Introduction
Proton beam therapy (PBT) is associated with the
emission of high radiation energy after penetration up to a certain
depth (1,2) and is widely used for the treatment of
various cancer types (3), such as
head and neck (4), prostate
(5), pediatric brain (6), liver (7)
and esophageal (8) cancer. PBT
techniques have advanced over the last few decades. One of the most
representative advances is the development of the spot-scanning
technique using pencil beams (9).
Spot-scanning features several dosimetric traits that, depending on
the circumstances, can further improve upon conventional
passive-scattered broad-beam irradiation; it provides a smaller
entrance dose and allows for intensity-modulated treatments that
enable dose painting to further spare normal tissue in the vicinity
of complex tumor structures. A decrease in the cost of the
manufacturing of patient-specific devices, such as compensators,
and shorter device adjustment times are other advantages (10–12). The
major disadvantage of PBT scanning is the larger lateral penumbra
compared with that of passive-scattered beams (13,14).
Nevertheless, the number of facilities offering spot-scanning PBT
is rapidly increasing worldwide.
Several studies have investigated how to improve the
plan quality of spot-scanning PBT using hardware or software
systems. Techniques to improve the optimization method and to
decrease the size of the penumbra are the main topics of focus.
Regarding the development of optimization technology, pencil beam
selection (resampling) (15–17) and modified pencil beam positioning,
such as contour scanning (18), have
been developed. Regarding technology to decrease the penumbra,
collimator (19,20) or aperture (21–23)
systems have been utilized at some facilities. In addition, a
system with four moveable trimmer plates, enabling energy
layer-specific collimation [dynamic multi-leaf collimator (MLC)],
has been developed (24,25). Furthermore, the combination of using
an MLC and contour scanning can reportedly decrease the dose to the
organ at risk (OAR) (26).
In contrast to the aforementioned benefits, devices
such as collimation systems have the disadvantage of generating
scattered particles when interacting with proton beams (27,28).
MELTHEA V (Hitachi, Ltd,) is the first commercially available PBT
system equipped with a synchrotron that allows switching between a
passive-scattered broad-beam and a spot-scanning pencil-beam using
the same nozzle. The MLC, which can potentially be used for
passive-scattered broad-beam irradiation, can also be used for
spot-scanning beam irradiation.
Kobe Proton Center (Kobe, Japan) has a treatment
nozzle that can deliver spot-scanning beams and is equipped with an
MLC. Dose calculation using the Monte Carlo (MC) method is also
available for PBT (29,30). A relatively large number of pediatric
patients with cancer are treated at the facility, and necrosis or
severe dysfunction of the nervous system are often mentioned as
adverse events that should be avoided particularly when treating
such patients. The present study investigated the dose distribution
benefits and caveats of spot-scanning PBT performed using a device
equipped with an MLC by simulating pediatric brain tumors in the
posterior fossa.
Materials and methods
Treatment planning system
The treatment planning system consisted of
RayStation ver. 9A (Ray Search Laboratories AB), which has a pencil
beam dose engine and its own MC dose engine; it transports primary
protons and secondary ions (up to α), but does not transport
secondary neutral particles, and does not generate δ-ray electrons.
MC dose engine starts the transportation upstream of the patient
transport grid or the most upstream beam modifier (MLC or range
shifter) when used, thus the edge scatter on MLC is included in the
calculation. The MC dose engine is described in further detail in
the studies by Saini et al (29) and Maes et al (30). The RayStation uses 1.06 times the
projected spot size as the spot distance by default, and this size
was not changed for the present study. The optimization algorithm
was a sequential quadratic programming method that uses
Broyden-Fletcher-Goldfarb-Shanno updates of the quasi-Newton
approximation of the Hessian of the Lagrangian (31).
