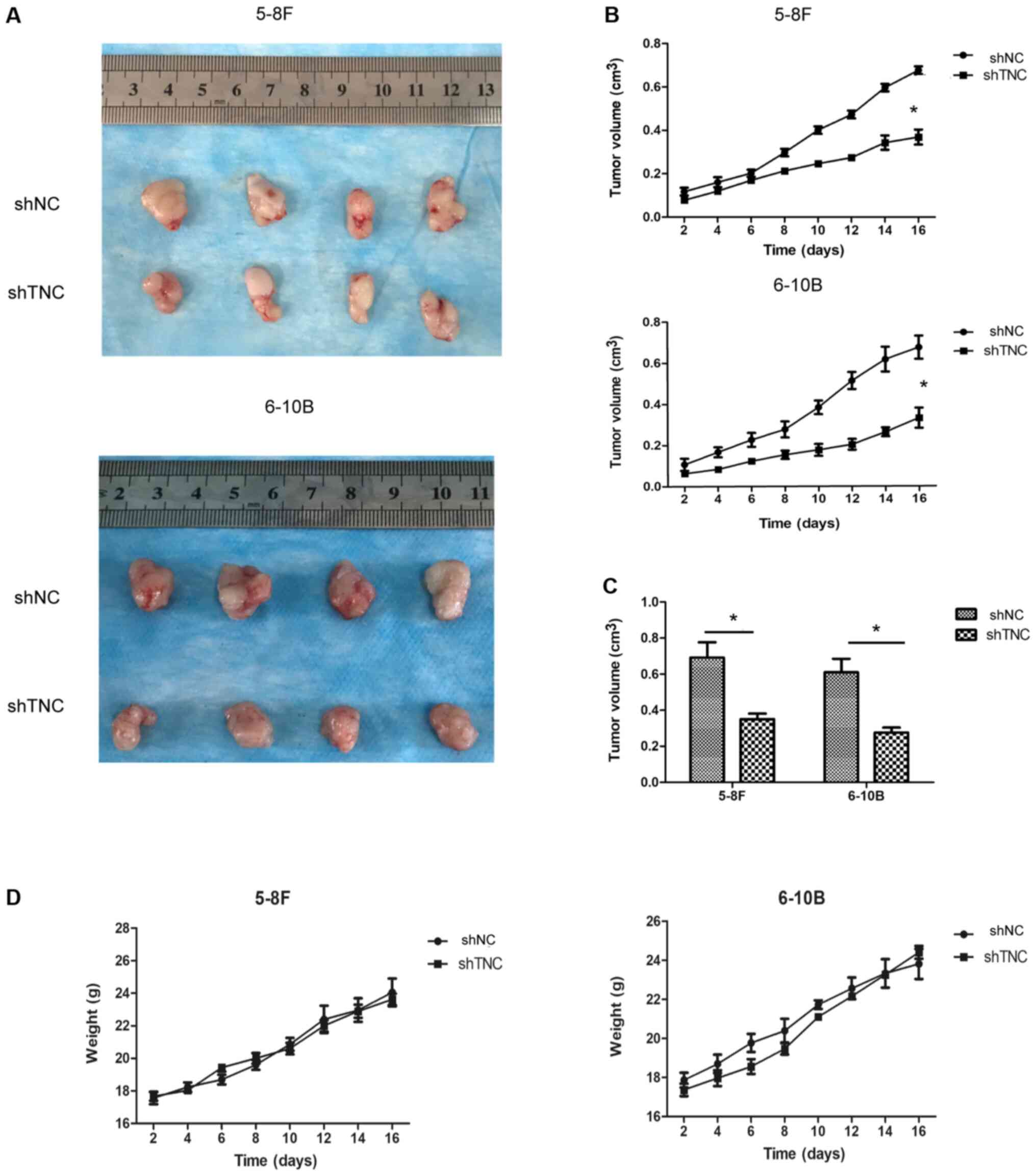
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adham M, Kurniawan AN, Muhtadi AI, Roezin
A, Hermani B, Gondhowiardjo S, Tan IB and Middeldorp JM:
Nasopharyngeal carcinoma in Indonesia: Epidemiology, incidence,
signs, and symptoms at presentation. Chin J Cancer. 31:185–196.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun XS, Li XY, Chen QY, Tang LQ and Mai
HQ: Future of radiotherapy in nasopharyngeal carcinoma. Br J
Radiol. 92:201902092019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kimura Y, Suzuki D, Tokunaga T,
Takabayashi T, Yamada T, Wakisaka N, Yoshizaki T, Murata H, Miwa K,
Shoujaku H, et al: Epidemiological analysis of nasopharyngeal
carcinoma in the central region of Japan during the period from
1996 to 2005. Auris Nasus Larynx. 38:244–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu S, Lin S, Tham IWK, Pan J, Lu J and Lu
JJ: Intensity-modulated radiation therapy in the salvage of locally
recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
83:676–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Co J, Mejia MB and Dizon JM: Evidence on
effectiveness of intensity-modulated radiotherapy versus
2-dimensional radiotherapy in the treatment of nasopharyngeal
carcinoma: Meta-analysis and a systematic review of the literature.
