Introduction
Lung cancer (LC) is a life-threatening disease with
1,735,350 new cancer cases diagnosed and 609,640 mortality cases in
2018 in the United States and ranks second among all types of
cancer worldwide (1,2). The major risk factor for LC is smoking,
which accounts for 75–80% of LC-associated mortality (3). Non-small cell lung cancer (NSCLC)
accounts for 85% of LC cases and can be further divided into two
main subtypes, including lung squamous cell carcinoma (LUSC) and
lung adenocarcinoma (LUAD) (4). The
occurrence and development of NSCLC involve a multistep
carcinogenic process, which includes numerous variations of gene
expression, for instance, V-Ki-ras2 Kirsten rat sarcoma viral
oncogene homolog, and signal transduction, including nuclear
factor-κB (NF-κB), ERK1/2 and AKT (5). The combination of surgery and
chemotherapy remains the primary treatment method for patients with
NSCLC (6). Despite significant
improvements in the detection, diagnosis and targeted therapy, the
5-year survival rate of patients with NSCLC is only ~15% (7). The underlying mechanisms promoting the
development of NSCLC are very specific and require further
investigation in order to develop novel therapeutic strategies.
MicroRNAs (miRNAs) are non-coding RNA molecules of
~20 nucleotides in length that bind to the 3′ untranslated region
(UTR) of the mRNA. This miRNA-mRNA interaction results in mRNA
degradation or translation repression, which regulates target gene
expression (8). Furthermore, it has
been verified that miRNAs serve as regulators in multiple
physiological and pathological conditions (9). The expression of specific miRNAs is
commonly dysregulated in tumor tissues, indicating that miRNAs may
serve as oncogenes or tumor suppressors according to their target
genes (10), leading to the
regulation of cell proliferation, cell death and metastasis
(11). Based on their functions,
miRNAs are considered as therapeutic agents (8,12).
Numerous miRNAs have been reported to be involved in
the development of NSCLC. For example, miR-486 was found to be
associated with the overall survival of patients with
advanced-stage NSCLC (13), whereas
miRNA-148a can inhibit NSCLC cell invasion and migration in
vitro as well as cancer metastasis in vivo (14). Furthermore, based on the analysis of
the miRNA expression profile from the peripheral blood of healthy
controls and patients with LC, miR-4491 was shown to be increased
(15). However, its role in the
development of NSCLC remains unknown.
Materials and methods
Collection of tissue samples
Tumor and matched normal tissues were collected from
78 patients (62 men and 16 women) diagnosed with NSCLC at the
Shanxi Provincial Cancer Hospital between March 2016 and October
2018. The age range of patients was 45–76 years, 33 patients were
≤65 years and 45 patients were >65 years. The
clinicopathological characteristics of patients are listed in
Table I. A total of 52 patients were
at I–II stages, 26 patients were at III–IV stages, 44 patients were
diagnosed with LUAD, 34 patients were diagnosed with LUSC, 37
patients had no lymph node metastasis and 41 patients exhibited
lymph node metastasis. All patients provided written informed
consent prior to study enrollment. The present study was approved
by the Ethics Committee of Shanxi Provincial Cancer Hospital (IRB
no. SXPCP20160302).
 | Table I.Clinicopathological characteristics
of patients with non-small cell lung cancer. |
Table I.
Clinicopathological characteristics
of patients with non-small cell lung cancer.
|
Characteristics | Patient number |
|---|
| Age |
|
|
≤65 | 33 |
|
>65 | 45 |
| Sex | 62 |
|
Male | 16 |
|
Female |
|
| TNM stage |
|
|
I–II | 52 |
|
III–IV | 26 |
| Pathological
type |
|
|
Adenocarcinoma | 44 |
|
Squamous cell carcinoma | 34 |
| Lymph node
metastasis |
|
| No | 37 |
|
Yes | 41 |
Cell lines
The bronchial epithelium transformed cell line
BEAS-2B and the LUAD cell lines A549 and NCI-H1650 were purchased
from the American Type Culture Collection. The cells were
maintained in RPMI-1640 medium (HyClone; Cytiva), which was
supplemented with 10% fetal bovine serum (HyClone) and placed at
37°C in a humidified incubator containing 5% CO2.
