM) of triptolide were selected for use in
the subsequent experiments. To investigate the effect of triptolide
on TPA-induced MMP-9 expression in MCF-7 cells, zymography, western
blot analysis, RT-qPCR and luciferase assays were used. Zymography
showed that TPA increased the activity of MMP-9 in MCF-7 cells, and
that triptolide blocked the TPA-induced activity of MMP-9 in a
concentration-dependent manner. Western blot analysis demonstrated
that triptolide suppressed the TPA-induced expression of MMP-9
protein (Fig. 1C). In addition,
RT-qPCR showed that triptolide treatment suppressed the TPA-induced
expression of MMP-9 at the mRNA level (Fig. 1D). Furthermore, a luciferase reporter
assay demonstrated that the treatment of MCF-7 cells with
triptolide suppressed TPA-induced MMP-9 promoter activity (Fig. 1E). Together, these results
demonstrate the inhibitory effects of triptolide on MMP-9
expression.
Triptolide inhibits TPA-induced ERK
activation
The mechanism by which triptolide affects signaling
was investigated using zymography and western blot analysis. MCF-7
cells were pretreated with inhibitors of JNK (SP600125), p38
(SB203580) and ERK (PD98059) and then treated with TPA. As shown in
Fig. 2A, the inhibition of ERK, JNK
and p38 suppressed TPA-induced MMP-9 protein expression and
activity in MCF-7 cells. In addition, TPA markedly increased the
phosphorylation levels of ERK, JNK and p38. Triptolide inhibited
the TPA-induced phosphorylation of ERK; however, the total protein
level of ERK remained unaltered (Fig.
2B). These results suggest that the inhibition of MMP-9
expression by triptolide is associated with a reduction in the
phosphorylation of ERK.
 | Figure 2.Effect of triptolide on MAPK
expression in MCF-7 cells. (A) Cells were pretreated with
inhibitors of ERK (PD98059), JNK (SP600125) and p38 MAPK
(SB203580), and TPA was added for 24 h. MMP-9 activity was analyzed
by gelatin zymography and MMP-9 protein expression was detected by
western blotting. (B) Cells were pretreated with triptolide and TPA
was added for 24 h. The phosphorylation of ERK, JNK and p38 was
analyzed by western blotting. β-actin was used as an internal
control. Data presented are the result of three independent
experiments. ERK, extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase; MMP-9, matrix metalloproteinase-9; p38 MAPK,
mitogen-activated protein kinase; TPA,
12-O-tetradecanoylphorbol-13-acetate; p-, phosphorylated; zymo,
zymography. |
Triptolide inhibits TPA-induced NF-κB
and AP-1 activation
To further examine the inhibitory mechanism
underlying the transcriptional regulation of MMP-9 by triptolide,
western blot analysis was performed to examine the effects of
triptolide on NF-κB and AP-1 activation in MCF-7 cells. MCF-7 cells
were pretreated with inhibitors of NF-κB (Bay 11-7092) and AP-1 (SR
11302). As shown in Fig. 3A, the
inhibition of NF-κB or AP-1 suppressed the TPA-induced expression
of MMP-9 protein in MCF-7 cells. The addition of triptolide
inhibited the TPA-induced nuclear translocation of NF-κB p65/p50
and phosphorylation of cytoplasmic IκBα and IKKα/β. Total c-Fos
expression in the nucleus and total IκBα, IKKα and IKKβ expression
in the cytosol did not exhibit any changes. In addition,
phosphorylation of the AP-1 subunit c-Fos in the nucleus of the
TPA-induced cells was decreased following treatment with triptolide
(Fig. 3B). Using luciferase assays,
the treatment of MCF-7 cells with triptolide was showed to suppress
TPA-induced NF-κB and AP-1 promoter activity (Fig. 3C and D). To investigate DNA-binding
activity, EMSAs were performed. The results showed that triptolide
markedly inhibited the TPA-induced binding activities of NF-κB and
AP-1 (Fig. 3E and F). These results
suggest that triptolide inhibits MMP-9 expression via the
modulation of its activation by the transcription factors NF-κB and
AP-1.
