Introduction
Thymic epithelial tumors are rare malignant tumors
of thymic origin that are mainly classified as thymoma, thymic
cancer and thymic neuroendocrine tumors (1). Thymic cancer accounts for 20% of thymic
epithelial tumors and exhibits a strong metastatic behavior
(1). The average age of onset of
thymic cancer is ~50 years, with ~50-65% of patients displaying
distant metastases on diagnosis in China from 1962–2003 (2). Late and poor prognosis, and
intrathoracic and distant metastases are the main causes of death
due to thymic cancer (3). Currently,
there is no effective treatment for thymic carcinoma, and
post-treatment recurrence and tumor metastasis are the most
important factors affecting patient prognosis and quality of life
(3,4). Hence, the discovery of biomarkers and
understanding of the molecular mechanisms of tumor recurrence and
metastasis are of great significance for the diagnosis and
treatment of thymic cancer.
High mobility group (HMG) proteins are a series of
chromatin-related proteins widely present in eukaryotes and
consisting of 3 families: HMGA, HMGB and HMGN (5). HMGA2 belongs to the non-histone
chromosome HMGA family that exerts its function as a transcription
factor by altering the structure of chromatin and the interaction
of DNA and target protein (6). HMGA2
is highly expressed in embryonic and immature tissues, but is
almost absent in differentiated and mature tissues (7). Studies have found that HMGA2 is also
highly expressed in a variety of malignant tumors, such as lung
cancer, ovarian cancer and colorectal cancer (8–12), and
its enhanced expression is closely related to increased tumor
invasiveness and disease prognosis (8–12).
Hence, HMGA2 is considered as a potential tumor marker for all
types of cancer.
Epithelial-mesenchymal transition (EMT) refers to
the loss of polarity, tight junctions and adhesion connections
between cells, and it is an effective way for epithelial cells to
acquire migration ability and is the phenomenon underlying
epithelial cell carcinoma invasion and metastasis (13). EMT is not only related to normal
embryonic development, but is also closely related to tumorigenesis
and tumor development (14). Studies
have demonstrated that EMT serves a pivotal role in the primary
invasion and secondary metastasis of numerous types of cancer,
including breast, colon, lung, prostate and liver cancer (15–17).
During the occurrence of EMT, epithelial cells lose their polarity
and contact with surrounding stromal cells is reduced. Meanwhile,
cell migration and motility are enhanced and cells take on a
mesenchymal phenotype while losing their epithelial phenotype
(13). EMT can be induced by a
variety of signaling pathways, such as the transforming growth
factor-β, Wnt and Notch pathways. Wnt signaling can induce EMT in
tumor cells by inhibiting glycogen synthase kinase 3β-mediated
phosphorylation and β-catenin degradation in the cytoplasm
(18,19).
The Wnt/β-catenin signaling pathway participates in
regulating embryonic development and plays an indispensable role in
tumor generation and development (19). A study has demonstrated that the
expression of factors associated with Wnt/β-catenin in ovarian
epithelial carcinoma tissues is significantly higher compared with
that in benign ovarian tumor tissues (20). Huang et al (21) observed that the expression of
Wnt/β-catenin in nasopharyngeal tissues of patients with
nasopharyngeal carcinoma was significantly higher compared with
that in normal control tissues. The aforementioned finding suggests
that the occurrence and development of nasopharyngeal carcinoma is
highly associated with abnormal Wnt/β-catenin signaling. To the
best of our knowledge, few studies have investigated the
association between HMGA2, WNT signaling and EMT in thymic cancer.
Studies have reported that HMGA2 can affect the EMT of gastric
cancer, tongue cancer and retinoblastoma by regulating the
Wnt/β-catenin signaling pathway (22–24). Our
previous study found that with the increase in clinical staging of
thymic cancer, the expression of β-catenin gradually increased,
suggesting that Wnt/β-catenin is closely related to thymic cancer
(25). Hence, it was speculated that
the abnormal expression of HMGA2 may activate the Wnt/β-catenin
pathway and promote tumorigenesis. The present study aimed to
investigate the effect and mechanism of HMGA2 on EMT in thymic
cancer cells. HMGA2 interference vectors [small interfering
(si)-HMGA2) were constructed to study the effect of HMGA2 on EMT in
thymic cancer cells. Further molecular experiments were performed
to verify whether these effects were achieved via the Wnt/β-catenin
signaling pathway. The findings of the present study will provide
some insights for the treatment of thymic cancer.
Materials and methods
Cell culture and transfection
IU-TAB-1, a thymic cancer cell line (cat. no.
T8001), was obtained from Applied Biological Materials Inc. Cell
lines 293T, A549 and HCT-116 (normal control cell lines) were
kindly provided by the Stem Cell Bank of the Chinese Academy of
Medical Sciences. IU-TAB-1, 293T, A549 and HTC 116 cells were
cultured in RPMI 1640 (cat. no. SH30022.01B; Hyclone; Cytiva),
Prigrow II (cat. no. TM002; Applied Biological Materials Inc.),
F-12K (cat. no. 21127-022; Gibco; Thermo Fisher Scientific, Inc.)
or McCoy's 5A (cat. no. 16600-082; Gibco; Thermo Fisher Scientific
Inc.) medium, respectively, supplemented with 10% fetal bovine
serum (FBS; cat. no. 10270-106; Gibco; Thermo Fisher Scientific
Inc.) in an atmosphere containing 5% CO2 and 95% air at
37°C. The medium was replaced every 24 h and the cells were
subcultured or cryopreserved in liquid nitrogen at −196.56°C when
the confluence reached 70–80%.