Cell transfections
miR-negative control mimic (NC, 50 nM,
5′-UAGUCUCGGGAGACUCACUACC-3′), miR-NC inhibitor (50 nM,
5′-UAACCGAAUUCACAUGGUCCUA-3′), miR-4491 inhibitor (50 nM,
5′-UUUGGUCACACCAGUCCACAUU-3′), miR-4491 mimic (50 nM,
5′-AAUGUGGACUGGUGUGACCAAA-3′), si-control (100 nM,
5′-TTCTCCGAACGTGTCACGTTT-3′) and si-Tripartite motif containing 7
(TRIM7, 100 nM, 5′-CGGAAAAGAAGGAGAGCAA-3′) were synthesized by
GenePharma. Cell transfections were performed by Lipofectamine
2000® solution (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Transfections
were performed at 37°C for 48 h and cells were collected for
subsequent experiments.
miR-4491 expression profile
The Starbase V2.0 project (http://starbase.sysu.edu.cn/) was used for the
analysis of miR-4491 expression levels in NSCLC and normal tissues
from The Cancer Genome Atlas (TCGA) datasets (TCGA-LUAD and
TCGA-LUSC) (16).
mRNA and miRNA quantification
Total RNA was isolated from tumor and normal tissues
derived from patients with NSCLC and from BEAS-2B, A549 and
NCI-H1650 cells using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA synthesis was performed using TaqMan
microRNA reverse transcription kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and TransScript First-Strand cDNA
Synthesis SuperMix (Beijing TransGen Biotech Co., Ltd.). Reverse
transcription-quantitative PCR (RT-qPCR) experiments were performed
on an ABI7500 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) under the following conditions: 95°C for 5 min, followed by
40 cycles of 95°C for 10 sec, 60°C for 30 sec and 72°C for 1 sec.
miR-4491 levels were normalized to those of U6. TRIM7 levels were
normalized to those of GAPDH. The primers were synthetized by
GenScript. The sequences of primers were as follows: GAPDH, forward
5′-GAAGGTGAAGGTCGGAGTC-3′, reverse 5′-GAAGATGGTGATGGGATTTC-3′; U6,
forward 5′-GCTTCGAGGCAGGTTACATG-3′, reverse
5′-GCAACACACAACATCTCCCA-3′; TRIM7, forward
5′-ATTATATAGGGTGTCCACATA-3′, reverse 5′-TATGTGGACACCCTATATAAT-3′;
miR-4491, forward 5′-AATGTGGACTGGTGTGACCAAA-3′ and reverse
5′-TTTGGTCACACCAGTCCACATT-3′. The relative expression levels were
normalized to endogenous control and were expressed as
2−ΔΔCq (17).
Western blotting
A549 and NCI-H1650 cells were lysed using RIPA on
ice buffer (Sigma-Aldrich; Merck KGaA). The protein concentration
was determined using the BCA method (Sigma-Aldrich; Merck KGaA).
Proteins (20 µg) were separated by 8% SDS-PAGE and were transferred
onto PVDF membranes. Membranes were blocked by 5% skimmed milk for
2 h at room temperature and incubated with primary antibodies
against TRIM7 (cat. no. ab105330; 1:1,000; Abcam), p65 (cat. no.
ab32536; 1:1,000; Abcam) and β-actin (cat. no. 4970; 1:1,000; Cell
Signaling Technology, Inc.) overnight at 4°C. Membranes were then
incubated with goat-anti-rabbit secondary antibody (cat. no. 7074;
1:2,000, Cell Signaling Technology, Inc.) for 2 h at room
temperature. The protein blots were developed by ECL Western Blot
Kit (Pierce; Thermo Fisher Scientific, Inc.). Band intensities were
analyzed by Image Lab™ (version 4.0; Bio-Rad Laboratories, Inc.).
Relative expression levels were normalized to endogenous control
β-actin using ImageLab™ (version 3.0; Bio-Rad Laboratories,
Inc.).
Cell proliferation assay
The proliferation of A549 and NCI-H1650 cells was
evaluated using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.). Briefly, A549 and NCI-H1650 cells
(3×104) were seeded in 96-well plates and incubated at
37°C for 48 h. Subsequently, cells were incubated with CCK-8
reagent (10 µl) for 3 h at 37°C. The absorbance was measured at 450
nm by spectrophotometry.
Cell apoptosis assay
A549 and NCI-H1650 cell apoptosis was assessed with
the Annexin V-FITC/propidium (PI) apoptosis detection kit
(Sigma-Aldrich; Merck KGaA). A549 and NCI-H1650 cells
(1×106) were seeded in 6-well plates and incubated at
37°C for 48 h. Subsequently, cells were stained with Annexin V (5
µl) and PI (1 µl) for 10 min at room temperature in the dark. The
number of apoptotic cells was estimated by a FACSCalibur flow
cytometer (BD Biosciences). The data were analyzed by the CellQuest
software (version 3.3; BD Biosciences).