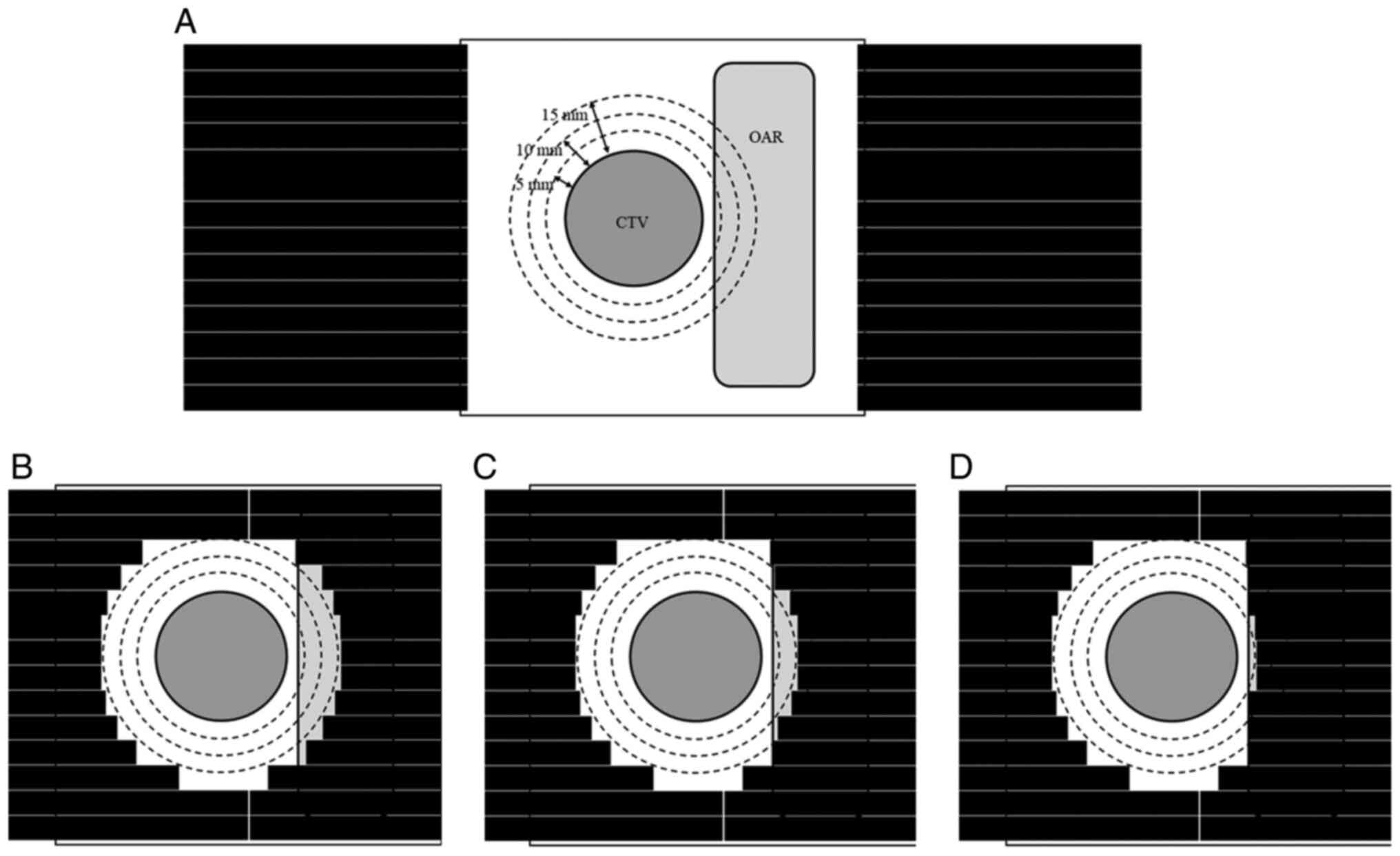
Virtual target study
Three rectangular virtual targets were created with
the following dimensions: 4×4×4, 6×6×4 and 8×8×4 cm. The MLC was
set at fully open (initial plan) or closed to create irradiation
areas with a border at 15, 10 or 5 mm from the target (15, 10- and
5-mm plans, respectively). The beam direction was frontal, and the
resulting dose distributions were calculated. Next, the dose
distributions were scaled so that the dose received by 50% of the
volume (D50) of the targets matched the prescription
dose. To assess the benefit of using MLC, lateral penumbras,
defined as 80 to 20% of the prescription dose at the target center
for each field, were compared for different MLC openings. Also, the
maximum dose (Dmax), which could be increased by
scattered particles from the MLC, was compared. In a preliminary
study, it was confirmed that spots with an energy deposit located
>15 mm away from the target were relatively sparse and had a
minimal effect on the dose distribution of the target in this
system [number of spots: −5.9%; D5 of the target: +0.11
Gy(RBE); D9 of the target: −0.17 Gy(RBE)]. Thus, the
present study was started with the MLC closed to create a border at
15 mm from the target.
Analyzing clinical data
Clinical data for a total of 18 pediatric patients
with brain tumors in the posterior fossa treated at Kobe Proton
Center were studied (age range, 8 months-12 years; mean, 5.8 years;
9 males and 9 females). The primary diseases were medulloblastoma
(n=10), ependymoma (n=5) and atypical teratoid/rhabdoid tumor
(AT/RT; n=3). The tumors had already been resected, and the
post-operative cavities were the targets. The beam directions were
left, right and top. Closing the MLC to shield the brainstem
impairs the dose of the area on the distant side of the brainstem
in the CTV. To minimize the volume of the low-dose area in the CTV,
the angle of the top beam was arranged so as to run parallel to the
brainstem. The prescription doses [measured in gray relative
biological effectiveness: Gy(RBE)] were the same as those used in
clinical practice: 27 Gy(RBE) for medulloblastoma, 59.4 Gy(RBE) for
ependymoma and 54 Gy(RBE) for AT/RT. For patients with
medulloblastoma, brain radiotherapy is usually performed in
combination with whole craniospinal irradiation (CSI). Thus, by
adding a dose of CSI [23.4 Gy(RBE)] to make a total dose of 50.4
Gy(RBE), the dose difference was decreased among the 3 diseases,
making it easier to review and compare.
