Introduction
PDAC remains one of the most invasive malignancies
worldwide, and had the highest cancer-associated mortality rate of
12.5% between 2009 and 2017 (1,2).
Although great improvements have been made in preventing and
treating PDAC, including neoadjuvant chemotherapy, 2D-conformal
radiotherapy etc. (3,4), the increase in survival rate is still
slow with a 5-year survival rate of just 4–6% (5). In addition, the molecular mechanisms
underlying the metastatic process of PDAC are complex and remain
undetermined (6). Hence, there is an
urgent need to explore the underlying mechanisms to identify novel
potential therapeutic targets for the development of new
therapeutic strategies and improve prognosis for patients with
PDAC.
Wnt signaling includes 3 distinct pathways: the
canonical Wnt/β-catenin transcription pathway, the planar cell
polarity pathway and the Wnt/Ca2+ pathway (7). Wnt signaling regulation of gene
transcription needs dynamic multiprotein complexes that contain
axin, adenomatous polyposis coli (APC), Dishevelled (DVL) and other
proteins, such as casein kinase1α (CK1α), glycogen synthase kinase
3 (GSK-3) (7,8). As a cytoplasm-nucleus shuttling
protein, DVL2 has been reported as a hub of Wnt signaling (9). DVL2 protein upregulation has been found
in numerous tumors including prostate cancer (10), hepatocellular carcinoma (11), ovarian cancer (12) and esophageal squamous cell carcinoma
(13). Our previous study
demonstrated that knockdown of DVL2 in PDAC cells CFPAC-1 and
SW1990 inhibited epithelial-mesenchymal transition (EMT) induced by
IQ motif-containing GTPase-activating protein 1 (IQGAP1)
overexpression (14). However, the
involvement of DVL2 in the progression and EMT of PDAC including
the relationship between DVL2 expression and the clinicopathologic
features of PDAC remain unclear.
The present study investigated the biological
function and mechanism of DVL2 in PDAC. The expression level of
DVL2 in PDAC tissues and in pancreatic cancer cell lines were
detected and the association between expression level and PDAC
clinicopathological features were analyzed. In addition, the roles
and mechanisms of DVL2 in PDAC were explored. DVL2 may have the
potential to be a predictive biomarker for patients with PDAC and a
therapeutic target in the treatment of PDAC.
Materials and methods
Gene datasets
RNA array datasets [GSE15471 (15) and GSE16515 (16)], which consisted of 36 pairs of tumor
and normal tissue samples and 36 tumor samples and 16 normal
samples, were downloaded from the Genome Expression Omnibus
database (www.ncbi.nlm.nih.gov/gds). The data were used to
investigate the expression level of DVL2 in PDAC tissues and normal
tissue samples.
Patients
A total of 97 pancreatic cancer samples and 85
adjacent normal samples (≥1 cm distance from the tumor edge) were
obtained from patients who were histopathologically diagnosed with
primary PDAC at The First Affiliated Hospital of Kangda College of
Nanjing Medical University (Lianyungang, China) between January
2010 and January 2019. These patients were not treated with
anticancer therapy, including chemotherapy and radiotherapy prior
to surgery. The tissues were surgically resected. A few adjacent
normal samples were difficult to obtain as they were too close to
tumor tissue. The tissues were routinely fixed at room temperature
overnight in 10% neutral formalin, embedded in paraffin and stored
at room temperature until use. Clinicopathological information
included age, sex, pathological grade, tumor location and size,
perineural invasion, lymph node metastasis, tumor stage and the
follow-up data (17). The
classification systems for stage and grade used in the present
study were from the AJCC Cancer Staging Manual 8th edition
(18). Among the 97 patients, there
were 59 males and 38 females with an age range of 37–92 years and a
median age of 63 years. Patients were followed-up via the telephone
every 3 months in the first year and every 6 months in the second
and third year until patient death. The last date of follow-up was
December 1, 2019. The overall survival (OS) time was defined as the
interval between surgery and death or the last follow-up. Approval
from ethics committees of The First Affiliated Hospital of Kangda
College of Nanjing Medical University (Lianyungang, China)
(approval no. KY20190924002) were obtained before enrollment into
the study. Written informed consent was also obtained from the
participants prior to enrollment in the study. The study was
performed in accordance with government policies and the
Declaration of Helsinki.
Reagents and antibodies
Primary antibodies for human DVL2 (1:1,000; cat. no.
3224), β-catenin (1:1,000; cat. no. 8480), cyclin D1 (1:1,000; cat.
no. 2978), vimentin (1:1,000; cat. no. 5741), E-cadherin (1:1,000;
cat. no. 3195), N-cadherin (1:1,000; cat. no. 13116), Snail
(1:1,000; cat. no. 3879), and matrix metalloproteinase (MMP-9)
(1:1,000; cat. no. 13667) were purchased from Cell Signaling
Technology, Inc. Horseradish peroxidase (HRP)-conjugated anti-mouse
IgG (1:1,000; cat. no. 7076) and anti-rabbit IgG (1:1,000; cat. no.
7074) secondary antibodies were purchased from Cell Signaling
Technology, Inc. The control antibody (anti-GAPDH and anti-Histone
H3) was acquired from Bioworld Technology, Inc. and Proteintech
Group, Inc., respectively. Anti-human DVL2 (1:100; cat. no.
sc-390303) was acquired for immunohistochemistry from Santa Cruz
Biotechnology, Inc. Cell counting kit-8 (CCK-8) was purchased from
Dojindo Molecular Technologies, Inc. TRIzol® reagent and
PrimeScript RT Master Mix (Perfect Real Time) were obtained from
Takara Bio Inc.
Haemotoxylin-eosin (H&E) staining,
immunohistochemistry (IHC) and scoring
Tissue samples were fixed in 10% buffered formalin
for 24 h at room temperature, then dehydrated and
paraffin-embedded. Paraffin sections (4-µm-thick) were dewaxed in
xylene at room temperature and rehydrated using a graded ethanol
series (incubated 50% ethanol for 10 min, 70% ethanol for 10 min,
80% ethanol for 10 min, 95% ethanol for 10 min, 100% ethanol for 10
min thrice). In order to ensure that cancer tissue was collected,
all slides were reviewed using H&E staining. Hematoxylin
staining was performed for 4 min and eosin staining for 1 min at
room temperature. DVL2 protein levels in PDAC tissues were detected
by immunohistochemistry using a standard immunoperoxidase staining
procedure. The slides were blocked in BSA blocking buffer (cat. no.