Prediction of target genes
miRWalk (http://mirwalk.umm.uni-heidelberg.de/) and TargetScan
(http://www.targetscan.org/vert_72/)
softwares were used to predict miR-4491 targets. The targets
predicted by miRWalk were further selected for score analysis. A
score of 1.0 was considered as optimal. The 50 top ranked targets
of miR-4491 predicted by TargetScan were also selected. The two
gene sets were overlapped by online Vein Map (http://bioinformatics.psb.ugent.be/webtools/Venn/).
The functions of the 7 overlapped genes were subsequently
investigated by literature review.
Dual luciferase reporter assays
Wild-type or mutant TRIM7 3′UTR were ligated into
pGL3-luciferase reporter plasmids (Promega Corporation),
co-transfected with miR-4491 mimic or miR-NC into A549 and
NCI-H1650 cells by Lipofectamine® 2000 solution
(Invitrogen; Thermo Fisher Scientific, Inc.) and incubated for 48 h
at 37°C. Subsequently, A549 and NCI-H1650 cells were trypsinized
and transferred to 24-well plates. Luciferase reporter activities
were examined by the Dual Luciferase Assay System (Promega
Corporation). The luciferase reporter activities were normalized to
the Renilla luciferase activity (Promega Corporation).
Statistical analysis
The data were analyzed by GraphPad Prism 6 (GraphPad
Software, Inc.) and were expressed as the means ± standard
deviation. The differences between two groups from tissues and cell
lines were analyzed using paired or unpaired Student's t-test,
respectively. The differences between three groups were analyzed by
one-way ANOVA and Newman Keuls analysis. The correlation between
miR-4491 and TRIM7 expression was analyzed by Pearson Correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-4491 levels are increased in
patients with NSCLC
The data derived from TCGA indicated that miR-4491
levels were significantly increased in cancer tissues from patients
with LUAD (n=512) compared with adjacent normal tissues from cancer
patients (n=20; Fig. 1A). In
addition, miR-4491 levels were significantly increased in cancer
tissues from patients with LUSC (n=475) compared with those in
adjacent normal tissues from cancer patients (n=38; Fig. 1B). The data derived from patient
tissues collected in the present study indicated that the miR-4491