The full-length cDNA of HMGA2 (NM_001300918.1) was
obtained from the National Center for Biotechnology Information
database (https://www.ncbi.nlm.nih.gov/search/all/?term=NM_001300918.1).
IU-TAB-1 cells were transfected with HMGA2 siRNA using
Lipofectamine® 2000 reagent (cat. no. 13778030;
Invitrogen; Thermo Fisher Scientific Inc.) according to the
manufacturer's instructions. Briefly, 100 pmol siRNA and 5 µl
Lipofectamine® RNAiMAX were added to 250 µl Opti-MEM at
4°C for 20 min, respectively, and then the above mixture was
incubated at 37°C in a 5% CO2 incubator for 48 h. The
cell experiments were divided into 5 groups: i) Control
(untransfected cells); ii) HMGA2-siRNA1; iii) HMGA2-siRNA2; iv)
HMGA2-siRNA3; and v) non-targeting negative control. The
transfection efficiency was detected by reverse
transcription-quantitative PCR (RT-qPCR). Wnt signaling antagonist
(XAV-939; cat. no. HY-15147; 4 µM) and agonist (SKL2001; cat. no.
HY-101085; 30 µM) were purchased from MCE. XAV-939 and SKL2001 were
added after the cells were stably transfected for 48 h and cultured
in a constant temperature incubator at 37°C for 48 h.
Cell Counting Kit-8 assay
IU-TAB-1 cells were seeded in a 96-well plate at a
density of 5×103 cells/ml in Prigrow II medium
containing 10% FBS. Following 48 h of treatment, 10 µl Cell
Counting Kit-8 (CCK-8) solution (cat. no. C1706; Bioswamp Wuhan
Bienle Biotechnology, Co., Ltd.) was added to each well and the
cells were cultured at 37°C for 4 h. Cell proliferation was
examined by measuring the optical density at 450 nm using a plate
reader (Multiskan FC; Thermo Fisher Scientific Inc.).
Transwell migration and invasion
assays
IU-TAB-1 cells were cultured in serum-free Prigrow
II medium for 24 h and resuspended in Prigrow II medium containing
1% FBS. Subsequently, the cells were seeded into Transwell chambers
at 1×105 cells/ml, while 0.75 ml of Prigrow II medium
containing 10% FBS was added into the lower chambers. The plate was
incubated in 5% CO2 at 37°C for 48 h. Subsequently, 1 ml
of 4% formaldehyde solution was added to each well, and the plate
was incubated at 4°C for 10 min for immobilization. Following 30
min of incubation at room temperature with 0.5% crystal violet
solution (cat. no. C1701; Bioswamp Wuhan Bienle Biotechnology Co.
Ltd.), the cells were observed under a fluorescent microscope
(magnification, ×200).
For cell invasion assay, the upper chambers were
pre-coated with 80 µl of Matrigel (cat. no. 356234; BD
Biosciences). The chambers were incubated at 37°C for 30 min for
gel formation and hydrated in 1% FBS for 4 h before use. In the
lower chambers, 750 µl Dulbecco's modified Eagle's medium (DMEM)
containing 10% FBS was added. Subsequently, IU-TAB-1 cells were
added to the upper chambers at a density of 1×105
cells/well and incubated for 48 h at 4°C. Next, 1 ml of 4%
paraformaldehyde (cat. no. 10010018; Sinopharm Chemical Reagent,
Co. Ltd.) and 1 ml of 0.5% crystal violet (cat. no. C1701; Bioswamp
Wuhan Bienle Biotechnology Co. Ltd.) were added at room
temperature, and the invaded cells were counted under a fluorescent
microscope.
Flow cytometry
Both early and late stages of apoptosis rate of
IU-TAB-1 cells was analyzed using flow cytometry according to the
manufacturer's instructions. IU-TAB-1 cells (1×106/ml)
were cultured for 24 h in 37°C and harvested. Subsequently, 1 ml of
pre-cooled PBS was added and the cells were centrifuged at 1,000 ×
g. Then, 10 µl of Annexin V-FITC (cat. no. 556547; BD Biosciences)
and 10 µl of PI (cat. no. 556547; BD Biosciences) were added. The
data were analyzed using CytExpert software v.2.0 (Beckman Coulter,
Inc.).
Immunofluorescence
IU-TAB-1 cells (1×106) were fixed in 4%
paraformaldehyde for 30 min at room temperature. After washing
twice with pre-cooled phosphate-buffered saline (PBS), the cells
were permeabilized in 5% Triton X-100 (cat. no. CB1701; Bioswamp
Wuhan Bienle Biotechnology Co. Ltd.) for 20 min and blocked with 5%
bovine serum albumin at 37°C for 1 h. The cells were then incubated
with antibodies against β-catenin (1:200; cat. no. MAB37201;
Bioswamp Wuhan Bienle Biotechnology Co., Ltd.) overnight at 4°C,
followed by incubation with Alexa Fluor 594-conjugated Goat
Anti-Rabbit (1:200; cat. no. PAB160018; Bioswamp Wuhan Bienle
Biotechnology Co. Ltd.) for 30 min at room temperature and
counterstained with 4′,6-diamidino-2-phenylindole to identify the
nuclei at 4°C for 5 min. Images were captured with a fluorescence
microscope (DMIL LED; Leica Microsystems GmbH).