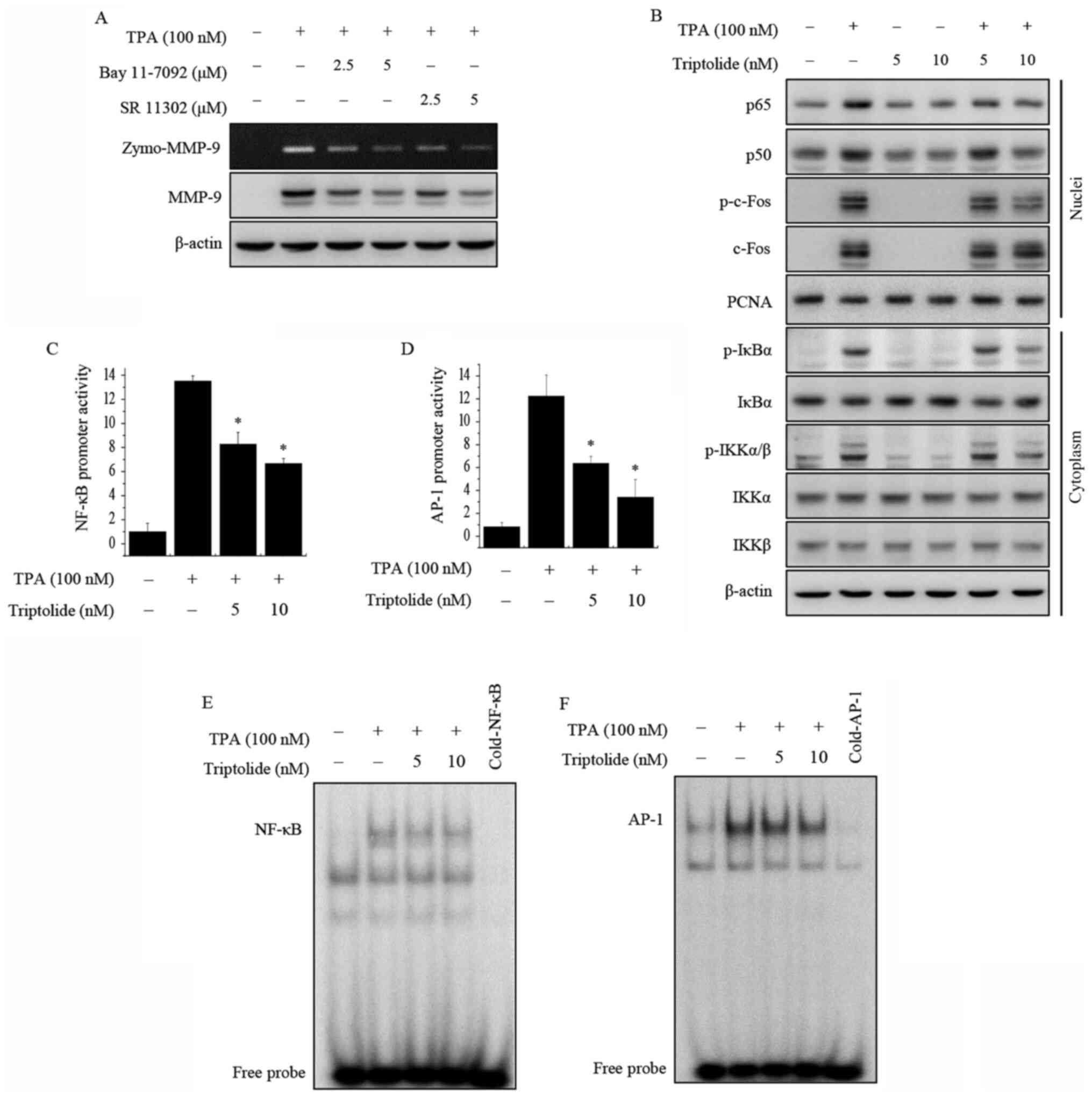 | Figure 3.Effects of triptolide on TPA-induced
NF-κB and AP-1 activation in MCF-7 cells. (A) MCF-7 cells were
pretreated with inhibitors of NF-κB (Bay 11-7092) and AP-1 (SR
11302), and then TPA was added for 24 h. MMP-9 activity was
detected by gelatin zymography and MMP-9 protein expression was
analyzed by western blotting. (B) Cells were treated with
triptolide and/or TPA. After a 3-h incubation, nuclear and
cytoplasmic extracts were prepared. Translocation of p65, p50 and
p-c-Fos to the nucleus and the levels of p-IκBα, p-IKKα/β, total
c-Fos, IκBα, IKKα and IKKβ were determined by western blotting.