Optimization was performed using a pencil beam dose
engine. After optimization, the dose distribution was calculated
using the MC dose engine for later evaluations. Each beam included
a range shifter of 0–6 mm water equivalent thickness made up of
polyethylene. Robust optimization with a 4-mm setup and 3.5% range
of uncertainty were used. The maximum number of iterations was 40.
The main parameters of the beam delivery system are shown in
Table I. The clinical goal was to
cover the CTV (uniform prescription dose), brainstem [≤52 Gy(RBE)
for <8 years; 54 Gy(RBE) for ≥8 years), cervical cord [≤40
Gy(RBE) for <8 years; 43 Gy(RBE) for ≥8 years], brain [≤60
Gy(RBE)] and cochlea [≤43 Gy(RBE)], all converted with an
equivalent dose of 2 Gy(RBE).
 | Table I.Beam parameters. |
Table I.
Beam parameters.
| Beam
parameters | Value |
|---|
| Energy, MeV | 70.7–235 |
| Energy steps,
n | 92 |
| Pulse frequency,
Hz | 1/2.8 |
| Field size, cm | 20×15 |
| Source-axis
distance (X, Y), m | 2.696, 3.029 |
| Scanning speed (X,
Y), mm/msec | 60, 120 |
| Spot size at
isocenter in air, mm (one sigma) | 3.3–12 |
| Dose rate,
Gy/min | 1 |
The first treatment plan was created with the MLC
fully open (initial plan). Next, the initial plan was modified by
closing the MLC to create an irradiation area with a border at 15
mm from the CTV (15-mm plan); then, only the leaves of the MLC for
which the OAR region overlapped with the border at 10 or 5 mm from
the CTV were closed (10- and 5-mm plans, respectively). Fig. 1 illustrates these four treatment
plans. The dose distribution was calculated for each plan in the
same way as that used for the virtual target study.
All the study procedures involving human
participants were conducted in accordance with the ethical
standards of the institutional research committee (approval no.
30-7), were in compliance with the Declaration of Helsinki, and
were approved by the Institutional Review Board of Kobe Proton
Center (Kobe, Japan).
Statistical analysis
Repeated measures single-factor ANOVA followed by
Bonferroni's post hoc test, analyzed using Statcel 4 software (OMS
Publishing Inc.), was used to compare the dose to the target and
OAR among the 15-, 10- and 5-mm and initial plans, and P<0.05
was considered to indicate a statistically significant
difference.
Results