37520; Thermo Fisher Scientific Inc.) at room temperature for 1 h.
The slides were then incubated at 4°C overnight with anti-DVL2
(1:100; cat. no. sc-390303; Santa Cruz Biotechnology, Inc.). The
slides were then incubated with horse radish peroxidase-conjugated
secondary antibodies (1:1,000; cat. no. 7076; Cell Signaling
Technology, Inc.) at room temperature for 2 h. The slides were then
developed using diaminobenzidine (DAB) staining. These slides were
observed by an inverted light microscope (magnification, ×200;
Olympus Corporation). All stained sections were independently
viewed by 2 pathologists (Department of Human Pathology, The First
Affiliated Hospital of Kangda College of Nanjing Medical
University, Lianyungang, China), who were blinded to patient's
clinical data. The ratio of positive tumor cells was quantified,
and tumor cell proportion was evaluated as previously described
(13): 0, no staining; 1+, staining
<10%; 2+, staining of 10–35%; 3+, staining of 35–70%; and 4+,
staining of >70%. The staining intensity was graded from 0 to 3,
(0, none; 1, weak; 2, moderate; and 3, strong staining) The IHC
score for each case was calculated as the ratio of positive tumor
cells × staining intensity score. An optimal cutoff value was
identified as follows: cases with IHC score >4 were regarded as
high expression of DVL2, whereas cases with IHC score of ≤4 as low
expression.
Cell culture and stable
transfection
Normal human pancreatic ductal cell line hTERT-HPNE,
pancreatic adenocarcinoma cell line SW1990, PDAC cell lines
CFPAC-1, PANC-1 and BxPc-3 were purchased from the American Type
Culture Collection. Cell culture was performed as previously
described (14). SW1990, CFPAC-1,
PANC-1 and BxPc-3 were maintained in Dulbecco's Modified Eagle's
Medium (DMEM; Gibco; Thermo Fisher Scientific Inc.) supplemented
with penicillin (100 U/ml), 10% fetal bovine serum (FBS, Gibco;
Thermo Fisher Scientific Inc.) and streptomycin (100 µg/ml) at 37°C
under air with 5% CO2. hTERT HPNE was cultured in medium
containing 3 vol of glucose-free DMEM (cat. no. D-5030; Sigma;
Merck KGaA with additional 1.5 g/l sodium bicarbonate and 2 mM
L-glutamine), 5% FBS (Gibco; Thermo Fisher Scientific Inc.), 750
ng/ml puromycin, 1 vol of Medium M3 Base (cat. no. M300F-500;
InCell Corporation LLC), 5.5 mM D-glucose and 10 ng/ml human
recombinant epidermal growth factor (cat. no. CM1013; Guangzhou
Cellcook Biotech, Co., Ltd.) at 37°C in 5% CO2. The cell
line was thawed every 2 months.
Lentiviral constructs expressing short hairpin
(sh)RNA-DVL2 and scrambled shRNA (shNC) were purchased from
Shanghai GenePharma Co., Ltd. The sequences of the 2 target shRNAs
were as follows: DVL2-shRNA1# 5′-AGTCAACCTGTCTCTCAAT-3′;
DVL2-shRNA2# 5′-TCCACAATGTCTCTCAATA-3′; and shNC,
5′-TTCTCCGAACGTGTCACGT-3′. The 3rd lentivirus generation system,
which consisted of the GenePharma Supersilencing Vector containing
the shRNA sequence and lentiviral packaging plasmids (Helper
vector-I, Helper vector-II, Helper vector-III; Shanghai GenePharma
Co., Ltd.) were used to produce lentiviral particles for the
transduction of shRNA-mediated knockdown system. 293T cells,
supplied by Shanghai GenePharma Co., Ltd., were used to produce the
lentivirus. A total of 20 µg plasmids were used for transfection,
and the ratio of the lentiviral plasmid, packaging vector and
envelope vector was 1:4:3. Cells were cultured in DMEM medium with
lentiviruses at a multiplicity of infection of 30 for 48 h at room
temperature. After 48 h of infection at room temperature, PANC-1
and CFPAC-1 cells were treated with puromycin (1 µg/ml) and
cultured for ~2 weeks to obtain stably transfected cells.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA of different PDAC cells (CFPAC-1, PANC-1,
BxPC-3 and SW1990) and HPNE cells were extracted using
TRIzol® reagent and reverse-transcribed into cDNA using
PrimeScript RT Master Mix according to the manufacturer's protocol.
The RT-qPCR amplification was detected by SYBR Green PCR master mix
(Takara Bio Inc.). The primer sequences used were as follows: DVL2
forward, 5′-CGTCACAGATTCCACAATGTCT-3′ and reverse,
5′-TCGTTGCTCATGTTCTCAAAGT-3′ and GAPDH forward,
5′-CCATGTTCGTCATGGGTGTGAACCA-3′; and reverse,
5′-GCCAGTAGAGGCAGGGATGATGTTC-3′. The thermocycling conditions were
as follows: initial denaturation at 95°C for 5 min followed by 40
cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 15
sec and extension at 72°C for 30 sec. Each experiment was performed
at least 3 times. GAPDH was used as the internal control. Relative
results of mRNA expression were analyzed using the
2−ΔΔCq method (19).
Western blotting
Total protein from PDAC cells (CFPAC-1, PANC-1,
BxPC-3 and SW1990) and HPNE cells was collected and lysed on ice by
using an NP40 lysis buffer containing protease and phosphatase
inhibitors (Thermo Fisher Scientific Inc.). The concentrations were
measured using a bicinchoninic acid (BCA) protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). An equivalent amount of
protein (0.6 mg/lane) from each protein lysates was separated with
10% SDS-PAGE electrophoresis and transferred to a PVDF membrane
(MilliporeSigma). The membranes were incubated in 0.05%
Tris-buffered saline with 5% skimmed milk for 1 h at room
temperature and then incubated overnight with the appropriate
primary antibodies at 4°C, followed by HRP-conjugated secondary
antibody at room temperature for 1 h. Signal detection was
performed using an enhanced chemiluminescence kit (Beyotime
Institute of Biotechnology) and western blotting detection system
(Tanon Science & Technology Co., Ltd.). Western blotting
analysis was used by standard methods.