RT-qPCR
Total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific
Inc.) according to the manufacturer's instructions. cDNA was
synthesized using a reverse transcriptase kit (cat. no. 639505;
Takara Bio Inc.). qPCR was performed with a CFX-Connect 96
real-time system (Bio-Rad Laboratories Inc.) using the SYBR Green
PCR kit (cat. no. KM4101; KAPA Biosystems; Roche Diagnostics). qPCR
was performed in duplicate and the thermocycling conditions were as
follows: 95°C for 3 min for denaturation; 39 cycles of denaturation
at 95°C for 5 sec, 56°C for 10 sec and 72°C for 25 sec; and 65°C
for 5 sec and 95°C for 50 sec for annealing and extension. The
results were analyzed by the 2−∆∆Cq method (26). GAPDH was used as the reference gene.
The primers were designed and configured by Wuhan Tianyi Huayu gene
Biotechnology Co. Ltd. and are listed in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Primer | Sequence
(5′-3′) |
|---|
| HMGA2-F |
TTCAGCCCAGGGACAA |
| HMGA2-R |
CCAGGCAAGGCAACAT |
| GAPDH-F |
CCACTCCTCCACCTTTG |
| GAPDH-R |
CACCACCCTGTTGCTGT |
Western blotting
Following 48 h of treatment, the IU-TAB-1
(1×106/ml) cells were washed with cold PBS and lysed
using a lysis buffer (cat. no. 180006; Bioswamp Wuhan Bienle
Biotechnology Co., Ltd.), and the proteins were quantified by the
bicinchoninic acid assay kit (cat. no. 180007; Bioswamp Wuhan
Bienle Biotechnology Co., Ltd.). The proteins (20 µg protein per
lane) were separated on a 12% gel using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
a buffer containing 5% skimmed milk in PBS with 0.05% Tween-20 for
2 h at room temperature and incubated overnight at 4°C with primary
antibodies against HMGA2 (1:1,000; cat. no. PAB40807; Bioswamp
Wuhan Bienle Biotechnology Co. Ltd.), Wnt3a (1:1,000; cat. no.
PAB30170; Bioswamp Wuhan Bienle Biotechnology Co. Ltd.), Wnt5a
(1:1,000 cat. no. PAB37965; Bioswamp Wuhan Bienle Biotechnology
Co., Ltd.), β-catenin (1:1,000; cat. no. MAB37201; Bioswamp Wuhan
Bienle Biotechnology Co., Ltd.), E-cadherin (1:1,000, cat. no.
PAB33542; Bioswamp Wuhan Bienle Biotechnology Co. Ltd.), vimentin
(1:1,000; cat. no. PAB40646; Bioswamp Wuhan Bienle Biotechnology
Co., Ltd.) and GAPDH (1:2,000; cat. no. PAB36264; Bioswamp Wuhan
Bienle Biotechnology Co., Ltd.). Following 3 washes with PBS/10%
Tween-20, the membranes were incubated with horseradish
peroxidase-conjugated secondary goat rabbit IgG (1:20,000; cat. no.
PAB160011; Bioswamp Wuhan Bienle Biotechnology Co., Ltd.) for 2 h
at room temperature. Protein bands were visualized by enhanced
chemiluminescence color detection (Tanon-5200; Tanon Science and
Technology Co., Ltd.) and analyzed using GIS software v.4.2 (Tanon
Science and Technology Co., Ltd.).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed by one-way analysis
of variance followed by the post-hoc Tukey's post-hoc test using
SPSS 19.0 software (IBM Corp.). All figures were prepared using
GraphPad Prism 5.0 software (Graph Pad Software Inc.). P<0.05
was considered to indicate statistical significance and all
statistical analyses were based on 3 independent experiments.
Results
HMGA2 expression in IU-TAB-1, A549,
HCT-116 and 293T cells
As shown in Fig. 1,
compared with that in 293T cells, the protein expression of HMGA2
was significantly increased in IU-TAB-1, A549 and HCT-116 cells
(P<0.05), with IU-TAB-1 cells demonstrating the highest
expression. Hence, IU-TAB-1 cells were used for subsequent
experimentation.