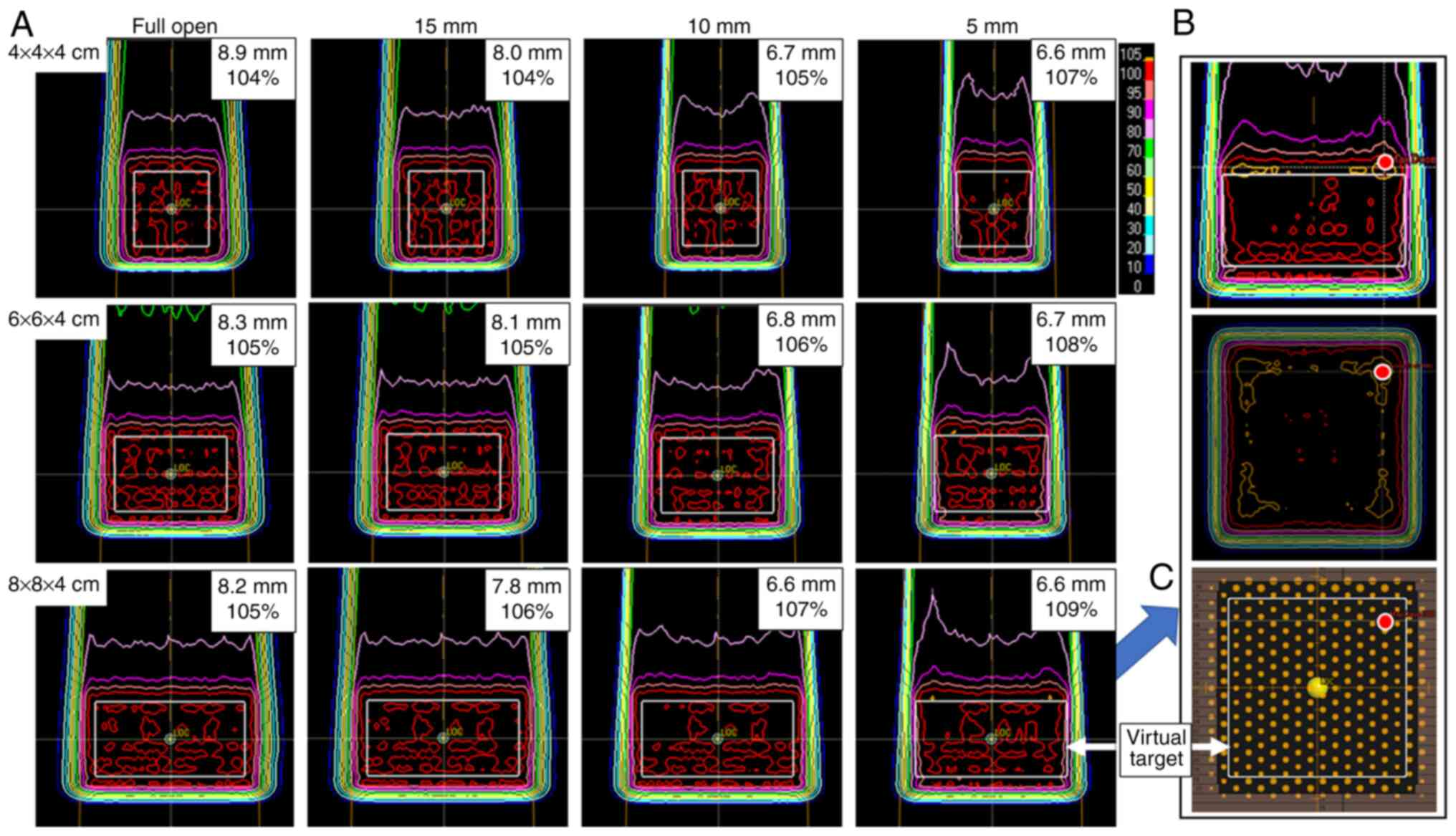
Virtual target study
The lateral penumbra sharpened as the MLC was
closed: From 8.9 to 6.6 mm for a 4×4×4-cm target, from 8.3 to 6.7
mm for a 6×6×4-cm target and from 8.2 to 6.6 mm for an 8×8×4-cm
target. For the 10-mm plan, the 95% dose line encompassed the
target. For the 5-mm plan, the 95% dose line did not cover the
entire target completely. The point of Dmax was located
1–3 mm upstream of the target close to the beam edge, and the
Dmax tended to increase as the MLC was closed: From 104
to 107% for a 4×4×4-cm target, from 105 to 108% for a 6×6×4-cm
target and from 105 to 109% for an 8×8×4-cm target. The dose in the
proximal region along the beam edge increased concomitantly as the
MLC was closed. All the data are shown in Fig. 2.
Analyzing clinical data
The difference in Dmean values of the
brainstem, cervical cord, brain and cochlea among the initial, 15-,
10- and 5-mm plan showed significant changes
(P=9.9×10−10, 1.3×10−17, 2.1×10−16
and 2.0×10−15, respectively). The values showed a
decreasing trend as the MLC was closed. The Dmax of the
cervical cord and cochlea also showed significant changes
(P=3.0×10−4 and P=1.1×10−3, respectively).
The Dmax decrease in the cervical cord was 8.3, followed
by 5.6 Gy(RBE) in medulloblastoma. Meanwhile, the maximal
Dmax decrease in the cochlea was 8.9, followed by 5
Gy(RBE) in AT/RT and ependymoma. None of the Dmean,
D95 or Dmax values of the CTV were changed,
and only the conformality index of the planning target volume (PTV)
(CTV + 5 mm) showed a significant increase (P=1.7×10−3).
The Dmax of the skin exhibited no change (Table II and Fig. 3). In 10 patients, the Dmax
of the brain in all MLC-closure plans had a higher value relative
to that of the initial plan. The maximum increase was 0.8 Gy(RBE)
[from 51.2 to 52 Gy(RBE)] in a patient with medulloblastoma.
Fig. 4 shows an example of a patient
with AT/RT (8 months old, female). The prescription dose in the
initial plan was 27 Gy(RBE) for the post-operative cavity +15 mm
and 54 Gy(RBE) for the post-operative cavity +10 mm, but excluding
the brainstem. The doses at the brainstem and cochlea were
prominently decreased as the MLC was closed, while the dose to the
CTV remained almost the same.
 | Table II.Comparison of dose parameters for
each plan. |
Table II.