Head Neck. 38 (Suppl 1):E2130–E2142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu ZJ, Zheng RS, Zhang SW, Zou XN and Chen
WQ: Nasopharyngeal carcinoma incidence and mortality in China in
2009. Chin J Cancer. 32:453–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Colaco RJ, Betts G, Donne A, Swindell R,
Yap BK, Sykes AJ, Slevin NJ, Homer JJ and Lee LW: Nasopharyngeal
carcinoma: A retrospective review of demographics, treatment and
patient outcome in a single Centre. Clin Oncol (R Coll Radiol).
25:171–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones FS and Jones PL: The tenascin family
of ECM glycoproteins: Structure, function, and regulation during
embryonic development and tissue remodeling. Dev Dyn An.
218:235–259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Midwood KS, Chiquet M, Tucker RP and Orend
G: Tenascin-C at a glance. J Cell Sci. 129:4321–4327.
2016.PubMed/NCBI
|
|
11
|
Van Obberghen-Schilling E, Tucker RP,
Saupe F, Gasser I, Cseh B and Orend G: Fibronectin and Tenascin-C:
Accomplices in vascular morphogenesis during development and tumor
growth. Int J Dev Biol. 55:511–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Midwood KS, Hussenet T, Langlois B and
Orend G: Advances in Tenascin-C biology. Cell Mol Life Sci.
68:3175–3199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida T, Akatsuka T and Imanaka-Yoshida
K: Tenascin-C and integrins in cancer. Cell Adh Migr. 9:96–104.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lange K, Kammerer M, Saupe F, Hegi ME,
Grotegut S, Fluri E and Orend G: Combined lysophosphatidic
acid/platelet-derived growth factor signaling triggers glioma cell
migration in a Tenascin-C microenvironment. Cancer Res.
68:6942–6952. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang W, Chiquet-Ehrismann R, Moyano JV,
Garcia-Pardo A and Orend G: Interference of Tenascin-C with
syndecan-4 binding to fibronectin blocks cell adhesion and
stimulates tumor cell proliferation. Cancer Res. 61:8586–8594.
2001.PubMed/NCBI
|
|
16
|
Oskarsson T, Acharyya S, Zhang XH,
Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K,
Brogi E and Massagué J: Breast cancer cells produce Tenascin C as a
metastatic niche component to colonize the lungs. Nat Med.
17:867–874. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beiter K, Hiendlmeyer E, Brabletz T,
Hlubek F, Haynl A, Knoll C, Kirchner T and Jung A: Beta-Catenin
regulates the expression of Tenascin-C in human colorectal tumors.
Oncogene. 24:8200–8204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lowy CM and Oskarsson T: Tenascin C in
metastasis: A view from the invasive front. Cell Adh Migr.
9:112–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoneura N, Takano S, Yoshitomi H, Nakata
Y, Shimazaki R, Kagawa S, Furukawa K, Takayashiki T, Kuboki S,
Miyazaki M and Ohtsuka M: Expression of annexin II and stromal
tenascin C promotes epithelial to mesenchymal transition and
correlates with distant metastasis in pancreatic cancer. Int J Mol
Med. 42:821–830. 2018.PubMed/NCBI
|
|
20
|
Nagaharu K, Zhang X, Yoshida T, Katoh D,
Hanamura N, Kozuka Y, Ogawa T, Shiraishi T and Imanaka-Yoshida K:
Tenascin C induces epithelial-mesenchymal transition-like change
accompanied by SRC activation and focal adhesion kinase
phosphorylation in human breast cancer cells. Am J Pathol.
178:754–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan Q, Tang J, Han Y and Wang D:
Co-expression modules construction by WGCNA and identify potential
prognostic markers of uveal melanoma. Exp Eye Res. 166:13–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao YN, Cao X, Luo DH, Sun R, Peng LX,
Wang L, Yan YP, Zheng LS, Xie P, Cao Y, et al: Urokinase-type
plasminogen activator receptor signaling is critical in
nasopharyngeal carcinoma cell growth and metastasis. Cell Cycle.
13:1958–1969. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bose S, Yap LF, Fung M, Starzcynski J,
Saleh A, Morgan S, Dawson C, Chukwuma MB, Maina E, Buettner M, et
al: The ATM tumour suppressor gene is down-regulated in
EBV-associated nasopharyngeal carcinoma. J Pathol. 217:345–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang XR, Li YQ, Liang SB, Jiang W, Liu F,
Ge WX, Tang LL, Mao YP, He QM, Yang XJ, et al: Development and
validation of a gene expression-based signature to predict distant
metastasis in locoregionally advanced nasopharyngeal carcinoma: A
retrospective, multicentre, cohort study. Lancet Oncol. 19:382–393.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Torlakovic EE, Nielsen S, Vyberg M and
Taylor CR: Getting controls under control: The time is now for
immunohistochemistry. J Clin Pathol. 68:879–882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liberzon A, Birger C, Thorvaldsdóttir H,
Ghandi M, Mesirov JP and Tamayo P: The molecular signatures
database (MSigDB) hallmark gene set collection. Cell Syst.