CCK-8 assay and colony formation
assay
CFPAC-1 and PANC-1 cells were plated at a density of
2.5×103 cells/well in 96-well plates. The relative
growth rate of transfected cells with small interfering (si)DVL2
and negative controls were measured by CCK-8 reagent (Dojindo
Molecular Technologies, Inc.) at different time points (24, 48, 72,
96 and 120 h). The absorbance value was measured in a microplate
reader at 450 nm. At least 3 wells were evaluated for each
group.
For the colony formation assay, CFPAC-1 and PANC-1
cells were seeded at a density of 500 cells/well in 6-well plates
and incubated for 10 days at room temperature. Cells were fixed
with 96% ethanol for 10 min and subsequently stained with 1%
crystal violet for 5 min at room temperature. The colonies were
counted manually and imaged using an inverted light microscope
(magnification, ×40; Olympus Corporation) and a digital camera
(Olympus Corporation).
Gap closure assay
CFPAC-1 and PANC-1 cells (1×105) were
seeded in Culture Inserts (EMD Millipore) as previously reported
(14). The culture inserts were
removed when cells reached 95% confluence. Cells were incubated in
serum-free DMEM and photographed at 0 and 24 h after scraping using
an inverted light microscope (magnification, ×200; Olympus
Corporation).
Transwell migration and matrigel
invasion assays
Transwell migration and invasion assays were
performed using transwell chambers fixed with or without Matrigel
at 37°C for 24 h, respectively. CFPAC-1 and PANC-1
(~2×104 cells) were added into the upper 8-µm-pore
chamber of transwell inserts (Merck KGaA) in a 200 µl serum-free
medium. The lower chamber contained 600 µl of 10% FBS culture
medium. After 48 h incubation at 37°C, the migrated or invaded
cells were fixed with paraformaldehyde at 37°C for 20 min, stained
with crystal violet at 37°C for 20 min and then counted using an
inverted light microscope (magnification, ×200; Olympus
Corporation).
Co-immunoprecipitation (co-IP) and
immunofluorescence imaging
PANC-1 or CFPAC-1 cells were lysed in NP40 lysis
buffer (Beyotime Institute of Biotechnology). Following
centrifugation at 13,000 × g for 15 min at 4°C, the 1/10 volume of
supernatant was collected as input and the remaining supernatant
was incubated with 20 µl/ml protein A/G sepharose beads (Beyotime
Institute of Biotechnology) at 4°C for 1 h to remove non-specific
hybrid proteins. Following centrifugation (12,000 × g at 4°C for 5
min), half of the supernatant was incubated with 2 µg anti-DVL2
(1:100; cat. no. sc-390303, Santa Cruz Biotechnology, Inc.), half
of the supernatant was incubated with 2 µg negative control rabbit
IgG (cat. no. A7016 Beyotime Institute of Biotechnology), together
with 20 µl protein A sepharose beads (Beyotime Institute of
Biotechnology) at 4°C overnight. The beads were washed 5 times with
cell lysis buffer and collected by centrifugation for 5 min at
3,000 × g at 4°C. The proteins were released by boiling the samples
and were analyzed by western blotting as previously described.
For immunofluorescence, cells were plated on
coverslips, washed 3 times with PBS, and were fixed by 4%
paraformaldehyde for 15 min at room temperature, permeabilized in
0.2% Triton X-100 for 10 min, blocked in 5% bovine serum albumin
for 40 min at room temperature and incubated with primary
antibodies (1:100; cat. no. 8480, Cell Signaling Technology, Inc.)
overnight at 4°C. Subsequently, Alexa Fluor-conjugated secondary
antibodies (1:1,000; cat. no. A31627; Thermo Fisher Scientific
Inc.) were incubated for 2 h at room temperature followed by
counterstaining with DAPI for 10 min at room temperature. Images
were obtained by confocal laser microscopy (magnification, ×20;
FV3000; Olympus Corporation). The β-catenin expression levels were
determined using Quantity One v.4.6.2 software (Bio-Rad
Laboratories, Inc.).
Xenograft tumorigenicity assays
Male BALB/c nude mice, 4–6 weeks of age, weight
15–20 g were obtained from the Model Animal Research Center at
Nanjing University (Nanjing, China). and were housed under specific
pathogen-free conditions, with food and water provided ad
libitum. The mice were randomly divided into 2 groups, 5 mice
in each group. All animal studies (including the mice euthanasia
procedure) were approved by the Animal Care Committee of The First
Affiliated Hospital of Kangda College of Nanjing Medical University
(Lianyungang, China) (approval no. KY20190924002) in compliance
with the regulations and guidelines of the Institutional Animal
Care.
Inhalation anesthesia was used with 1.5% isoflurane
in this experiment. A total of 1×106 PANC-1 cells with
shDVL2 PANC-1 cells or non-target shRNA suspending in 50 µl
phosphate-buffered saline were orthotopically injected (in the
pancreatic tail) in the mice through a median lateral laparotomy
(20,21). Three months after the laparotomy, the
mice were euthanized by cervical dislocation and examined for tumor
volume and suspected metastatic liver tissues.
Statistical analysis
All statistical results were calculated by SPSS 23
statistical software (IBM Corp.). Data are presented as the mean ±
SD of 3 independent experiments. A Chi-square (χ2) test
and Fisher's exact test were performed to analyze the association
between DVL2 expression and clinicopathological features. A
Kaplan-Meier analysis and log-rank test were used to assess and
compare the survival curves. Unpaired two-tailed Student's t-tests
were used to assess the differences between 2 independent groups.
ANOVA with the post hoc Tukey's test was performed to compare
differences between multiple groups. P<0.05 was considered
indicate a statistically significant difference.