Comparison of dose parameters for
each plan.
| Parameter | Initial plan | 15-mm plan | 10-mm plan | 5-mm plan | P-value |
|---|
| CTV |
|
|
|
|
|
|
Dmean | 53.2 | 53.2 | 53.2 | 53.2 | 0.68 |
|
D95 | 49.4 | 49.4 | 49.5 | 49.5 | 0.10 |
|
Dmax | 55.5 | 55.5 | 55.5 | 55.5 | 0.09 |
| PTV |
|
|
|
|
|
|
Conformity index | 0.4 | 0.4 | 0.4 | 0.39 |
1.7×10−3a |
| Brainstem |
|
|
|
|
|
|
Dmean | 46.0 | 46.0 | 45.4 | 42.4 |
9.9×10−10a |
|
D5 | 50.3 | 50.3 | 50.4 | 50.3 | 0.06 |
|
D2 | 50.7 | 50.8 | 50.8 | 50.8 | 0.08 |
|
Dmax | 51.0 | 51.0 | 51.1 | 51.0 | 0.11 |
| Cervical cord |
|
|
|
|
|
|
Dmean | 19.7 | 19.5 | 19.0 | 18.3 |
1.3×10−17a |
|
D5 | 38.6 | 38.6 | 38.2 | 34.5 |
6.8×10−4a |
|
D2 | 40.8 | 40.9 | 40.6 | 39.0 |
9.9×10−5a |
|
Dmax | 41.7 | 41.7 | 41.4 | 40.0 |
3.0×10−4a |
| Brain |
|
|
|
|
|
|
Dmean | 22.2 | 22.1 | 22.0 | 21.6 |
2.1×10−16a |
|
D5 | 51.7 | 51.7 | 51.6 | 51.5 | 0.3 |
|
D2 | 54.1 | 54.2 | 54.1 | 54.1 | 0.34 |
|
Dmax | 54.6 | 54.7 | 54.6 | 54.6 | 0.35 |
| Cochlea |
|
|
|
|
|
|
Dmean | 27.8 | 27.6 | 27.2 | 26.3 |
2.0×10−5a |
|
D5 | 32.0 | 31.9 | 31.4 | 30.6 |
4.9×10−4a |
|
D2 | 33.2 | 33.1 | 32.7 | 31.7 |
1.0×10−3a |
|
Dmax | 33.7 | 33.7 | 33.3 | 32.3 |
1.1×10−3a |
| Skin |
|
|
|
|
|
|
Dmax | 25.8 | 25.7 | 25.7 | 25.7 | 0.41 |
Discussion
As the MLC closure increased, from the 15-mm plan to
the 5-mm plan, the dose-volume histogram (DVH) curve of the OAR
decreased. In particular, when the OAR was shielded to within 10 mm
from the CTV (10-mm plan), noticeable decreases were observed in
the DVH curves for the brainstem, cervical cord and cochlea. The
degree of the volume decrease was more prominent for the low to
middle dose level. This can be seen from the fact that the
Dmean was significantly decreased in most organs in all
MLC-closure plans; however, a decrease in Dmax or
D2 was only observed in the cervical cord and cochlea.
This trend is consistent with the study by Sugiyama et al
(20), in which the use of an MLC
decreased the dose mainly in the low-dose area of the lacrimal
gland during the treatment of maxillary sinus cancer. Prior to the
present study, it was predicted that the Dmax of the
brainstem would also decrease with MLC closure; however, no
significant changes in the high-dose parameters were observed in
the brainstem. We hypothesize that the robustness of the CTV in the
treatment planning strongly influenced this result. Since the
robustness of the CTV is prioritized in the treatment planning,
this method appears to be limited to decreasing the
Dmean of the brainstem, which is in full contact with
the CTV, and this method might not be sufficiently effective to
decrease the Dmax. Regarding the target, the
Dmean and D95 change remained within 1% of
the value of the initial plan. Only the conformity index showed a
significant difference, but when comparing the individual values,
there was no tendency to match the opening and closing of the MLC.
Partial permanent hair loss is a serious problem after PBT,
especially for female patients. The technique used in the present
study is not useful for skin protection as the distance between the
skin and CTV is >15 mm in numerous cases. In summary, this OAR
shielding technique using an MLC is effective for lowering the OAR
dose without affecting the target dose. In particular, in the
cervical cord and cochlea, tightening the shielding has the effect
of decreasing not only the Dmean, but also the
Dmax.
A total of 10 patients were found to have an
elevated Dmax in the brain. This circumstance can also
be inferred from the results of the virtual target study. In
Fig. 2, an increased dose was
observed in the proximal region along the beam edge. In situations
where the MLC was closed, the dose at the beam edge increased in a
manner that pulled the distribution toward the proximal side, as
represented by the bimodal peak of the 80% dose line. As the target
size increased, this tendency became stronger, and the two peaks
became clearer. This can be interpreted as each peak being
generated at the edge of the beam and being released from fusion as
the peaks separate from each other. This phenomenon can be
considered an effect of the scattering produced by the MLC. Hyer
et al (32) reported a 2-peak
dose profile in a simulation study using the MC method when the
collimation system was set to within 1 cm of the target, which is
similar to the present data. The clinical impact of an increased
dose caused by scattered radiation is important, but the maximum
increase was only 0.8 Gy(RBE) in a patient with medulloblastoma.