1:417–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang SH and O'Sullivan B: Overview of the
8th edition TNM classification for head and neck cancer. Curr Treat
Options Oncol. 18:402017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beckerman P, Qiu C, Park J, Ledo N, Ko YA,
Park AD, Han SY, Choi P, Palmer M and Susztak K: Human kidney
tubule-specific gene expression based dissection of chronic kidney
disease traits. EBioMedicine. 24:267–276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang J, Kong D, Cui Q, Wang K, Zhang D,
Gong Y and Wu G: Prognostic genes of breast cancer identified by
gene Co-expression network analysis. Front Oncol. 8:3742018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosen EY, Wexler EM, Versano R, Coppola G,
Gao F, Winden KD, Oldham MC, Martens LH, Zhou P, Farese RV Jr and
Geschwind DH: Functional genomic analyses identify pathways
dysregulated by progranulin deficiency, implicating Wnt signaling.
Neuron. 71:1030–1042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akabani G, Reardon DA, Coleman RE, Wong
TZ, Metzler SD, Bowsher JE, Barboriak DP, Provenzale JM, Greer KL,
DeLong D, et al: Dosimetry and radiographic analysis of
131I-labeled anti-tenascin 81C6 murine monoclonal antibody in newly
diagnosed patients with malignant gliomas: A phase II study. J Nucl
Med. 46:1042–1051. 2005.PubMed/NCBI
|
|
34
|
Reardon DA, Akabani G, Coleman RE,
Friedman AH, Friedman HS, Herndon JE II, Cokgor I, McLendon RE,
Pegram CN, Provenzale JM, et al: Phase II trial of murine
(131)I-labeled antitenascin monoclonal antibody 81C6 administered
into surgically created resection cavities of patients with newly
diagnosed malignant gliomas. J Clin Oncol. 20:1389–1397. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cokgor I, Akabani G, Kuan CT, Friedman HS,
Friedman AH, Coleman RE, McLendon RE, Bigner SH, Zhao XG,
Garcia-Turner AM, et al: Phase I trial results of
iodine-131-labeled antitenascin monoclonal antibody 81C6 treatment
of patients with newly diagnosed malignant gliomas. J Clin Oncol.
18:3862–3872. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sarli B, Topsakal R, Kaya EG, Akpek M, Lam
YY and Kaya MG: Tenascin-C as predictor of left ventricular
remodeling and mortality in patients with dilated cardiomyopathy. J
Investig Med. 61:728–732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aoki M and Fujishita T: Oncogenic roles of
the PI3K/AKT/mTOR axis. Current Topics in Microbiology and
Immunology. 407. Springer Verlag; pp. 153–189. 2017, PubMed/NCBI
|
|
38
|
Jhanwar-Uniyal M, Amin AG, Cooper JB, Das
K, Schmidt MH and Murali R: Discrete signaling mechanisms of mTORC1
and mTORC2: Connected yet apart in cellular and molecular aspects.
Adv Biol Regul. 64:39–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim LC, Cook RS and Chen J: MTORC1 and
mTORC2 in cancer and the tumor microenvironment. Oncogene.
36:2191–2201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang F, Meng M, Mo B, Yang Y, Ji Y, Huang
P, Lai W, Pan X, You T, Luo H, et al: Crosstalks between mTORC1 and
mTORC2 variagate cytokine signaling to control NK maturation and
effector function. Nat Commun. 9:48742018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee YJ and Han HJ: Troglitazone
ameliorates high glucose-induced EMT and dysfunction of SGLTs
through PI3K/Akt, GSK-3β, Snail1, and β-catenin in renal proximal
tubule cells. Am J Physiol Renal Physiol. 298:F1263–F1275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Byers LA, Diao L, Wang J, Saintigny P,
Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, et al: An
epithelial-mesenchymal transition gene signature predicts
resistance to EGFR and PI3K inhibitors and identifies Axl as a
therapeutic target for overcoming EGFR inhibitor resistance. Clin
Cancer Res. 19:279–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao YR, Wang JL, Xu C, Li YM, Sun B and
Yang LY: HEG1 indicates poor prognosis and promotes hepatocellular
carcinoma invasion, metastasis, and EMT by activating Wnt/β-catenin
signaling. Clin Sci. 133:1645–1662. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu W, Wang Z, Zhang W, Qian K, Li H, Kong
D, Li Y and Tang Y: Mutated K-ras activates CDK8 to stimulate the
epithelial-to-mesenchymal transition in pancreatic cancer in part
via the Wnt/β-catenin signaling pathway. Cancer Lett. 356:613–627.
2015. View Article : Google Scholar : PubMed/NCBI
|