PCNA was used as a loading control for the nucleus, and β-actin was
used as an internal control for the cytoplasm. (C) NF-κB-luc and
(D) AP-1-luc reporters were co-transfected with a Renilla
luciferase thymidine kinase reporter into the cells. The cells were
treated with TPA alone or with triptolide, and the NF-κB and AP-1
promoter activities were measured using a dual-luciferase reporter
assay. The DNA binding of (E) NF-κB and (F) AP-1 was analyzed using
electrophoretic mobility shift assays. Data are presented as the
mean ± SEM of three independent experiments. *P<0.05 vs. TPA
alone. AP-1, activator protein-1; MMP-9, matrix
metalloproteinase-9; NF-κB, nuclear factor-κB; p-, phosphorylated;
IKKα/β, IκB kinase α/β; IκBα, NF-κB inhibitor α; TPA,
12-O-tetradecanoylphorbol-13-acetate; zymo, zymography; PCNA,
proliferating cell nuclear antigen. |
Triptolide inhibits TPA-induced
invasion in vitro
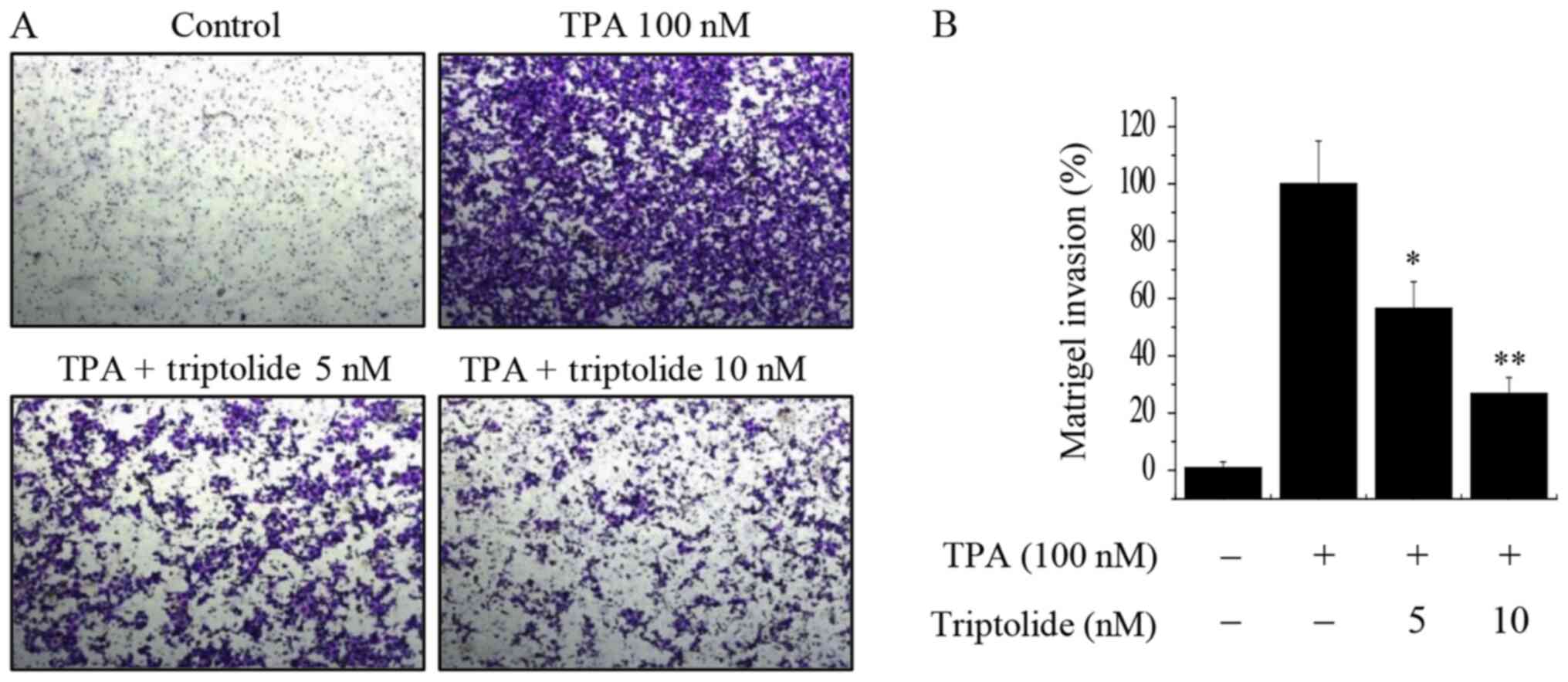
Using a Matrigel invasion assay, the effect of
triptolide on the invasive ability of MCF-7 breast cancer cells was
investigated. The results showed that a 10 nM concentration of
triptolide effectively inhibited the invasion ability of MCF-7
cells by almost 70% compared with that of the untreated control
cells (Fig. 4). These results
suggest that triptolide suppresses the invasive potential of breast
cancer cells.