levels in NSCLC tissues (n=78) were significantly higher compared
with those in the adjacent normal tissues (Fig. 1C). A similar expression profile of
miR-4491 was also observed in patients with LUAD (n=42; Fig. 1D) and LUSC (n=36; Fig. 1E).
Downregulation of miR-4491 negatively
affects the proliferation and induces the apoptosis of NSCLC
cells
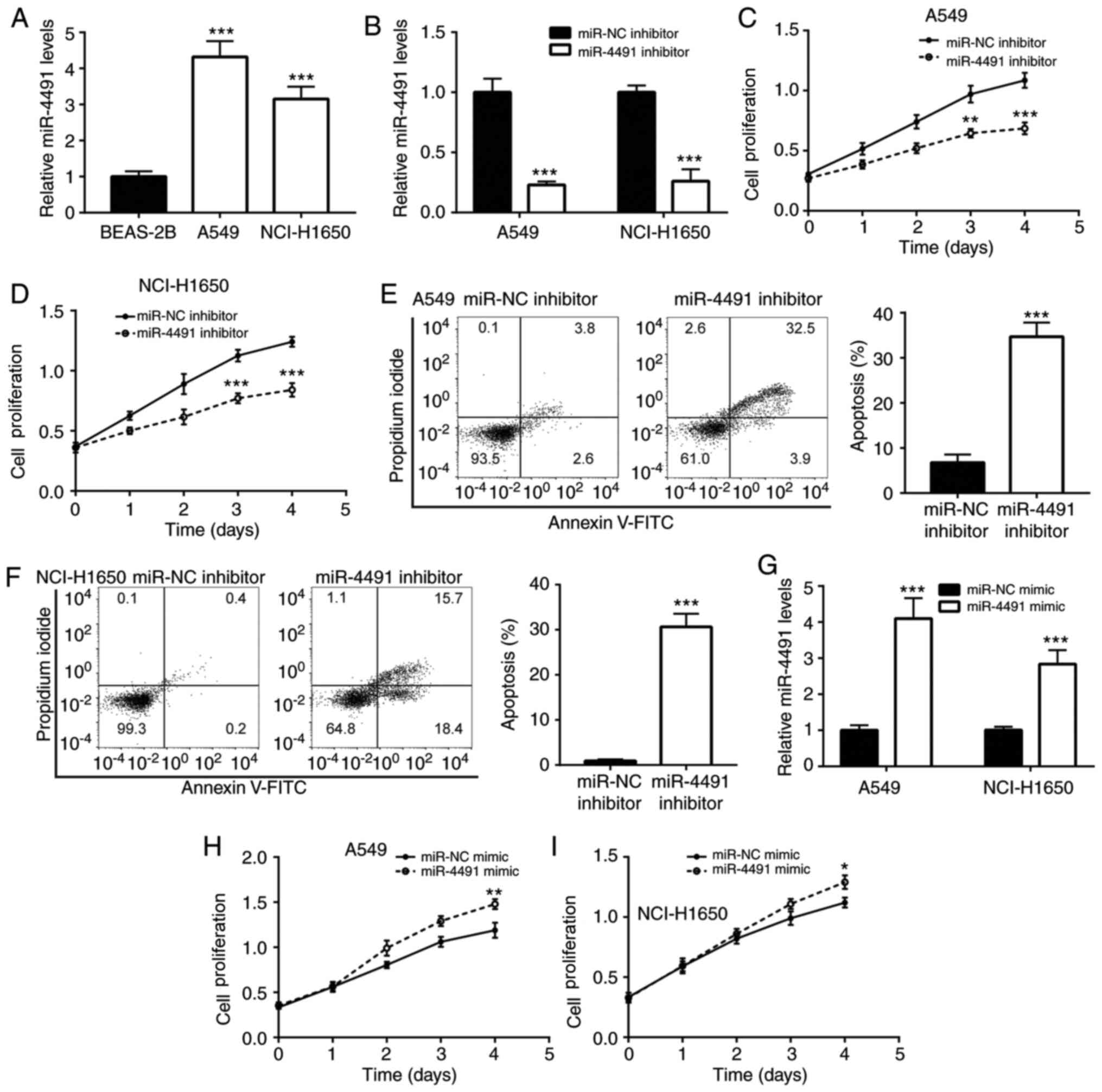
To detect the expression profile of miR-4491 in
NSCLC cell lines, A549 and NCI-H1650 cells were selected. The
results indicated that miR-4491 expression was significantly
increased in A549 and NCI-H1650 cells compared with BEAS-2B cells
(Fig. 2A).
Furthermore, the effects of the downregulation of
miR-4491 in NSCLC cell lines were determined. The downregulation of
miR-4491 expression by the miR-4491 inhibitor was confirmed in A549
and NCI-H1650 cells (Fig. 2B).
Subsequently, transfection of A549 and NCI-H160 cell with miR-4491
inhibitor resulted in a decrease in cell proliferation (Fig. 2C and D) in a time-dependent manner
compared with that of the miR-NC group. In addition, miR-4491
inhibitor induced apoptosis of A549 and NCI-H1650 cells (Fig. 2E and F).
In addition, the effects of miR-4491 overexpression
were determined in NSCLC cell lines. The overexpression of miR-4491
in A549 and NCI-H1650 cells using miR-4491 mimic was verified by
RT-qPCR analysis (Fig. 2G). The
effects caused by the miR-4491 mimic were opposite to those caused
by the miR-4491 inhibitor. miR-4491 mimic increased A549 and
NCI-H1650 cell proliferation (Fig. 2H
and I) in a time-dependent manner.
TRIM7 is targeted by miR-4491 in NSCLC
cell lines
The gene sets from miRWalk (n=2,231) and TargetScan
(n=51) were retrieved and 7 overlapping genes (Fig. 3A) were identified. The complementary
sites between TRIM7 and miR-4491 are presented in Fig. 3B.
Cell transfection with miR-4491 inhibitor induced a
significant upregulation of TRIM7 mRNA and protein levels (Fig. 3C and D) and a concomitant significant
downregulation of the protein levels of p65 (Fig. 3E) in A549 and NCI-H1650 cells.
Furthermore, miR-4491 mimic significantly decreased the relative
luciferase activity of TRIM7 wild-type (Fig. 3F) but not TRIM7 mutant (Fig. 3G) in A549 and NCI-H1650 cells.