Controls for interference
expression
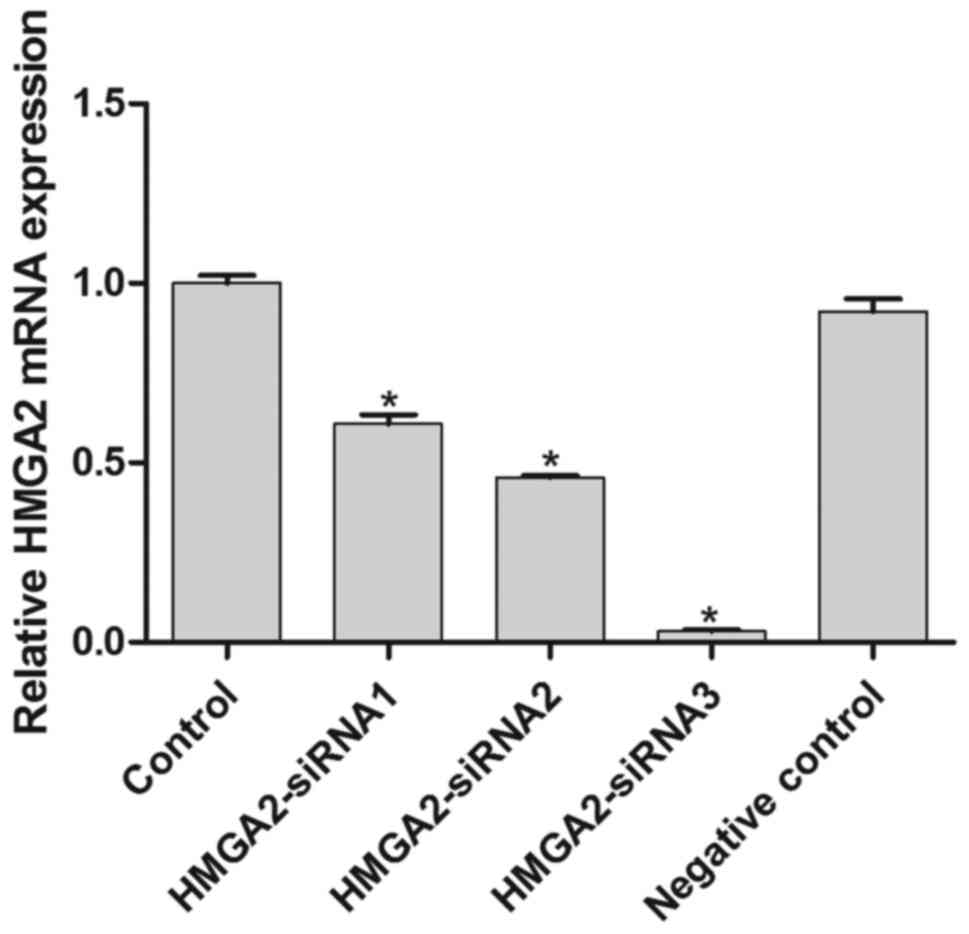
mRNA expression of HMGA2 in the control,
HMGA2-siRNA1, HMGA2-siRNA2, HMGA2-siRNA3 and negative control
groups was observed to confirm HMGA2 inhibition by the interference
vector (Fig. 2). The results
confirmed that all 3 HMGA2 siRNAs significantly downregulated the
expression of HMGA2 compared with the control and negative control
IU-TAB-1 cells (P<0.05; Fig. 2).
HMGA2-siRNA3 induced the lowest HMGA2 expression among the 3 siRNAs
and was hence used to silence HMGA2 in the subsequent
experiments.
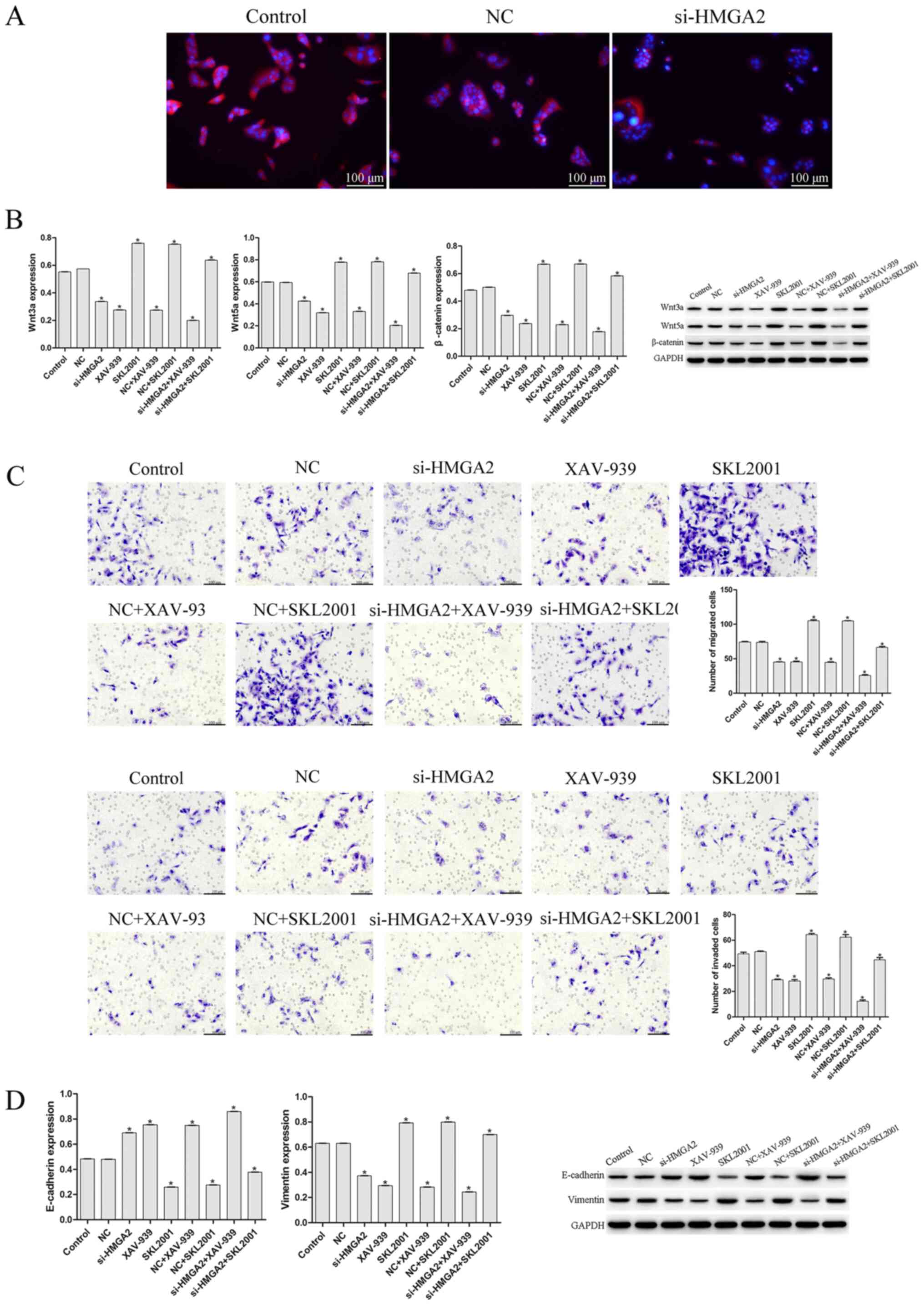
si-HMGA2 attenuates EMT in IU-TAB-1
cells
To observe the effect of HMGA2 on cell
proliferation, migration and invasion, CCK-8 and Transwell assays
were performed (Fig. 3A-C). Compared
with the control, si-HMGA2 significantly suppressed cell
proliferation, migration and invasion (P<0.05; Fig. 3A, B and D). Flow cytometry revealed
that si-HMGA2 increased the apoptotic rate of IU-TAB-1 cells
compared with control and negative control cells (Fig. 3D). The expression of EMT-related
proteins (E-cadherin, vimentin, Wnt3a, Wnt5a, and β-catenin) was
further assessed (Fig. 3E). The
protein expression of vimentin, Wnt3a, Wnt5a and β-catenin were
significantly decreased by si-HMGA2 (P<0.05; Fig. 3E), whereas that of E-cadherin was
increased significantly compared with the control group (P<0.05;
Fig. 3E), which indicated that HMGA2
silencing inhibited EMT in IU-TAB-1 cells.
si-HMGA2 attenuates EMT in IU-TAB-1
cells via the Wnt/β-catenin pathway
Immunofluorescence was conducted to observe the
protein expression of β-catenin. Fig.
4A reveals that si-HMGA2 induced lower expression of β-catenin
compared with that in the control and negative control groups.
Proteins associated with the Wnt/β-catenin pathway were
subsequently assessed using western blotting, which demonstrated
that si-HMGA2 significantly downregulated the expression of Wnt3a,
Wnt5a, vimentin and β-catenin compared with the control group
(P<0.05; Fig. 4B), and