Results
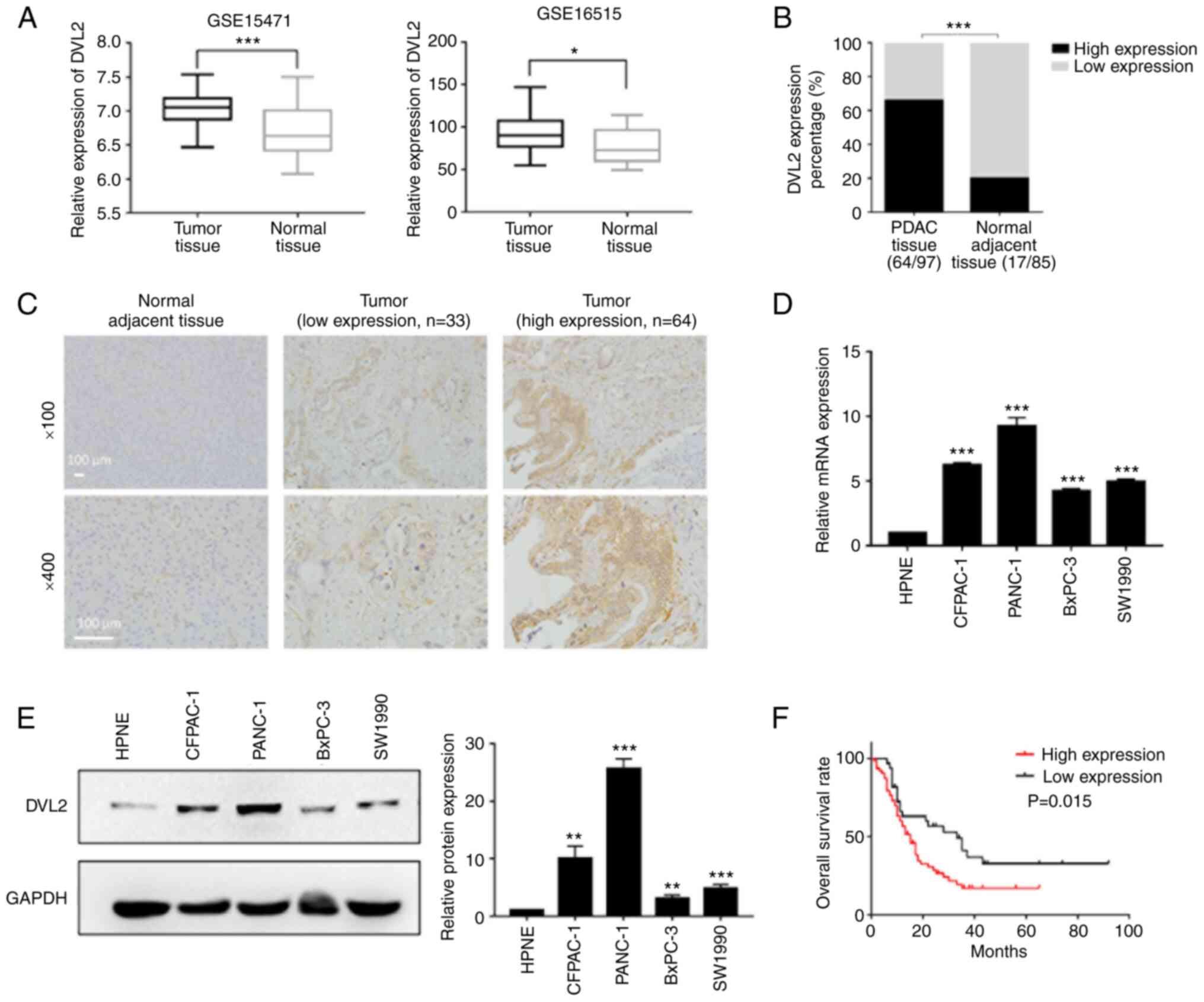
DVL2 is significantly upregulated in
PDAC tissues and cells
mRNA expression and prognostic values of DVL2 in
PDAC were analyzed by using the open Gene Expression Omnibus (GEO)
database (https://www.ncbi.nlm.nih.gov/geo/). The GEO database
was examined and the data from 2 mRNA expression profile datasets
(GSE15471 and GSE16515), which consisted of 36 pairs of tumor and
normal tissue samples, and 36 tumor samples and 16 normal samples,
respectively were extracted. Compared with adjacent normal tissues,
a significant upregulation of DVL2 in PC tissues was found after
analyzing the data from the 2 datasets (P<0.05; Fig. 1A). In addition, 97 PDAC tissues and
85 adjacent normal samples from The First Affiliated Hospital of
Kangda College of Nanjing Medical University (Lianyungang, China)
were used for IHC staining to analyze the association of DVL2 with
clinicopathological features. DVL2 was highly expressed in PDAC
tissues (66.0%, 64/97) compared with adjacent normal tissues
(20.0%, 17/85) (P<0.001; Fig. 1B and
C).
Expression levels of DVL2 were also detected in 4
pancreatic cancer cell lines (BxPC-3, SW1990, CFPAC-1, PANC-1) and
a normal cell line (hTERT-HPNE) by western blotting and RT-qPCR.
The protein and mRNA expression of DVL2 was highly upregulated in
pancreatic cancer cells, especially in PANC-1 and CFPAC-1 compared
with hTERT-HPNE cells (P<0.01; Fig.
1D and E).
In order to ascertain the prognostic value of DVL2
in PDAC, we assessed the relationship between DVL2 gene expression
and OS. The Kaplan-Meier analysis demonstrated that the patients
with a high level of DVL2 expression had poorer OS rate compared
with those with a low level of DVL2 expression (P<0.05; Fig. 1F). The aberrantly high expression of
DVL2 in PDAC tissues and cells, and the Kaplan-Meier analysis
results indicated that DVL2 might be a prognostic predictor in the
OS of PDAC patients.
DVL2 protein expression is associated
with PDAC clinicopathological features
According to immunohistochemical scores, the 97 PDAC
samples were divided into high (n=64) and low (n=33) expression
groups. The representative samples revealed that majority of the
DVL2 expression was positively immunostained in the cytoplasm of
PDAC tissues (images in the middle and the right 2 columns of
Fig. 1C). χ2 and Fisher's
exact test was performed to assess the association between DVL2
expression and clinicopathologic variables in PDAC tissues. The
results demonstrated that DVL2 expression levels were significantly
associated with tumor node metastasis (TNM) stage (P=0.015) and
pathological node (N) stage (P=0.046), but not with sex, age,
histological differentiation, T classification, perineural
invasion, blood vessel invasion, tumor size and location (Table I). These results suggest that DVL2
expression level is associated with metastatic ability of PDAC.
 | Table I.Association between DVL2 expression
and clinicopathological variables in patients with PDAC (n=97). |
Table I.