Downregulation of miR-4491 decreases
p65 levels by targeting TRIM7 in NSCLC cells
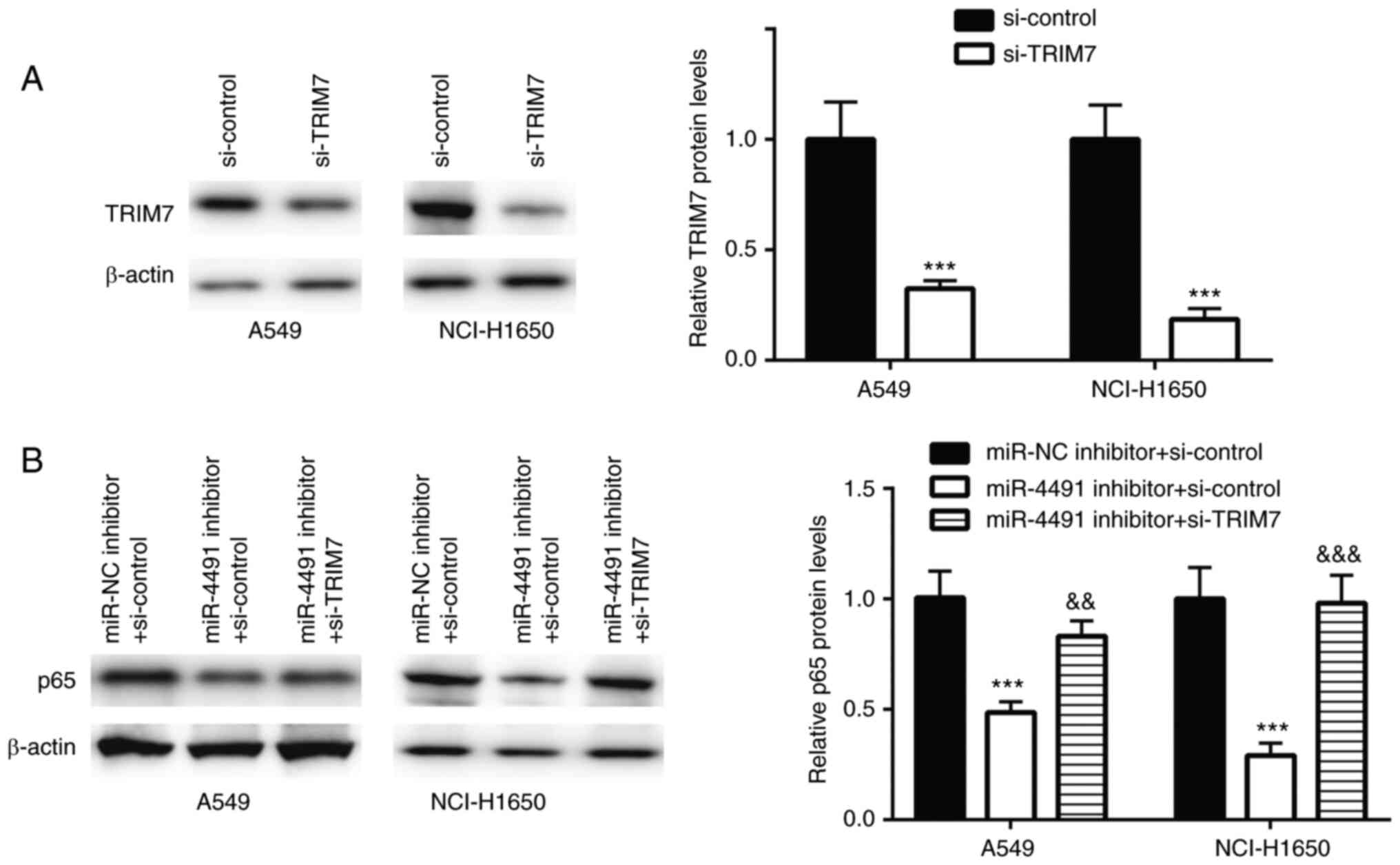
TRIM7 was successfully downregulated using siRNA
transfection in A549 and NCI-H1650 cells (Fig. 4A).
In A549 and NCI-H1650 cells transfected with
miR-4491 inhibitor, p65 protein expression was significantly
decreased, an effect that was rescued following co-transfection
with si-TRIM7 (Fig. 4B).