upregulated the levels of E-cadherin, suggesting that si-HMGA2
inhibited the activation of Wnt/β-catenin signaling. To further
study whether si-HMGA2 affects EMT in IU-TAB-1 cells by regulating
the Wnt/β-catenin pathway, Wnt/β-catenin agonists or inhibitors
were applied (Fig. 4B and C).
Compared with the control and NC, the migration, invasion and
expression of vimentin of cells treated with Wnt/β-catenin agonists
were increased (P<0.05; Fig. 4B and
C), whereas E-cadherin expression was significantly decreased
(P<0.05; Fig. 4B and C). On the
other hand, the combination of si-HMGA2 with Wnt/β-catenin
inhibitors suppressed the migration, invasion, and expression of
vimentin of IU-TAB-1 cells (P<0.05; Fig. 4B and C), whereas E-cadherin
expression was increased significantly compared to the control and
NC group (P<0.05; Fig. 4B and
C).
Discussion
The human HMGA2 gene is located on the chromosome
band 12q14-15 and contains at least 5 exons distributed in the
≥160-kb genomic region (6). HMGA2 is
almost undetectable in healthy adult tissues; however, HMGA2
upregulation has been detected in breast cancer (27), sarcoma (28), pancreatic cancer (29) and non-small cell lung cancer
(30) tissues. Whether HMGA2 is also
upregulated in thymic cancer has not been studied to the best of
our knowledge. IU-TAB-1, A549 and HCT-116 are common tumor cell
lines (31–33). Studies have demonstrated that HMGA2
is highly expressed in a variety of malignant tumors, including
lung, colon and ovarian cancer (8–12), and
its enhanced expression is closely related to enhanced tumor
aggressiveness and disease prognosis (8–12).
Hence, we speculated that the occurrence and development of thymic
cancer is also related to the abnormally high expression of HMGA2.
Hence, in the present study, A549, HCT-116 and 293T cells were used
as controls. The results of the present study demonstrated that
HGMA2 expression in IU-TAB-1 cells was significantly higher
compared with that in A549 and HCT-116 cells, which suggested that
HMGA2 expression was significantly increased in thymic cancer
cells.