This degree of dose escalation is usually not clinically important.
Therefore, MLC can be considered to have a strong effect,
suppressing the dose delivered to the area surrounding the target,
such as the OAR, and the dose increase arising from scattered
radiation is likely to have little clinical significance.
Several studies attempting to improve the plan
quality for spot-scanning PBT using collimation and aperture
systems have been performed. Wang et al (22) performed a virtual target study and
reported that the target dose homogeneity with a 5-mm aperture
margin plan was equivalent to the plan without an aperture, but the
normal tissue dose was decreased. Dowdell et al (21) performed a simulation study using a
patient-specific aperture and observed a decrease in the
out-of-field dose. Hyer et al (10) performed a simulation study using a
novel dynamic collimator system. The study reported that it was
possible to decrease the OAR dose without changing the target dose,
and the dose decrease effect became more marked as the complexity
of the target shape increased. In clinical studies, Yasui et
al (23) reported that a dose
decrease in the OAR was obtained in 10 patients with head and neck
cancer with no decrease in the CTV dose using an aperture system,
and Sugiyama et al (20)
reported that Dmean and D2 values of the OAR,
including the optic nerve and chiasm, were successfully decreased
for 26 patients with maxillary cancer using an MLC system.
Winterhalter et al (26)
reported that energy-specific collimation decreased the volume
receiving >30% of the prescription dose (V30) outside
the PTV by 19.8% in 4 patients with brain and skull base cancer.
Regarding pediatric brain tumors, researchers at the University of
Iowa and Pennsylvania developed a dynamic collimation and a fixed
aperture system, and reported a clear trimming effect, decreasing
the OAR dose and increasing the conformity index of the target in 5
patients with brain tumors (24,25).
Smith et al (24) reported
decreases in the Dmean of normal tissue adjacent to the
target of 13.65 and 5.18% for a dynamic collimation and a fixed
aperture system, respectively, and the conformity index improved by
21.35 and 8.38%, respectively. Moignier et al (25) reported that the average decrease in
the Dmean of the brain was 25.1%, and they concluded
that a 24.8% decrease in brain necrosis and a 25.1% decrease in the
risk of secondary cancer could be expected. Moteabbed et al
(33) performed a simulation study
using a custom-fabricated beam-shaping aperture and range
compensator for 14 patients with brain tumor and sarcoma. It was
revealed that the device contributed to a decreased OAR dose and
that a smaller spot size could further improve the plan quality.
Comparing past studies, it is common not only to decrease the dose
delivered to the OAR, but also to maintain the dose to the target
within the range of a 5- to 10-mm aperture and collimation system.
The novelty of the present study is as follows. First, the dose
trimming effect and scattered radiation dose derived from the MLC
was evaluated while maintaining the target dose for spot-scanning
PBT equipped with MLC, which is a relatively new technique, and
support for the effect was further verified in a virtual target
study. Second, the method can be implemented with existing
equipment. This technique does not require a large capital
investment, such as a dynamic collimation system, and the
irradiation field can be trimmed easily and arbitrarily according
to the shape of the lesions and organs, rather than using a
custom-fabricated aperture system. Third, it is noteworthy that the
usefulness of the technique was verified in pediatric patients with
brain tumors. Based on data reported by Moignier et al
(25), spot-scanning PBT with MLC
can greatly decrease the risk of necrosis or secondary cancer. As
pediatric tumors are rare diseases, it is difficult to accumulate
and analyze multiple cases. In fact, very few studies applying
similar techniques have dealt with pediatric brain tumors. Since
Kobe Proton Center is adjacent to one of the largest pediatric
hospitals in Japan, it is possible to study numerous cases of
pediatric cancer and to obtain data on therapeutic efficacy and
safety. We consider it meaningful and novel to examine the dose
distribution effect in the largest number of cases so far and to
examine the possibility of applying this method to decrease future
risks.
As the present study was a simulation study, the
various beam parameters were unified. In clinical practice, a total
of 12 patients were treated using broad beams before the scanning
system was installed, and 3 patients were treated using scanning
beams without MLC before the collimation system was equipped with
the scanning beam irradiation system; the remaining 3 patients were
treated using scanning beams with MLC. In total, 17 of the 18
patients are still alive as of April 2021. The follow-up period was
0.4–2.9 years (median, 1.6 years). Local recurrences occurred in 2
patients. Neurological disorders associated with radiation, such as
necrosis, paralysis, audiovisual impairment or abnormal MRI
findings, have not been observed.