Discussion
Triptolide has been used in the treatment of
autoimmune and inflammatory diseases, such as rheumatoid arthritis
(26). Numerous studies have
investigated the antitumor effects of triptolide in various types
of cancer, and have demonstrated that triptolide can induce
apoptosis and inhibit the proliferation of cancer cells in
vitro and reduce the growth and metastasis of tumors in
vivo (23,24,26–29).
Notably, certain studies have revealed that triptolide inhibits the
proliferation of breast cancer cells in vitro, induces
apoptosis and modulates the expression of several signaling
molecules (29,30). Triptolide has been shown to induce
apoptosis by increasing caspase-3 activity (30), downregulating estrogen receptor α
(31) and the Wnt/β-catenin pathway
(32) and regulating DNA
repair/damage (33,34) in various subtypes of breast cancer,
including basal and triple-negative types. These previous findings
suggest that triptolide is a promising treatment for various types
of breast cancer. Li et al (22) revealed that triptolide was cytotoxic
to human breast cancer stem cells and primary breast cancer cells
in vitro and in vivo. Furthermore, studies have shown
that triptolide is a multi-target anticancer agent that modulates
various molecular pathways, for example, by reducing the
transcriptional activity of NF-κB and AP-1 and inhibiting the
expression of heat shock protein 70 (24,35,36).
Triptolide also exerts effects via autophagy and p38/ERK/mTOR
phosphorylation (37) and modulates
the expression of ERK, NF-κB, focal adhesion kinase, vascular
endothelial growth factor, β-catenin and AKT (38). However, no previous studies have
investigated whether triptolide inhibits MAPK or transcription
factors such as AP-1 and NF-κB. Thus, triptolide has been
demonstrated to be an antitumor agent that inhibits proliferation
and induces apoptosis in breast cancer cells. However, there is
little data available regrating the inhibitory effect of triptolide
on the invasion and migration of human breast cancer cells.
Therefore, the present study investigated the effects of triptolide
on MMP-9 activity in human breast cancer cells and examined the
underlying molecular mechanisms of this activity.
MMP-9 is a key enzyme in tumor metastasis and
invasion, and its activation is associated with the progression of
breast tumors (7). In the present
study, the results revealed that triptolide inhibited the
TPA-induced expression of MMP-9 in MCF-7 human breast cancer cells
at the protein and mRNA levels, which suggests that triptolide may
have potent anti-metastatic activity. In addition, the present
study demonstrated that triptolide inhibited TPA-induced MMP-9
expression by suppressing ERK pathways and, subsequently, NF-κB and
AP-1 activity in human breast cancer cells.