Downregulation of miR-4491 negatively
affects the proliferation and triggers the apoptosis of NSCLC cells
by targeting TRIM7
The miR-4491 inhibitor inhibited the proliferation
of A549 and NCI-H1650 cells (Fig. 5A and
B) in a time-dependent manner compared with that of the miR-NC
+ si-control group, which was rescued following the co-transfection
with si-TRIM7. In addition, the miR-4491 inhibitor induced A549 and
NCI-H1650 cell apoptosis (Fig. 5C and
D), which was reversed following the co-transfection with
si-TRIM7.
miR-4491 expression is negatively
correlated with TRIM7 expression in NSCLC tissues
The data derived from patient tissues collected in
the present study indicated that TRIM7 expression in NSCLC tissues
(n=78) was significantly lower compared with that in the adjacent
normal tissues (Fig. 6A). A similar
expression profile of TRIM7 was also observed in patients with LUAD
(n=42; Fig. 6B) and LUSC (n=36;
Fig. 6C). In addition, miR-4491
expression was negatively correlated with TRIM7 expression in NSCLC
tissues (Fig. 6D), LUAD tissues
(Fig. 6E) and LUSC tissues (Fig. 6F).
Discussion
miRNAs are emerging biomarkers used in the diagnosis
and treatment of patients with NSCLC (18,19). In
particular, miR-21 (20), miR-9
(21) and miR-143 (22) have been used as biomarkers of NSCLC.
miR-4491 has shown potential diagnostic value for several diseases,
as it was reported to be downregulated in ischemic stroke (23), while it is upregulated in gastric
cancer (24) and chronic heart
failure (25). A recent study
reported a diagnostic value of miR-4491 in LC (15). However, its therapeutic value in
NSCLC has yet to be reported.
In the present study, the data from TCGA and from
the collected tumor tissues indicated that miR-4491 expression was
increased in tumor tissues of patients with NSCLC compared with
normal matched tissues. Furthermore, miR-4491 downregulation
negatively affected the proliferation and triggered the apoptosis
of NSCLC cells. These findings suggested that miR-4491 may have an
oncogenic role in NSCLC.
The miRNA-mRNA interaction regulates target gene
expression by inducing mRNA degradation or translational repression
(8). This interaction might
therefore regulate cell proliferation, cell death and metastasis
(11). The involvement of miR-4491
target genes in NSCLC requires thus further investigation in order
to determine the function of miR-4491 in NSCLC.
In the present study, miRWalk and TargetScan were
used to identify 7 overlapping genes, including INTS3 and NABP
interacting protein (INIP), protein O-mannose kinase (POMK), muscle
RAS oncogene homolog (MRAS), alpha-1,3-mannosyl-glycoprotein
4-beta-N-acetylglucosaminyltransferase B (MGAT4B), angiotensin II
receptor type 1 (AGTR1), chromosome 1 open reading frame 116
(C1orf116) and TRIM7, which could all be targeted by miR-4491.
Among these genes, there has not been any reports about the
function of INIP or POMK in the development of cancer. MRAS and
MGAT4B however were reported as oncogenic genes, and MRAS can
initiate tumor formation in lungs (26). MGAT4B transcripts are also
upregulated in diethylnitrosamine-induced mouse model for
hepatocellular carcinoma (27).
There are only few reports about the expression profile of AGTR1
and C1orf116 in patients with lung cancer. AGTR1 expression is
shown to be decreased in LUAD tissues compared with adjacent normal
tissues (28), and downregulation of
C1orf116 is associated with a poor prognosis of patients with lung
cancer (29). TRIM7 belongs to the
TRIM protein family, which is involved in cell proliferation
(30), cell apoptosis (31) and immunity (32), and is a well-known tumor suppressor
involved in the development of various types of cancer. For
example, TRIM7 inhibits the progression of hepatocellular carcinoma
by negatively regulating Src (33),
and TRIM7 expression is decreased in tumor tissues from patients
with LC (34). Subsequently, TRIM7
was selected for the subsequent experimentations in the present
study.
In the present study, TRIM7 was targeted and
negatively regulated by miR-4491. However, the downstream targets
of TRIM7 were not detected.
NF-κB consists of 5 subunits, including NF-κB1 (p50
and its precursor p105), NF-κB2 (p52 and its precursor p100), RelA
(p65), RelB and c-Rel. In addition, the p50/65 heterodimer is
enriched in nearly all types of cells (35,36).