E-cadherin loss is a prominent feature of cellular
EMT (34). Decreased E-cadherin
levels can lead to reduced cell adhesion and allow cells to acquire
characteristics that enable invasion and metastasis (35). Studies have reported mutations in the
E-cadherin gene or downregulation of E-cadherin expression in lung,
breast, gastric and other epithelial cancer types (36,37).
Vimentin is one of the main components of the medium fibers of
fibroblasts (38). When E-cadherin
is lost, the expression of vimentin and N-cadherin increases and
cells acquire an interstitial phenotype (39). The present study demonstrated that
HMGA2 silencing upregulated the expression of E-cadherin and
downregulated that of vimentin, which suggested that inhibition of
HMGA2 suppressed EMT in thymic cancer cells. Cell infiltration is
an important feature of malignant tumors, wherein EMT serves an
important role (13). Cells
undergoing EMT can grow on and penetrate Matrigel, revealing that
EMT may be an important factor for tumor cells to break through the
basement membrane (40). In clinical
treatment, tumor cells metastasize to other sites through blood
vessels and lymphatic vessels, representing further tumor
deterioration and a poor clinical prognosis. E-cadherin expression
is inversely related to the degree of tumor differentiation and
lymph node metastasis (39). After
injection of E-cadherin-deficient tumor cells into nude mice,
carcinogenicity and metastasis were significantly enhanced
(41). In the present study, HMGA2
silencing inhibited the proliferation, migration and invasion of
thymic cancer cells, while promoting cell apoptosis. In the present
study, further evaluation of the expression of EMT-associated
proteins demonstrated that when HMGA2 was suppressed, E-cadherin
was upregulated and vimentin was downregulated, which suggested
that inhibition of HMGA2 suppressed EMT in thymic cancer cells.
β-catenin is the core molecule of the Wnt pathway
and its accumulation in the cytoplasm is the key to Wnt/β-catenin
activation (42). When Wnt signaling
is activated, the Wnt protein binds to the extracellular domain of
frizzled protein. β-catenin cannot be degraded, and a large amount
of free β-catenin accumulates in the cytoplasm, enters the nucleus
and combines with the transcription factor T cytokine/lymphocyte
enhancing factor to regulate cell proliferation and apoptosis
(43). Qin et al (44) demonstrated that the expression of
β-catenin in SNK-6 and YTS cell lines was significantly higher
compared with that in normal natural killer cells and was
significantly higher in natural-killer/T cell lymphoma tissues
compared with reactive hyperplasia of lymph nodes. Ebert et
al (45) demonstrated that
β-catenin expression was increased in gastric cancer tissues, and a
β-catenin gene mutation was detected compared to the normal gastric
tissue. Shi and Yin (46) studied
the expression of β-catenin and hepatocyte nuclear factor-1α in
hepatocellular carcinoma tissues and their effects on the prognosis
of hepatocellular carcinoma. The results of the aforementioned
study revealed that the clinical prognosis of patients with
abnormal β-catenin expression was significantly worse compared with
that of patients with normal β-catenin expression, which suggested
that abnormal expression of β-catenin was related to the
development of hepatocellular carcinoma (46). The findings of the present study
revealed that when the expression of HMGA2 was silenced, β-catenin
was downregulated, suggesting that HMGA2 inhibition reduced
β-catenin accumulation of thymic cancer cells. The present study
further examined the effect of si-HMGA2 on the Wnt/β-catenin
signaling pathway and demonstrated that HMGA2 silencing inhibited
Wnt/β-catenin signaling. This finding indicated that the effect of
HMGA2 on EMT in thymic cancer cells may be achieved by regulating
the activity of Wnt/β-catenin signaling. Inhibiting HMGA2 activity
inhibited the EMT activity of HMGA2 thymogenic cancer cells in the
present study. In the present study, cells were treated with
si-HMGA2 in combination with Wnt/β-catenin agonists (SKL2001) or
inhibitors (XAV-939), and the changes in EMT in thymic cancer cells
were observed. In the present study, cell migration and invasion
and vimentin expression were significantly enhanced by
Wnt/β-catenin agonists compared with that in control cells, while
the expression of E-cadherin was significantly decreased.
Correspondingly, Wnt/β-catenin inhibitors demonstrated the opposite
effect. These findings demonstrated that HMGA2 inhibition
suppressed Wnt/β-catenin activation and inhibited EMT in thymic
cancer cells, providing a potential therapeutic strategy for the
clinical treatment of thymic cancer.
The present study had several limitations. Firstly,
E-cadherin is the only bona fide Wnt target investigated in
the present study, hence the association between HMGA-2 and Wnt
signaling requires further investigation. T cell factor/lymphoid
enhancer factor activity should be detected in future studies.
Secondly, the use of just one thymic cell line is a limitation of
the present study. More cell lines and clinical samples should be
investigated in future studies to verify the findings of the
present study.
In conclusion, HMGA2 may be a key protein that
regulates EMT in thymic cancer cells. In the present study,
inhibition of HMGA2 significantly attenuated cell proliferation,
migration and invasion, and promoted apoptosis, and this mechanism
may be related to Wnt/β-catenin signaling.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data used in this study are available from the
corresponding author upon request.
Authors' contributions
ST and JC performed the experiments, collected and
analyzed the data. ST drafted the manuscript. JC revised the
manuscript for important intellectual content. ST and JC confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Noriyuki T: Thymic epithelial tumors.