MMPs are involved in numerous signaling pathways,
including pathways involving NF-κB and AP-1, as well as MAPK, PI3K
and protein kinase C. However, no systemic research focusing on
NF-κB and AP-1 in a triptolide-treated MCF-7 model has previously
been reported. In the present study, the results indicate that
NF-κB and AP-1 are important factors associated with MMP-9. A
previous study examined the effects of TPA on the expression of
MMP-2 and MMP-9 in MCF-7 and MDA-MB-231 cell lines, and revealed
that TPA induced MMP-9 enzyme activity and protein expression but
had no effect on MMP-2 expression (39). MAPK pathways, the predominant cascade
modulating MMP-9 expression, are involved in cellular proliferation
and survival (12). Tang et
al (40) reported that the
activation of ERK mediated apoptosis and cell cycle arrest after
DNA damage, independent of p53. Tan et al (36) demonstrated that the treatment of
breast cancer cells with triptolide activated ERK in a dose- and
time-dependent manner. These studies indicate that ERK activation
is crucial in the mediation of triptolide-induced caspase-dependent
apoptosis. The present study revealed that the inhibition of MMP-9
expression by triptolide is associated with reduced phosphorylation
of ERK. Triptolide has been shown to exert an anti-invasive effect
in breast cancer cells via inhibition of the ERK pathway (36).
The present study investigated the effects of
triptolide on the DNA-binding activity of TPA-induced NF-κB and
AP-1 to determine the molecular signaling pathways by which
triptolide influences the migration and invasion of breast cancer
cells. The NF-κB and AP-1 elements of the MMP-9 promoter have been
demonstrated to serve a prominent role in the TPA- and
cytokine-induced expression of the MMP-9 gene and the associated
invasion of tumor cells (7,13,17).
Chung et al (41) reported
that the anti-metastatic and antitumor effects of caffeic acid and
its phenyl ester are mediated through the selective suppression of
MMP-9 activity and the inhibition of NF-κB and MMP-9
transcriptional activities. In addition, Weng et al
(13) reported that the
anti-invasive effects of lucidenic acid against the TPA-induced
invasion of human hepatoma cells proceeded via inactivation of the
MAPK signal transduction pathway and attenuation of the binding
activities of NF-κB and AP-1. Furthermore, another study revealed
that triptolide affected the proliferation and metastasis of
melanoma cells via the inhibition of NF-κB expression, which
consequently suppressed MMP-9 and MMP-2 expression (42). These signaling patterns have also
been reported for other metalloproteinases. For example, triptolide
was demonstrated to regulate the MAPK/ERK/JNK/AP-1 signaling
pathway and directly affect the activation of MMP-1/3/13 in
rheumatoid arthritis (43). However,
no studies have explored these pathways in breast cancer models,
and therefore further investigation is required. In the present
study, triptolide inhibited the transcriptional activity of MMP-9
in TPA-induced MCF-7 breast cancer cells by suppressing NF-κB and
AP-1 DNA-binding activities.
TPA has been well identified as a tumor promotor in
a variety of human cell lines and has demonstrated the ability to
increase the expression of nuclear factors associated with
metastasis in selected tumor cell lines. TPA signaling has an
association with AP-1 as well as MMP-1, −3 and −9, which have
TPA-responsive elements, and TPA-sensitive MMPs are stimulated by
cytokines including IL-1 and TNF-α (39). Therefore, the results of the present
suggest that the effects of TPA, as a promoter of ERK signaling and
NF-κB and AP-1 activation-mediated breast cancer metastasis, were
effectively inhibited by triptolide administration in the MCF-7
breast cancer cell line. MMP-9 expression was not evident in MCF-7
cells in the normal state in the present study or our previous
study (9). Thus, TPA is a selective
agent for increasing the expression of MMP-9, and served to
establish a model for examining the ability of triptolide to
suppress the metastasis of MCF-7 cells. Triptolide was selected for
evaluation due to its ability to inhibit TPA-induced MMP-9
expression in MCF-7 cells (9).
However, systemic research with various transcription factors, such
as NF-κB and AP-1 and the associated signaling pathways, in an
MCF-7 cell model is lacking. Although the present study suggests
the potential of triptolide as a potential anticancer drug
candidate, the mechanism requires further investigation; for
example, the involvement of MMP-2 (44), MMP-3 and MMP-5 could be examined.