NF-κB can target genes that stimulate cell proliferation,
inflammation, angiogenesis, metastasis and cancer cell resistance
to chemotherapy and radiotherapy (36). NF-κB p65 is commonly activated in LC
(37). TRIM7 has been initially
identified to degrade p65 in LC (34). The present study demonstrated that
miR-4491 inhibitor decreased p65 protein expression in NSCLC cells.
This effect was reversed following transfection with si-TRIM7.
These observations were consistent with previous findings.
However, whether TRIM7 could affect the role of
miR-4491 in the proliferation and apoptosis of NSCLC cells remains
unknown. Cell transfection with miR-4491 inhibitor negatively
affected cell proliferation and triggered apoptosis, whereas these
effects were reversed by si-TRIM7 in NSCLC cells.
The present study demonstrated also that TRIM7
expression in cancer tissues from patients with NSCLC, including
LUAD and LUSC, was downregulated compared with the adjacent normal
tissues. In addition, miR-4491 expression was negatively correlated
with TRIM7 expression in NSCLC tissues, including LUAD and
LUSC.
Taken together, the results from the present study
suggested that miR-4491 may enhance cell proliferation and
resistance to apoptosis as well as the activation of p65 in NSCLC
by targeting TRIM7.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data and materials are available from the
corresponding author on reasonable request.
Authors' contributions
FH, GC, YG, BL, YS, XQ, HT and XZ conducted the
experimentations, data interpretation and data analysis. HZ
designed the experimentations, performed the data interpretation
and data analysis, and wrote the article. FH, GC, YG, BL, YS, XQ,
HT, XZ and HZ confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanxi Provincial Cancer Hospital (IRB no.
SXPCP20160302). All patients provided written informed consent
prior to study enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cersosimo RJ: Lung cancer: A review. Am J
Health Syst Pharm. 59:611–642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inamura K: Lung cancer: Understanding its
molecular pathology and the 2015 WHO classification. Front Oncol.
7:1932017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalari KR, Rossell D, Necela BM, Asmann
YW, Nair A, Baheti S, Kachergus JM, Younkin CS, Baker T, Carr JM,
et al: Deep sequence analysis of non-small cell lung cancer:
Integrated analysis of gene expression, alternative splicing, and
single nucleotide variations in lung adenocarcinomas with and
without oncogenic KRAS mutations. Front Oncol. 2:122012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA,
Atkins MB, Leming PD, et al: Five-year survival and correlates
among patients with advanced melanoma, renal cell carcinoma, or
non-small cell lung cancer treated with nivolumab. JAMA Oncol.
5:1411–1420. 2019.(Online ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shah MY, Ferrajoli A, Sood AK,
Lopez-Berestein G and Calin GA: Microrna therapeutics in cancer-an
emerging concept. EBioMedicine. 12:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buchan JR and Parker R: Molecular biology.
The two faces of miRNA. Science. 318:1877–1878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goh JN, Loo SY, Datta A, Siveen KS, Yap
WN, Cai W, Shin EM, Wang C, Kim JE, Chan M, et al: microRNAs in
breast cancer: Regulatory roles governing the hallmarks of cancer.
Biol Rev Camb Philos Soc. 91:409–428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henry JC, Azevedo-Pouly ACP and Schmittgen
TD: MicroRNA replacement therapy for cancer. Pharm Res.
28:3030–3042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu
Y, Chen Y, Xu L, Zen K, Zhang C and Shen H: Serum microRNA
signatures identified in a genome-wide serum microRNA expression
profiling predict survival of non-small-cell lung cancer. J Clin
Oncol. 28:1721–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Yu T, Cao J, Liu L, Liu Y, Kong HW,
Zhu MX, Lin HC, Chu DD, Yao M and Yan MX: MicroRNA-148a suppresses
invasion and metastasis of human non-small-cell lung cancer. Cell
Physiol Biochem. 37:1847–1856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He Q, Fang Y, Lu F, Pan J, Wang L, Gong W,
Fei F, Cui J, Zhong J, Hu R, et al: Analysis of differential
expression profile of miRNA in peripheral blood of patients with
lung cancer. J Clin Lab Anal. 33:e230032019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cancer Genome Atlas Research Network, ;
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Q, Huang SX, Zhang F, Li SJ, Liu C,
Xi YY, Wang L, Wang X, He QQ, Sun CC and Li DJ: MicroRNAs: A novel
potential biomarker for diagnosis and therapy in patients with
non-small cell lung cancer. Cell Prolif. 50:e123942017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petrek H and Yu AM: MicroRNAs in non-small
cell lung cancer: Gene regulation, impact on cancer cellular
processes, and therapeutic potential. Pharmacol Res Perspect.