Association between DVL2 expression
and clinicopathological variables in patients with PDAC (n=97).
|
|
| DVL2
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Number | Low | High | P-value |
|---|
| Sex |
|
|
| 0.363 |
|
Male | 59 | 18 | 41 |
|
|
Female | 38 | 15 | 23 |
|
| Agea, years |
|
|
| 0.474 |
|
≤63 | 49 | 15 | 34 |
|
|
>63 | 48 | 18 | 30 |
|
| Tumor location |
|
|
| 0.342b |
|
Pancreatic head | 82 | 30 | 52 |
|
|
Non-head | 15 | 3 | 12 |
|
| Tumor size, cm |
|
|
| 0.913 |
|
<4 | 61 | 21 | 40 |
|
| ≥4 | 36 | 12 | 24 |
|
| Histological
differentiation |
|
|
| 0.259 |
| I
/I–II/II | 66 | 20 | 46 |
|
|
II–III/III | 31 | 13 | 18 |
|
| TNM stage |
|
|
| 0.015c |
|
I–IIA | 51 | 23 | 28 |
|
|
IIB-IV | 46 | 10 | 36 |
|
| T
classification |
|
|
| 0.940 |
| T1 or
T2 | 26 | 9 | 17 |
|
| T3 or
T4 | 71 | 24 | 47 |
|
| N
classification |
|
|
| 0.046c |
| N0 | 54 | 23 | 31 |
|
| N1 | 43 | 10 | 33 |
|
| M
classification |
|
|
| 1.000b |
| M0 | 93 | 32 | 61 |
|
| M1 | 4 | 1 | 3 |
|
| Perineural
invasion |
|
|
| 0.451 |
|
Present | 14 | 6 | 8 |
|
|
Absent | 83 | 27 | 56 |
|
| Blood vessel
invasion |
|
|
| 1.000b |
|
Present | 8 | 3 | 5 |
|
|
Absent | 89 | 30 | 59 |
|
Knockdown of DVL2 inhibits cellular
proliferation in CFPAC-1 and PANC-1 cells
To further assess the potential role of DVL2 in
PDAC, 2 cell lines (PANC-1 and CFPAC-1) with relatively higher DVL2
expression levels were used for DVL2 knockdown using 2 shRNAs. The
interference efficiency of the 2 shRNAs was confirmed by western
blotting and RT-qPCR. Both protein and mRNA expression levels of
DVL2 were decreased in shRNA#1- and shRNA#2-transfected cells
compared with negative controls (P<0.001; Fig. 2A and B). ShRNA#2 (shDVL2) displayed
the highest knockdown efficiency (P<0.001; Fig. 2A and B) and was selected for
subsequent experimentation. In addition, the results from the CCK-8
assays demonstrated that DVL2 knockdown significantly suppressed
the pancreatic cell proliferation rate compared with negative
control cells (P<0.001; Fig. 2C and
D). The same trend from the colony formation assays was
observed in the pancreatic cell lines. DVL2 knockdown significantly
suppressed the colony-forming capacity of pancreatic cell compared
with control cells (P<0.01; Fig. 2E
and F). Together, these results demonstrated that DVL2
knockdown inhibited the growth of PDAC cells in vitro.
DVL2 knockdown inhibits the migration
and invasion of PC cells in vitro
Given that upregulation of DVL2 was positively
associated with TNM stage and lymph node metastasis in PDAC tissues
(Table I), we speculated that DVL2
may contribute to the migration and invasion of PC. Indeed,
migratory and invasion abilities were markedly decreased in cells
with DVL2 knockdown compared with control cells (Fig. 3). DVL2 knockdown cells exhibited
significantly slower wound closure in wound healing assays compared
with control cells (P<0.001; Fig. 3A
and B). In addition, the results from transwell migration
(without Matrigel) or invasion (with Matrigel) assays revealed that
knockdown of DVL2 expression significantly reduced the migration
and invasion abilities of both cell lines compared with control
cells (P<0.01; Fig. 3C and D).
Our previous study demonstrated that DVL2 was involved in EMT
induced by IQGAP1 overexpression in PDAC (14). Hence, DVL2-mediated changes in EMT of
pancreatic cells was examined. EMT markers, including E-cadherin,
N-cadherin, vimentin and Snail (22,23),
induced by DVL2 knockdown were analyzed in the PANC-1 and CFPAC-1
cell lines by western blotting. The epithelial marker E-cadherin
(22,23) was significantly upregulated in DVL2
knockdown group compared with control group (Fig. 3E). By contrast, the mesenchymal
markers N-cadherin and vimentin (11), as well as the EMT-activating
transcription factor Snail, were significantly decreased on DVL2
knockdown in both PANC-1 and CFPAC-1 cell lines compared with
control cells (P<0.05; Fig. 3E).
These results demonstrated that DVL2 could promote migration and
invasion of PDAC via induction of the EMT pathway.
Decreased DVL2 promotes Wnt/β-catenin
signaling inactivation in PDAC
Several studies have reported that the Wnt/β-catenin
signaling pathway is significantly involved in EMT, tumorigenesis
and progression of PDAC (14,22,23).
It has been reported that knockdown of DVL2 reduces the expression
of β-catenin in esophageal cancer cells and lung cancer (13,24). In
contrast, DVL2 knockdown does not affect the expression levels of
β-catenin in hepatocellular carcinoma and human gliomas (11,25).
Hence, the present study examined whether the expression of
β-catenin could be regulated by changes of DVL2 in PDAC. The
expression levels of β-catenin were tested by western blotting and
immunofluorescence analysis. The results revealed that β-catenin
expression as well as the specific Wnt target genes Cyclin D1 and
matrix metalloproteinase 9 (MMP-9) (13) were downregulated in silenced DVL2
cells compared with control cells (P<0.01; Fig. 4A). In addition, it was demonstrated
that DVL2 interacted with β-catenin in PDAC cells (Fig. 4B). Immunofluorescence analysis
further revealed that the decreased expression levels of β-catenin
and nuclear translocation of β-catenin were observed in shDVL2
cells compared with in shNC cells as the fluorescence in the
nucleus decreased (P<0.001; Fig.
4C). These results suggested that DVL2 modulated both protein
expression and nuclear translocation of β-catenin.
DVL2 knockdown inhibits the proliferation and
invasion of PC cells in vivo. Finally, it was investigated
whether DVL2 could participate in tumorigenesis of PC cells in
vivo. Pancreatic tails of nude mice were orthotopically
injected with PANC-1-shDVL2 and corresponding negative control
cells. The mice were euthanized three months after the injection.