In conclusion, the present study demonstrated that
triptolide effectively decreased the expression of MMP-9 and cell
invasion through inhibition of the TPA-induced phosphorylation of
ERK and the downregulation of NF-κB and AP-1 activity. These
findings suggest that triptolide is a potent inhibitor of
TPA-induced MMP-9 expression and shows promise as a potential
therapeutic agent for preventing the metastasis and invasion of
breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Biomedical
Research Institute, Jeonbuk National University Hospital and Basic
Science Research Program through the National Research Foundation
of Korea funded by the Ministry of Education (nos.
2016R1D1A1B03930499 and 2020R1I1A1A01054100)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HSC and OYH conceived and designed the study and
were major contributors to writing the manuscript. OYH performed
the experiments and analyzed the data. JSK and YJJ contributed to
conception and design, and acquisition of funding. KHP and HYJ were
involved in the additional experiments and revision process. JSK
and HSC confirm the authenticity of all the raw data. All authors
read and approved the final manuscript, and agree to be accountable
for all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
MTT
|
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide
|
|
PCR
|
polymerase chain reaction
|
|
TPA
|
12-O-tetradecanoylphorbol-13-acetate
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
p38
|
mitogen-activated protein kinase
|
|
AP-1
|
activator protein-1
|
|
NF-κB
|
nuclear factor-κB
|
|
p-IKKα/β
|
phosphorylated IκB kinase α/β
|
|
p-IκBα
|
phosphorylated nuclear factor of κ
light polypeptide gene enhancer in B-cells inhibitor, α
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian D, Li Y, Li X and Tian Z: Aloperine
inhibits proliferation, migration and invasion and induces
apoptosis by blocking the Ras signaling pathway in human breast
cancer cells. Mol Med Rep. 18:3699–3710. 2018.PubMed/NCBI
|
|
3
|
Di Leo A, Curigliano G, Diéras V, Malorni
L, Sotiriou C, Swanton C, Thompson A, Tutt A and Piccart M: New
approaches for improving outcomes in breast cancer in Europe.
Breast. 24:321–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
García Rodríguez J, García Colmenero C,
Clèries Soler R and Oleaga Sánchez I: Five years survival of women
diagnosed with breast cancer during the period 1997-1999 in
Toledo-Centro and Mancha Area, Spain. Rev Esp Salud Publica.
84:843–850. 2010.(In Spanish).
|
|
5
|
Rose DP, Connolly JM and Coleman M: Effect
of omega-3 fatty acids on the progression of metastases after the
surgical excision of human breast cancer cell solid tumors growing
in nude mice. Clin Cancer Res. 2:1751–1756. 1996.PubMed/NCBI
|
|
6
|
Nakajima M, Welch DR, Belloni PN and
Nicolson GL: Degradation of basement membrane type IV collagen and
lung subendothelial matrix by rat mammary adenocarcinoma cell
clones of differing metastatic potentials. Cancer Res.
47:4869–4876. 1987.PubMed/NCBI
|
|
7
|
Hwang JK, Yu HN, Noh EM, Kim JM, Hong OY,
Youn HJ, Jung SH, Kwon KB, Kim JS and Lee YR: DHA blocks
TPA-induced cell invasion by inhibiting MMP-9 expression via
suppression of the PPAR-γ/NF-κB pathway in MCF-7 cells. Oncol Lett.
13:243–249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehner C, Hockla A, Miller E, Ran S,
Radisky DC and Radisky ES: Tumor cell-produced matrix
metalloproteinase 9 (MMP-9) drives malignant progression and
metastasis of basal-like triple negative breast cancer. Oncotarget.
5:2736–2749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noh EM, Lee YR, Hong OY, Jung SH, Youn HJ
and Kim JS: Aurora kinases are essential for PKC-induced invasion
and matrix metalloproteinase-9 expression in MCF-7 breast cancer
cells. Oncol Rep. 34:803–810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rao JS, Steck PA, Mohanam S,
Stetler-Stevenson WG, Liotta LA and Sawaya R: Elevated levels of
M(r) 92,000 type IV collagenase in human brain tumors. Cancer Res.
53 (Suppl):2208–2211. 1993.PubMed/NCBI
|
|
11
|
Sato H and Seiki M: Regulatory mechanism
of 92 kDa type IV collagenase gene expression which is associated
with invasiveness of tumor cells. Oncogene. 8:395–405.