7:e005282019. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bica-Pop C, Cojocneanu-Petric R, Magdo L,
Raduly L, Gulei D and Berindan-Neagoe I: Overview upon miR-21 in
lung cancer: Focus on NSCLC. Cell Mol Life Sci. 75:3539–3551. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei W, Dong Z, Gao H, Zhang YY, Shao LH,
Jin LL, Lv YH, Zhao G, Shen YN and Jin SZ: MicroRNA-9 enhanced
radiosensitivity and its mechanism of DNA methylation in non-small
cell lung cancer. Gene. 710:178–185. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang Q, Yuan Y, Gong Y, Luo X, Su X, Hu X
and Zhu W: Therapeutic delivery of microRNA-143 by cationic
lipoplexes for non-small cell lung cancer treatment in vivo. J
Cancer Res Clin Oncol. 145:2951–2967. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu X, Liu X, Liu Y, Chang W, Song Y and
Zhu S: Uncovering the potential differentially expressed miRNAs and
mRNAs in ischemic stroke based on integrated analysis in the gene
expression omnibus database. Eur Neurol. 9:404–414. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang B, Jing C, Wang J, Guo X, Chen Y, Xu
R, Peng L, Liu J and Li L: Identification of microRNAs associated
with lymphangiogenesis in human gastric cancer. Clin Transl Oncol.
16:374–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li H, Fan J, Yin Z, Wang F, Chen C and
Wang DW: Identification of cardiac-related circulating microRNA
profile in human chronic heart failure. Oncotarget. 7:33–45. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mishra J, Kumar A, Sinha A, Das S and
Srivastava A: Ingenuity in pattern recognition: A novel
bioinformatics approach towards lung cancer identification. Int J
Bioinform Res Appl. 6:531–541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blomme B, Heindryckx F, Stassen JM, Geerts
A, Colle I and Van Vlierberghe H: Serum protein N-glycan
alterations of diethylnitrosamine-induced hepatocellular carcinoma
mice and their evolution after inhibition of the placental growth
factor. Mol Cell Biochem. 372:199–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goldstein B, Trivedi M and Speth RC:
Alterations in gene expression of components of the
renin-angiotensin system and its related enzymes in lung cancer.
Lung Cancer Int. 2017:69149762017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parsana P, Amend SR, Hernandez J, Pienta
KJ and Battle A: Identifying global expression patterns and key
regulators in epithelial to mesenchymal transition through
multi-study integration. BMC Cancer. 17:4472017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen L, Chen DT, Kurtyka C, Rawal B, Fulp
WJ, Haura EB and Cress WD: Tripartite motif containing 28 (Trim28)
can regulate cell proliferation by bridging HDAC1/E2F interactions.
J Biol Chem. 287:40106–40118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zaman MM, Nomura T, Takagi T, Okamura T,
Jin W, Shinagawa T, Tanaka Y and Ishii S:
Ubiquitination-deubiquitination by the TRIM27-USP7 complex
regulates tumor necrosis factor alpha-induced apoptosis. Mol Cell
Biol. 33:4971–4984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Versteeg GA, Benke S, Garcia-Sastre A and
Rajsbaum R: InTRIMsic immunity: Positive and negative regulation of
immune signaling by tripartite motif proteins. Cytokine Growth
Factor Rev. 25:563–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu L, Qin C, Li T, Ma X, Qiu Y, Lin Y, Ma
D, Qin Z, Sun C, Shen X, et al: The E3 ubiquitin ligase TRIM7
suppressed hepatocellular carcinoma progression by directly
targeting Src protein. Cell Death Differ. 27:1819–1831. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin J, Lu Z, Wang X, Liu Y, Han T, Wang Y,
Wang T, Gan M, Xie C, Wang J and Yu B: E3 ubiquitin ligase TRIM7
negatively regulates NF-kappa B signaling pathway by degrading p65
in lung cancer. Cell Signal. 69:1095432020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park MH and Hong JT: Roles of NF-κB in
cancer and inflammatory diseases and their therapeutic approaches.
Cells. 5:152016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bassères DS, Ebbs A, Levantini E and
Baldwin AS: Requirement of the NF-kappaB subunit p65/RelA for
K-Ras-induced lung tumorigenesis. Cancer Res. 70:3537–3546. 2010.
View Article : Google Scholar
|