Although the treatment system used in this study is
commercially available, most PBT facilities can use either a
scattered broad beam with MLC or a scanning beam without MLC. The
development of a collimator system and its external attachment are
both expensive and laborious. The development of systems that will
allow MLC to be applied to scanning beam irradiation is widely
anticipated. The present study investigated the utility of spot
scanning using a MLC system for the treatment of brain tumors
located in the posterior fossa, assuming the brainstem, cervical
cord, brain, cochlea, and skin as OARs. Other organs, such as the
optic nerve and the gastrointestinal tract, are also clinically
important, and radiotherapy can cause serious adverse events,
potentially complicating treatment. Examining the effects at
different sites and for different diseases will be a clinically
important future prospect.
In conclusion, the present study investigated the
effect of spot-scanning PBT combined with MLC using a commercially
available system to calculate the dose distribution in pediatric
cases with brain tumors located in the posterior fossa. The
Dmean was decreased in the brainstem, cervical cord,
brain and cochlea, and the Dmax was decreased in the
cervical cord and cochlea in the MLC-closure plan, while the dose
distribution of the target was maintained. Dose elevation in the
brain, probably caused by the scattered beams, was 0.8 Gy(RBE) at
most; this value was determined to be small enough not to have a
significant clinical impact. Ideally, the leaf margin should be
determined by maintaining the target dose, decreasing the OAR dose
and minimizing the dose increase to the surrounding organs caused
by scattered radiation. The settings differ depending on the
system, but in the present system, a setting between 5 and 15 mm
was considered ideal.
Acknowledgements
Not applicable.
Funding
This study was supported by Kawano Masanori Memorial
Public Interest Incorporated Foundation for Promotion of
Pediatrics.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NF and TY conceived and designed the study. MM, YD
and TSu analyzed the clinical data. NF and TSo analyzed and checked
all data and wrote the manuscript. All authors read and approved
the final manuscript. NF and TSo confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
All the study procedures involving human
participants were conducted in accordance with the ethical
standards of the institutional research committee (approval no.
30-7), were in compliance with the Declaration of Helsinki and were
approved by the Institutional Review Board of Kobe Proton Center
(Kobe, Japan). The requirement for informed consent was waived, as
the study was a retrospective simulation study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lawrence JH, Tobias CA, Born JL, Linfoot
JA, Kling RP and Gottschalk A: Alpha and proton heavy particles and
the bragg peak in therapy. Trans Am Clin Climatol Assoc.
75:111–116. 1964.PubMed/NCBI
|
|
2
|
Bortfeld T and Schlegel W: An analytical
approximation of depth-dose distributions for therapeutic proton
beams. Phys Med Biol. 41:1331–1339. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foote RL, Stafford SL, Petersen IA, Pulido
JS, Clarke MJ, Schild SE, Garces YI, Olivier KR, Miller RC, Haddock
MG, et al: The clinical case for proton beam therapy. Radiat Oncol.
7:1742012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holliday EB and Frank SJ: Proton radiation
therapy for head and neck cancer: A review of the clinical
experience to date. Int J Radiat Oncol Biol Phys. 89:292–302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Royce TJ and Efstathiou JA: Proton therapy
for prostate cancer: A review of the rationale, evidence, and
current state. Urol Oncol. 37:628–636. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haas-Kogan D, Indelicato D, Paganetti H,
Esiashvili N, Mahajan A, Yock T, Flampouri S, MacDonald S, Fouladi
M, Stephen K, et al: National cancer institute workshop on proton
therapy for children: Considerations regarding brainstem injury.
Int J Radiat Oncol Biol Phys. 101:152–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klein J and Dawson LA: Hepatocellular
carcinoma radiation therapy: Review of evidence and future
opportunities. Int J Radiat Oncol Biol Phys. 87:22–32. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chuong MD, Hallemeier CL, Jabbour SK, Yu
J, Badiyan S, Merrell KW, Mishra MV, Li H, Verma V and Lin SH:
Improving outcomes for esophageal cancer using proton beam therapy.
Int J Radiat Oncol Biol Phys. 95:488–497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lomax A: Intensity modulation methods for
proton radiotherapy. Phys Med Biol. 44:185–205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hyer DE, Hill PM, Wang D, Smith BR and
Flynn RT: A dynamic collimation system for penumbra reduction in
spot-scanning proton therapy: Proof of concept. Med Phys.
41:0917012014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeLaney TF: Proton therapy in the clinic.