1993.PubMed/NCBI
|
|
12
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weng CJ, Chau CF, Hsieh YS, Yang SF and
Yen GC: Lucidenic acid inhibits PMA-induced invasion of human
hepatoma cells through inactivating MAPK/ERK signal transduction
pathway and reducing binding activities of NF-kappaB and AP-1.
Carcinogenesis. 29:147–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho HJ, Kang JH, Kwak JY, Lee TS, Lee IS,
Park NG, Nakajima H, Magae J and Chang YC: Ascofuranone suppresses
PMA-mediated matrix metalloproteinase-9 gene activation through the
Ras/Raf/MEK/ERK- and Ap1-dependent mechanisms. Carcinogenesis.
28:1104–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kajanne R, Miettinen P, Mehlem A, Leivonen
SK, Birrer M, Foschi M, Kähäri VM and Leppä S: EGF-R regulates MMP
function in fibroblasts through MAPK and AP-1 pathways. J Cell
Physiol. 212:489–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srivastava AK, Qin X and Wedhas N: Amush
m, Linkhart TA, Chadwick RB and Kumar A: Tumor necrosis
factor-alpha augments matrix metalloproteinase-9 production in
skeletal muscle cells through the activation of transforming growth
factor-beta-activated kinase 1 (TAK1)-dependent signaling pathway.
J Biol Chem. 282:35113–35124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SO, Jeong YJ, Kim M, Kim CH and Lee
IS: Suppression of PMA-induced tumor cell invasion by capillarisin
via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem
Biophys Res Commun. 366:1019–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
Subunit of NF-kappa B through utilization of the Ikappa B kinase
and activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cohen M, Meisser A, Haenggeli L and
Bischof P: Involvement of MAPK pathway in TNF-alpha-induced MMP-9
expression in human trophoblastic cells. Mol Hum Reprod.
12:225–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng X, Shi W, Zhao C, Zhang D, Liang P,
Wang G and Lu L: Triptolide sensitizes human breast cancer cells to
tumor necrosis factor-α-induced apoptosis by inhibiting activation
of the nuclear factor-κB pathway. Mol Med Rep. 13:3257–3264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xi C, Peng S, Wu Z, Zhou Q and Zhou J:
Toxicity of triptolide and the molecular mechanisms involved.
Biomed Pharmacother. 90:531–541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Liu R, Yang Y, Huang Y, Li X, Liu R
and Shen X: Triptolide-induced in vitro and in vivo cytotoxicity in
human breast cancer stem cells and primary breast cancer cells.
Oncol Rep. 31:2181–2186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang S, Gu C, Zhang G, Kang J, Wen H, Lu Q
and Huang J: Inhibitive effect of triptolide on invasiveness of
human fibrosarcoma cells by downregulating matrix
metalloproteinase-9 expression. Asian Pac J Trop Med. 4:482–485.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Phillips PA, Dudeja V, McCarroll JA,
Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM and Saluja AK:
Triptolide induces pancreatic cancer cell death via inhibition of
heat shock protein 70. Cancer Res. 67:9407–9416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan D, Guo Q, Shen J, Zheng K, Lu C, Zhang
G, Lu A and He X: The effect of triptolide in rheumatoid arthritis:
from basic research towards clinical translation. Int J Mol Sci.
19:3762018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu W, Ou Y, Li Y, Xiao R, Shu M, Zhou Y,
Xie J, He S, Qiu P and Yan G: A small-molecule triptolide
suppresses angiogenesis and invasion of human anaplastic thyroid
carcinoma cells via down-regulation of the nuclear factor-kappa B
pathway. Mol Pharmacol. 75:812–819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan EW, Cheng SC, Sin FW and Xie Y:
Triptolide induced cytotoxic effects on human promyelocytic
leukemia, T cell lymphoma and human hepatocellular carcinoma cell
lines. Toxicol Lett. 122:81–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei
XF, Yang J, Underhill CB and Zhang L: Triptolide inhibits the
growth and metastasis of solid tumors. Mol Cancer Ther. 2:65–72.