Their metastatic liver nodules and orthotopic pancreatic tumors
were counted. Mice with silenced DVL2 had a reduction in tumor
weight compared with control mice (P<0.001; Fig. 5A). IHC staining demonstrated that
tumors in the PANC-1-shDVL2 group had lower DVL2 expression
compared with those in the control group (Fig. 5A). The DVL2 positive cells with brown
stain almost disappeared in shDVL2 mice. The PANC-1-shDVL2 group
also exhibited fewer visible liver metastatic nodules compared with
the control group (P<0.001; Fig.
5B). Taking together, these in vivo results indicated
that DVL2 silencing inhibited the proliferation and metastasis of
pancreatic cells.
 | Figure 5.Effects of DVL2 knockdown on the
proliferation and metastasis of PC cells in vivo. (A) Left,
representative photographs of orthotopic pancreatic tumors (black
arrows) from PANC-1-shDVL2 group and shNC group 3 months after
injection (n=5 per group). Middle, the tumor tissues were treated
with IHC staining of DVL2. Right, the weight of orthotopic
pancreatic tumors. (B) Hepatic metastasis model. Left,
representative liver metastatic nodules (black arrows) from DVL2
knockdown group and control group. Middle, typical images of
metastatic nodules in the liver by H&E staining. Right, the
numbers of metastatic liver nodules from the mice with the
indicated treatments (n=5 per group). ***P<0.001. DVL-2,
Dishevelled-2; NC, negative control; sh, short hairpin; IHC,
immunohistochemistry. |
Discussion
The present study demonstrated that the DVL2
expression was significantly elevated in PDAC tissues compared with
adjacent normal tissues. In addition, it also demonstrated that
DVL2 expression was positively linked to the TNM stage and N
classification in PDAC. Previous studies have reported that an
elevated DVL2 expression level is significantly associated with
poor prognosis in hepatocellular carcinoma and esophageal squamous
cell cancer (11,13). To the best of our knowledge, the
present study is the first study that reported that patients with
PDAC with high DVL2 expression had shorter OS times compared with
patients with low DVL2 expression, thus suggesting that DVL2 acts
as a potential tumor promoter.
To elucidate the possible functional significance of
DVL2 in pancreatic cancer, loss-of-function assays were performed
in vitro using pancreatic cell lines. The present study,
revealed that knockdown of DVL2 in PANC-1 and CFPAC-1 cells
inhibited cell proliferation, migration, invasion and suppressed
tumor growth and metastasis in vivo. Taken together, the
results of the present study indicated that DVL2 was critical for
the proliferation and invasion of PDAC. In addition, the present
study also evaluated the effect of DVL2 knockdown on normal
hTERT-HPNE cells to determine whether the effect is cancer-cell
specific. However, hTERT-HPNE cells transfected with shDVL2 were
prone to death (data not shown). DVL2 was reported to be a signal
transducing protein that participates in canonical and noncanonical
WNT signaling, which is involved in pancreas development, islet
function, insulin production and secretion, and the attenuation of
which perturbed pancreatic growth (26). The expression level of DVL2 needs to
be maintained within a certain range. The aberrant high expression
of DVL2 in pancreatic cells made it a potential therapeutic target,
while the absence or knockdown of DVL2 in normal pancreatic cells
was hazardous for cell survival (26).
There are 3 highly conserved domains in DVL (DVL1-3)
protein sequences: DIX (Dis/Axinhomologous domain), DEP and PDZ
(PSD-95 and ZO-1 domain) (27,28). The
PDZ domain can bind DVL2 with Frizzled proteins (29). The DEP domain is required for DVL
membrane recruitment to Frizzled proteins after Wnt3a treatment
(30). The Wnt signalosome
transduction is associated with DIX polymerization and DEP
dimerization (31). Studies have
suggested that the deletion of PDZ domain can reduce cytosolic
β-catenin levels, decrease T cell factor-dependent transcriptional
activity of β-catenin and suppress tumorigenesis of mesothelioma
(32). In addition, DVL2 has been
proven to enhance tumorigenesis by binding with the
ataxia-telangiectasia group D complementing gene through
stabilizing β-catenin (33).
Downregulation of DVL reduces the binding of β-catenin to the c-myc
promoter and the transcription of Wnt target genes, including
c-myc, Axin2 and Fgf8 (34). The
nuclear translocation of DVL is believed to display functional
activity in Wnt/β-catenin signaling (35). Suppression of DVL2 activates the
destruction complex including APC, axin, casein kinase Iα (CKIα)
and Glycogen synthase kinase 3β (GSK3β), decreases the
phosphorylation GSK-3β and reduces the accumulation of β-catenin in
esophageal cancer cells and lung cancer (13,24),
while DVL2 knockdown does not affect the expression levels of
β-catenin in hepatocellular carcinoma and human gliomas (11,25). The
transfection of DVL2 siRNA doesn't alter the total protein levels
and the nuclear protein levels of β-catenin in anaplastic lymphoma
kinase-positive anaplastic large cell lymphoma (36). The downregulation of DVL2 expression
has less effect on Wnt/β-catenin signaling compared with the other
2 isoforms (DVL1 and DVL3) in mouse F9 teratocarcinoma cells
(37). The results of the present
study revealed that DVL2 forms a complex with β-catenin by co-IP
assays. In addition, the findings of the present study indicated
that knockdown of DVL2 in PANC-1 and CFPAC-1 cells decreased
β-catenin levels, as well as that of specific Wnt target genes,
including cyclin D1 and MMP9. In the present study, the knockdown
of DVL2 diminished β-catenin nuclear translocation, suggesting that
DVL2 serves a vital role in the aberrant activation of the
canonical Wnt/β-catenin pathway. The function of this interaction
may be that DVL2 promotes the binding of β-catenin to the c-myc
promoter and the transcription of Wnt target genes in the nucleus,
while downregulation of DVL2 activates the destruction complex,
decreases the phosphorylation GSK-3β and reduces the accumulation
of β-catenin (24).