Front Radiat Ther Oncol. 43:465–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bert C and Durante M: Motion in
radiotherapy: Particle therapy. Phys Med Biol. 56:R113–R144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schippers JM and Lomax AJ: Emerging
technologies in proton therapy. Acta Oncol. 50:838–850. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Engelsman M, Schwarz M and Dong L: Physics
controversies in proton therapy. Semin Radiat Oncol. 23:88–96.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van de Water S, Kraan AC, Breedveld S,
Schillemans W, Teguh DN, Kooy HM, Madden TM, Heijmen BJ and
Hoogeman MS: Improved efficiency of multi-criteria IMPT treatment
planning using iterative resampling of randomly placed pencil
beams. Phys Med Biol. 58:6969–6983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van de Water S, Safai S, Schippers JM,
Weber DC and Lomax AJ: Towards FLASH proton therapy: The impact of
treatment planning and machine characteristics on achievable dose
rates. Acta Oncol. 58:1463–1469. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosas S, Belosi FM, Bizzocchi N, Böhlen T,
Zepter S, Morach P, Lomax AJ, Weber DC and Hrbacek J: Benchmarking
a commercial proton therapy solution: The Paul Scherrer Institut
experience. Br J Radiol. 93:201909202020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meier G, Leiser D, Besson R, Mayor A,
Safai S, Weber DC and Lomax AJ: Contour scanning for penumbra
improvement in pencil beam scanned proton therapy. Phys Med Biol.
62:2398–2416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bues M, Newhauser WD, Titt U and Smith AR:
Therapeutic step and shoot proton beam spot-scanning with a
multi-leaf collimator: A Monte Carlo study. Radiat Prot Dosimetry.
115:164–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugiyama S, Katsui K, Tominaga Y, Waki T,
Katayama N, Matsuzaki H, Kariya S, Kuroda M, Nishizaki K and
Kanazawa S: Dose distribution of intensity-modulated proton therapy
with and without a multi-leaf collimator for the treatment of
maxillary sinus cancer: A comparative effectiveness study. Radiat
Oncol. 14:2092019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dowdell SJ, Clasie B, Depauw N, Metcalfe
P, Rosenfeld AB, Kooy HM, Flanz JB and Paganetti H: Monte Carlo
study of the potential reduction in out-of-field dose using a
patient-specific aperture in pencil beam scanning proton therapy.
Phys Med Biol. 57:2829–2842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang D, Smith BR, Gelover E, Flynn RT and
Hyer DE: A method to select aperture margin in collimated spot
scanning proton therapy. Phys Med Biol. 60:N109–N119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yasui K, Toshito T, Omachi C, Hayashi K,
Tanaka K, Asai K, Shimomura A, Muramatsu R and Hayashi N:
Evaluation of dosimetric advantages of using patient-specific
aperture system with intensity-modulated proton therapy for the
shallow depth tumor. J Appl Clin Med Phys. 19:132–137. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smith B, Gelover E, Moignier A, Wang D,
Flynn RT, Lin L, Kirk M, Solberg T and Hyer DE: Technical Note: A
treatment plan comparison between dynamic collimation and a fixed
aperture during spot scanning proton therapy for brain treatment.
Med Phys. 43:46932016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moignier A, Gelover E, Wang D, Smith B,
Flynn R, Kirk M, Lin L, Solberg T, Lin A and Hyer D: Theoretical
benefits of dynamic collimation in pencil beam scanning proton
therapy for brain tumors: Dosimetric and radiobiological metrics.
Int J Radiat Oncol Biol Phys. 95:171–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Winterhalter C, Meier G, Oxley D, Weber
DC, Lomax AJ and Safai S: Contour scanning, multi-leaf collimation
and the combination thereof for proton pencil beam scanning. Phys
Med Biol. 64:0150022018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith BR, Hyer DE, Hill PM and Culberson
WS: Secondary neutron dose from a dynamic collimation system during
intracranial pencil beam scanning proton therapy: A monte carlo
investigation. Int J Radiat Oncol Biol Phys. 103:241–250. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimstrand P, Traneus E, Ahnesjo A and
Tilly N: Parametrization and application of scatter kernels for
modelling scanned proton beam collimator scatter dose. Phys Med
Biol. 53:3405–3429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saini J, Traneus E, Maes D, Regmi R, Bowen
SR, Bloch C and Wong T: Advanced Proton Beam Dosimetry Part I:
Review and performance evaluation of dose calculation algorithms.
Transl Lung Cancer Res. 7:171–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maes D, Saini J, Zeng J, Rengan R, Wong T
and Bowen SR: Advanced proton beam dosimetry part II: Monte Carlo
vs. Pencil beam-based planning for lung cancer. Transl Lung Cancer
Res. 7:114–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gill P, Murray W and Saunders M: Software
for large-scale nonlinear programming. User's guide for SNOPT
Version. 7:1–116. 2008.
|
|
32
|
Hyer DE, Hill PM, Wang D, Smith BR and
Flynn RT: Effects of spot size and spot spacing on lateral penumbra
reduction when using a dynamic collimation system for spot scanning
proton therapy. Phys Med Biol. 59:N187–N196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moteabbed M, Yock TI, Depauw N, Madden TM,
Kooy HM and Paganetti H: Impact of spot size and beam-shaping
devices on the treatment plan quality for pencil beam scanning
proton therapy. Int J Radiat Oncol Biol Phys. 95:190–198. 2016.
View Article : Google Scholar : PubMed/NCBI
|