2003.PubMed/NCBI
|
|
30
|
Lu L, Kanwar J, Schmitt S, Cui QC, Zhang
C, Zhao C and Dou QP: Inhibition of tumor cellular proteasome
activity by triptolide extracted from the Chinese medicinal plant
‘thunder god vine’. Anticancer Res. 31:1–10. 2011.PubMed/NCBI
|
|
31
|
Li H, Pan GF, Jiang ZZ, Yang J, Sun LX and
Zhang LY: Triptolide inhibits human breast cancer MCF-7 cell growth
via downregulation of the ERα-mediated signaling pathway. Acta
Pharmacol Sin. 36:606–613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shao H, Ma J, Guo T and Hu R: Triptolide
induces apoptosis of breast cancer cells via a mechanism associated
with the Wnt/β-catenin signaling pathway. Exp Ther Med. 8:505–508.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z, Sun C, Zhang L, Chi X, Ji J, Gao
X, Wang Y, Zhao Z, Liu L, Cao X, et al: Triptolide interferes with
XRCC1/PARP1-mediated DNA repair and confers sensitization of
triple-negative breast cancer cells to cisplatin. Biomed
Pharmacother. 109:1541–1546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng Y, Li F, He P, Yang Y, Yang J, Zhang
Y, Liu J, Tong Y, Li Q, Mei X, et al: Triptolide sensitizes breast
cancer cells to Doxorubicin through the DNA damage response
inhibition. Mol Carcinog. 57:807–814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang XH, Wong BC, Lin MC, Zhu GH, Kung
HF, Jiang SH, Yang D and Lam SK: Functional p53 is required for
triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB
activation in gastric cancer cells. Oncogene. 20:8009–8018. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tan BJ and Chiu GNC: Role of oxidative
stress, endoplasmic reticulum stress and ERK activation in
triptolide-induced apoptosis. Int J Oncol. 42:1605–1612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao H, Zhang Y, Dong L, Qu XY, Tao LN,
Zhang YM, Zhai JH and Song YQ: Triptolide induces autophagy and
apoptosis through ERK activation in human breast cancer MCF-7
cells. Exp Ther Med. 15:3413–3419. 2018.PubMed/NCBI
|
|
38
|
Sarkar S and Paul S: Triptolide mediated
amelioration of breast cancer via modulation of molecular pathways.
Pharmacogn J. 9:838–845. 2017. View Article : Google Scholar
|
|
39
|
Mackay AR, Ballin M, Pelina MD, Farina AR,
Nason AM, Hartzler JL and Thorgeirsson UP: Effect of phorbol ester
and cytokines on matrix metalloproteinase and tissue inhibitor of
metalloproteinase expression in tumor and normal cell lines.
Invasion Metastasis. 12:168–184. 1992.PubMed/NCBI
|
|
40
|
Tang D, Wu D, Hirao A, Lahti JM, Liu L,
Mazza B, Kidd VJ, Mak TW and Ingram AJ: ERK activation mediates
cell cycle arrest and apoptosis after DNA damage independently of
p53. J Biol Chem. 277:12710–12717. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chung TW, Moon SK, Chang YC, Ko JH, Lee
YC, Cho G, Kim SH, Kim JG and Kim CH: Novel and therapeutic effect
of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma
cells: Complete regression of hepatoma growth and metastasis by
dual mechanism. FASEB J. 18:1670–1681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jao HY, Yu FS, Yu CS, Chang SJ, Liu KC,
Liao CL, Ji BC, Bau DT and Chung JG: Suppression of the migration
and invasion is mediated by triptolide in B16F10 mouse melanoma
cells through the NF-kappaB-dependent pathway. Environ Toxicol.
31:1974–1984. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song X, Zhang Y and Dai E: Therapeutic
targets of thunder god vine (Tripterygium wilfordii hook) in
rheumatoid arthritis (Review). Mol Med Rep. 21:2303–2310.
2020.PubMed/NCBI
|
|
44
|
Lin CW, Shen SC, Hou WC, Yang LY and Chen
YC: Heme oxygenase-1 inhibits breast cancer invasion via
suppressing the expression of matrix metalloproteinase-9. Mol
Cancer Ther. 7:1195–1206. 2008. View Article : Google Scholar : PubMed/NCBI
|