Haigan. 47:181–185. 2007. View Article : Google Scholar
|
|
2
|
Ji W, Feng QF, Zhou ZM, Wang M, Chen DF,
Zhang HX, Xiao ZF, Wang LH and Yin WB: Treatment and prognosis
analysis of 73 cases of thymic carcinoma. Chin J Radia Oncol.
15:97–100. 2006.
|
|
3
|
Kondo K and Monden Y: Therapy for thymic
epithelial tumors: A clinical study of 1,320 patients from Japan.
Ann Thorac Surg. 76:878–884; discussion 884–885. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamarca A, Moreno V and Feliu J: Thymoma
and thymic carcinoma in the target therapies era. Cancer Treat Rev.
39:413–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozturk N, Singh I, Mehta A, Braun T and
Barreto G: HMGA proteins as modulators of chromatin structure
during transcriptional activation. Front Cell Dev Biol. 2:52014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narita M, Narita M, Krizhanovsky V, Nuñez
S, Chicas A, Hearn SA, Myers MP and Lowe SW: A novel role for
high-mobility group a proteins in cellular senescence and
heterochromatin formation. Cell. 126:503–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cleynen I and Van de Ven WJ: The HMGA
proteins: A myriad of functions (Review). Int J Oncol. 32:289–305.
2008.PubMed/NCBI
|
|
8
|
Di Cello F, Hillion J, Hristov A, Wood LJ,
Mukherjee M, Schuldenfrei A, Kowalski J, Bhattacharya R, Ashfaq R
and Resar LM: HMGA2 participates in transformation in human lung
cancer. Mol Cancer Res. 6:743–750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malek A, Bakhidze E, Noske A, Sers C,
Aigner A, Schäfer R and Tchernitsa O: HMGA2 gene is a promising
target for ovarian cancer silencing therapy. Int J Cancer.
123:348–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang ML, Chen CC and Chang LC: Gene
expressions of HMGI-C and HMGI(Y) are associated with stage and
metastasis in colorectal cancer. Int J Colorectal Dis.
24:1281–1286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hristov AC, Cope L, Reyes MD, Singh M,
Iacobuzio-Donahue C, Maitra A and Resar LM: HMGA2 protein
expression correlates with lymph node metastasis and increased
tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol.
22:43–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartuma H, Panagopoulos I, Collin A,
Trombetta D, Domanski HA, Mandahl N and Mertens F: Expression
levels of HMGA2 in adipocytic tumors correlate with morphologic and
cytogenetic subgroups. Mol Cancer. 8:362009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng P, Zheng CN, Ben W, Gao HJ, Niu WB,
He ZB, Gao C, Zou ZJ, Ma C, Niu J, et al: Norcantharidin inhibit
colon cancer cell EMT process through blocking αvβ6-ERK-ETS1 signal
pathway. Chin J Curr Adv Gen Surg. 10:757–762. 2014.(In
Chinese).
|
|
17
|
Lin Q, Zhou CR, Bai MJ, Zhu D, Chen JW,
Wang HF, Li MA, Wu C, Li ZR and Huang MS: Exosome-mediated miRNA
delivery promotes liver cancer EMT and metastasis. Am J Transl Res.
12:1080–1095. 2020.PubMed/NCBI
|
|
18
|
Zha L, Zhang J, Tang WX, Zhang N, He M,
Gao Y and Wang ZW: HMGA2 elicits EMT by activating the
Wnt/β-catenin pathway in gastric cancer. Dig Dis Sci. 58:724–733.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eugenio Z, Gabri VDP, Gray PC and Marianna
KJ: Epithelial plasticity in cancer: unmasking a microRNA network
for TGF-β- and Notch-, and Wnt-mediated EMT. J Oncol.
2015:1989672015. 2015.PubMed/NCBI
|
|
20
|
Li Y, Ma L, Zhang YL, Jiang T, Bi GB and
Sao C: Expression and clinical significance of β-catenin and axin
in ovarian epithelial cell carcinoma. J Modern Integ Med.
26:2871–2873+2957. 2017.PubMed/NCBI
|
|
21
|
Huang XW, Cui WW, Wang BF, Song YL, Liang
YJ and Dong Y: Abnormal activation of wnt/β-catenin signaling
pathway in nasopharyngeal carcinoma. J Tongji Med Univ. 46:295–298.
2017.
|
|
22
|
Zha L, Zhang J, Tang W, Zhang N, He M, Guo
Y and Wang Z: HMGA2 elicits EMT by activating the Wnt/β-catenin
pathway in gastric cancer. Dig Dis Sci. 58:724–733. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan CB, Zhao XP and Zhang H: High
expression of HMGA2 promotes metastasis of tongue carcinoma through
EMT. The 13th National Conference on Oral and Maxillofacial
Surgery.
|
|
24
|
Li W, Wang J, Zhang D, Zhang X, Xu J and
Zhao L: MicroRNA-98 targets HMGA2 to inhibit the development of
retinoblastoma through mediating Wnt/β-catenin pathway. Cancer
Biomark. 25:79–88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan S, Zhang QG, Zhang L, Liu N and Cheng
Y: Expression and clinical significance of β-catenin and c-myc in
invasive thymoma. J Chin Med Univ. 34:464–465. 2005.
|
|
26
|
Livak KJ and Schmittgen TDL: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rogalla P, Drechsler K, Kazmierczak B,
Rippe V, Bonk U and Bullerdiek J: Expression of HMGI-C, a member of
the high mobility group protein family, in a subset of breast
cancers: Relationship to histologic grade. Mol Carcinog.