EMT initiates tumor metastasis with the loss of
tumor cell-cell adhesion and the gain of tumor invasion (38). DVL2 is involved in the regulation of
EMT in breast cancer (39) and has
been found to increase Snail transcription through recruitment of
Forkhead box El (40). It has been
previously demonstrated that the knockdown of DVL2 inhibited EMT
induced by IQGAP1 overexpression (14). In the present study, DVL2
downregulation augmented the expression of epithelial marker
E-cadherin, decreased the mesenchymal markers (N-cadherin and
vimentin) and EMT-activating transcription factor Snail, further
confirming its role on EMT regulation in pancreatic cancer. In
addition, the present study demonstrated that DVL2 interacted with
β-catenin, modulated its expression level and facilitated β-catenin
translocation into the nucleus, suggesting that DVL2 was involved
in the regulation of EMT mediated by Wnt/β-catenin signaling.
Previous research found that DVL2 phosphorylation was essential for
the activation of canonical Wnt signaling (41). However, the phosphorylation level of
DVL2 in PC tissues and cells was not determined in the present
study. Further work is needed to elucidate the role of DVL2
phosphorylation in Wnt/β-catenin signaling mediated EMT.
In the present study, the effects of aberrantly
expressed DVL2 on PDAC were investigated. DVL2 expression was
upregulated in PDAC tissues and was positively associated with
advanced clinical stage and lymph node metastasis in patients with
PDAC. DVL2 knockdown impaired its oncogenic functions including
cell proliferation, migration, invasion and epithelial-mesenchymal
transition. DVL2 interacted with β-catenin and knockdown of DVL2
reduced the expression level of β-catenin and inhibited β-catenin
translocation into the nucleus. However, there were several
limitations in the present study. The molecular mechanisms by which
DVL2 was aberrantly regulated in PDAC cells and DVL2-knockdown
induced downregulation of β-catenin were not very clear. Future
mechanistic studies are required to verify the findings of the
present study. Further studies are needed to further explore the
precise oncogenic function and molecular mechanism of DVL2-induced
carcinogenesis.
In conclusion, the findings of the present study
suggested that DVL2 enhanced pancreatic tumorigenesis and
development, making it a promising new therapeutic target for PDAC.
Both in vitro and in vivo results of the present
study improved understanding of the underlying cellular mechanism
of DVL2 in the progression of EMT and metastasis of PDAC mediated
by Wnt/β-catenin signaling.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81900772) and the Foundation
of Health and Family planning Commission of Lianyungang (grant nos.
201802 and 201906) and the Startup Fund for Doctoral Research of
the First Affiliated Hospital of Kangda College of Nanjing Medical
University (grant no. BS202003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH, ML and JW designed and performed the
experiments. WH, ML, HC and TZ contributed to the analysis of data
and wrote the article. CZ and ZW made contributions to conception,
discussed the results and revised the manuscript. WH and ML confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Approval from Ethics committees of the First
Affiliated Hospital of Kangda College of Nanjing Medical University
(Lianyungang, China) (approval no. KY20190924002) were obtained
before enrollment into the study. Informed consent was obtained
from all individual participants included in the study. The study
was performed in accordance with government policies and the
Helsinki Declaration. All animal studies were approved by the
Animal Care Committee of the First Affiliated Hospital of Kangda
College of Nanjing Medical University (Lianyungang, China)
(approval no. KY20190924002) in compliance with the regulations and
guidelines of the Institutional Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu SL, Cao SG, Li Y, Sun B, Chen D, Wang
DS and Zhou YB: Pancreatic stellate cells facilitate pancreatic
cancer cell viability and invasion. Oncol Lett. 17:2057–2062.
2019.PubMed/NCBI
|
|
2
|
Xiao M, Li T, Ji Y, Jiang F, Ni W, Zhu J,
Bao B, Lu C and Ni R: S100A11 promotes human pancreatic cancer
PANC-1 cell proliferation and is involved in the PI3K/AKT signaling
pathway. Oncol Lett. 15:175–182. 2018.PubMed/NCBI
|
|
3
|
Tajima H, Makino I, Ohbatake Y, Nakanuma
S, Hayashi H, Nakagawara H, Miyashita T, Takamura H and Ohta T:
Neoadjuvant chemotherapy for pancreatic cancer: Effects on cancer
tissue and novel perspectives. Oncol Lett. 13:3975–3981. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buwenge M, Cilla S, Cammelli S, Macchia G,
Arcelli A, Farina E, Frakulli R, Panni V, Wondemagegnhu T, Uddin
AFMK, et al: Feasibility of 2D-conformal radiotherapy for
pancreatic carcinoma. Oncol Lett. 16:5939–5945. 2018.PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiou SH, Risca VI, Wang GX, Yang D,
Gruner BM, Kathiria AS, Ma RK, Vaka D, Chu P, Kozak M, et al:
BLIMP1 induces transient metastatic heterogeneity in pancreatic
cancer. Cancer Discov. 7:1184–1199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yokoyama N and Malbon CC: Dishevelled-2
docks and activates Src in a Wnt-dependent manner. J Cell Sci.
122:4439–4451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Zhang H, Zhang Y, Ng SS, Ren F,
Wang Y, Duan Y, Chen L, Zhai Y, Guo Q and Chang Z: Dishevelled-DEP
domain interacting protein (DDIP) inhibits Wnt signaling by
promoting TCF4 degradation and disrupting the TCF4/beta-catenin
complex. Cell Signal. 22:1753–1760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Jiao L, Hou J, Xu C, Wang L, Yu Y,
Li Y, Yang C, Wang X and Sun Y: Dishevelled-2 silencing reduces
androgen-dependent prostate tumor cell proliferation and migration
and expression of Wnt-3a and matrix metalloproteinases. Mol Biol
Rep. 40:4241–4250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang C, Li C, Chen X, Zhou Y, Yin B, Ni
R, Zhang Y and Liu J: Overexpression of dishevelled 2 is involved
in tumor metastasis and is associated with poor prognosis in
hepatocellular carcinoma. Clin Transl Oncol. 19:1507–1517. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu R, Cheng J, Chen Y, Wang W, Chen J and
Mao G: Potential role and prognostic importance of dishevelled-2 in
epithelial ovarian cancer. Int J Gynaecol Obstet. 138:304–310.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou G, Ye J, Sun L, Zhang Z and Feng J:
Overexpression of Dishevelled-2 contributes to proliferation and
migration of human esophageal squamous cell carcinoma. J Mol
Histol. 47:287–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu W, Wang Z, Zhang S, Lu X, Wu J, Yu K,
Ji A, Lu W, Wang Z, Wu J and Jiang C: IQGAP1 promotes pancreatic
cancer progression and epithelial-mesenchymal transition (EMT)
through Wnt/β-catenin signaling. Sci Rep. 9:75392019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Badea L, Herlea V, Dima SO, Dumitrascu T
and Popescu I: Combined gene expression analysis of whole-tissue
and microdissected pancreatic ductal adenocarcinoma identifies
genes specifically overexpressed in tumor epithelia.