19:153–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berner JM, Meza-Zepeda LA, Kools PF, Forus
A, Schoenmakers EF, Van de Ven WJ, Fodstad O and Myklebost O:
HMGIC, the gene for an architectural transcription factor, is
amplified and rearranged in a subset of human sarcomas. Oncogene.
14:2935–2941. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abe N, Watanabe T, Suzuki Y, Matsumoto N,
Masaki T, Mori T, Sugiyama M, Chiappetta G, Fusco A and Atomi Y: An
increased high-mobility group A2 expression level is associated
with malignant phenotype in pancreatic exocrine tissue. Br J
Cancer. 89:2104–2109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meyer B, Loeschke S, Schultze A, Weigel T,
Sandkamp M, Goldmann T, Vollmer E and Bullerdiek J: HMGA2
overexpression in non-small cell lung cancer. Mol Carcinog.
46:503–511. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arsianti A, Fadilah F, Bahtiar A and
Tanimoto H: Phytochemical analysis and in vitro cytotoxicity of
seaweed sargassum sp. Against colon HCT-116 and Lung-A549 cancer
cells. Proceedings of The 18th World Congress of Basic and Clinical
Pharmacology (WCP2018). pp. WCP2018Kyoto: 2018
|
|
32
|
Gökmen-Polar Y, Sanders KL, Goswami CP,
Cano OD, Zaheer NA, Jain RK, Kesler KA, Nelson RP Jr, Vance GH,
Smith D, et al: Establishment and characterization of a novel cell
line derived from human thymoma AB tumor. Lab Invest. 92:1564–1573.
2012. View Article : Google Scholar
|
|
33
|
Lee HS, Jang HJ, Lo EM, Truong CY, Groth
SS, Friedberg JS, Sugarbaker DJ and Burt BM: Povidone-iodine
results in rapid killing of thymic epithelial tumour cells through
cellular fixation†. Interact Cardiovasc Thorac Surg. 28:353–359.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Theys J, Jutten B, Habets R, Paesmans K,
Groot AJ, Lambin P, Wouters BG, Lammering G and Vooijs M:
E-Cadherin loss associated with EMT promotes radioresistance in
human tumor cells. Radiother Oncol. 99:392–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fransvea E, Angelotti U, Antonaci S and
Giannelli G: Blocking transforming growth factor-beta up-regulates
E-cadherin and reduces migration and invasion of hepatocellular
carcinoma cells. Hepatology. 47:1557–1566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bussemakers MJG, van Moorselaar RJ,
Giroldi LA, Ichikawa T, Isaacs JT, Takeichi M, Debruyne FM and
Schalken JA: Decreased expression of E-cadherin in the progression
of rat prostatic cancer. Cancer Res. 52:2916–2922. 1992.PubMed/NCBI
|
|
37
|
Fearon ER: Cancer: Context is key for
E-cadherin in invasion and metastasis. Curr Biol. 29:R1140–R1142.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun S, Sun W, Xia L, Liu L, Du R, He L, Li
R, Wang H and Huang C: The T-box transcription factor Brachyury
promotes renal interstitial fibrosis by repressing E-cadherin
expression. Cell Commun Signal. 12:762014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Z, Jin H, Yang S, Zhang ZY, Wen B and
Zhou SB: SDC1 knockdown induces epithelial-mesenchymal
transition and invasion of gallbladder cancer cells via the
ERK/Snail pathway. J Inter Med Res.
8:3000605209478832020.PubMed/NCBI
|
|
41
|
Telford BJ, Chen A, Beetham H, Frick J,
Brew TP, Gould CM, Single A, Godwin T, Simpson KJ and Guilford P:
Synthetic lethal screens identify vulnerabilities in GPCR signaling
and cytoskeletal organization in E-Cadherin-Deficient cell. Mol
Cancer Ther. 14:1213–1223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Clevers H: Wnt/β-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang D, Nai MY, Chen JZ and Wei LX:
Effect of silencing HMGA2 on signal transduction pathway of
Wnt/β-catenin in gastric cancer cells. World Chin J Dig.
21:1062–1069. 2013.(In Chinese). View Article : Google Scholar
|
|
44
|
Qin BB, Li YQ, Li XL, Lu LS, Zhang XD and
Zhang MZ: Expression of Wnt/β-catenin pathway key molecules in NK/T
cell lymphoma and its clinical significance. J Jilin Univ.
41:230–234. 2015.
|
|
45
|
Ebert MP, Fei G, Kahmann S, Müller O, Yu
J, Sung JJ and Malfertheiner P: Increased beta-catenin mRNA levels
and mutational alterations of the APC and beta-catenin gene are
present in intestinal-type gastric cancer. Carcinogenesis.
23:87–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi JF and Yin F: Expression of β-catenin
and hepatocyte nuclear factor-1 α in HCC and its effect on
prognosis. Chin J Oncol. 36:587–591. 2014.PubMed/NCBI
|