Hepatogastroenterology. 55:2016–2027. 2008.PubMed/NCBI
|
|
16
|
Pei H, Li L, Fridley BL, Jenkins GD,
Kalari KR, Lingle W, Petersen G, Lou Z and Wang L: FKBP51 affects
cancer cell response to chemotherapy by negatively regulating Akt.
Cancer Cell. 16:259–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng YP, Zhu Y, Yin LD, Zhang JJ, Wei JS,
Liu X, Liu XC, Gao WT, Jiang KR and Miao Y: PEG10 overexpression
induced by E2F-1 promotes cell proliferation, migration, and
invasion in pancreatic cancer. J Exp Clin Cancer Res. 36:302017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chun YS, Pawlik TM and Vauthey JN: 8th
Edition of the AJCC cancer staging manual: Pancreas and
Hepatobiliary cancers. Ann Surg Oncol. 25:845–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aiello NM, Rhim AD and Stanger BZ:
Orthotopic injection of pancreatic cancer cells. Cold Spring Harb
Protoc 2016. pdb.prot078360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu J, Liu X, Liao YP, Salazar F, Sun B,
Jiang W, Chang CH, Jiang J, Wang X, Wu AM, et al: Nano-enabled
pancreas cancer immunotherapy using immunogenic cell death and
reversing immunosuppression. Nat Commun. 8:18112017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou P, Li Y, Li B, Zhang M, Liu Y, Yao Y
and Li D: NMIIA promotes tumor growth and metastasis by activating
the Wnt/beta-catenin signaling pathway and EMT in pancreatic
cancer. Oncogene. 38:5500–5515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu F, Xia Z, Zhang M, Ding J, Feng Y, Wu
J, Dong Y, Gao W, Han Z, Liu Y, et al: SMARCAD1 promotes pancreatic
cancer cell growth and metastasis through Wnt/β-catenin-Mediated
EMT. Int J Biol Sci. 15:636–646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo K, Gu X, Liu J, Zeng G, Peng L, Huang
H, Jiang M, Yang P, Li M, Yang Y, et al: Inhibition of disheveled-2
resensitizes cisplatin-resistant lung cancer cells through
down-regulating Wnt/β-catenin signaling. Exp Cell Res. 347:105–113.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pulvirenti T, Van Der Heijden M, Droms LA,
Huse JT, Tabar V and Hall A: Dishevelled 2 signaling promotes
self-renewal and tumorigenicity in human gliomas. Cancer Res.
71:7280–7290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papadopoulou S and Edlund H: Attenuated
Wnt signaling perturbs pancreatic growth but not pancreatic
function. Diabetes. 54:2844–2851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma M, Molehin D, Castro-Piedras I,
Martinez EG and Pruitt K: Acetylation of conserved DVL-1 lysines
regulates its nuclear translocation and binding to gene promoters
in triple-negative breast cancer. Sci Rep. 9:162572019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharma M, Castro-Piedras I, Simmons GJ and
Pruitt K: Dishevelled: A masterful conductor of complex Wnt
signals. Cell Signal. 47:52–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong HC, Bourdelas A, Krauss A, Lee HJ,
Shao Y, Wu D, Mlodzik M, Shi DL and Zheng J: Direct binding of the
PDZ domain of Dishevelled to a conserved internal sequence in the
C-terminal region of Frizzled. Mol Cell. 12:1251–1260. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paclikova P, Bernatik O, Radaszkiewicz TW
and Bryja V: The N-Terminal part of the Dishevelled DEP domain is
required for Wnt/β-Catenin signaling in mammalian Cells. Mol Cell
Biol. 37:e00145–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gammons MV, Renko M, Johnson CM,
Rutherford TJ and Bienz M: Wnt signalosome assembly by DEP domain
swapping of Dishevelled. Mol Cell. 64:92–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uematsu K, Kanazawa S, You L, He B, Xu Z,
Li K, Peterlin BM, McCormick F and Jablons DM: Wnt pathway
activation in mesothelioma: Evidence of Dishevelled overexpression
and transcriptional activity of beta-catenin. Cancer Res.
63:4547–4551. 2003.PubMed/NCBI
|
|
33
|
Wang L, Heidt DG, Lee CJ, Yang H, Logsdon
CD, Zhang L, Fearon ER, Ljungman M and Simeone DM: Oncogenic
function of ATDC in pancreatic cancer through Wnt pathway
activation and beta-catenin stabilization. Cancer Cell. 15:207–219.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP and Li
L: Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading
to stabilization of beta-catenin-TCF interaction. J Cell Biol.
180:1087–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Itoh K, Brott BK, Bae GU, Ratcliffe MJ and
Sokol SY: Nuclear localization is required for Dishevelled function
in Wnt/beta-catenin signaling. J Biol. 4:32005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hegazy SA, Alshareef A, Gelebart P, Anand
M, Armanious H, Ingham RJ and Lai R: Disheveled proteins promote
cell growth and tumorigenicity in ALK-positive anaplastic large
cell lymphoma. Cell Signal. 25:295–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YN, Gao Y and Wang HY: Differential
mediation of the Wnt canonical pathway by mammalian Dishevelleds-1,
−2, and −3. Cell Signal. 20:443–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Geng Y, Ju Y, Ren F, Qiu Y, Tomita Y,
Tomoeda M, Kishida M, Wang Y, Jin L, Su F, et al: Insulin receptor
substrate 1/2 (IRS1/2) regulates Wnt/β-catenin signaling through
blocking autophagic degradation of dishevelled 2. J Biol Chem.
289:11230–11241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu Y, Chang R, Peng Z, Wang Y, Ji W, Guo
J, Song L, Dai C, Wei W and Wu Y: Loss of polarity protein AF6
promotes pancreatic cancer metastasis by inducing Snail expression.
Nat Commun. 6:71842015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Esaki N, Enomoto A, Takagishi M, Mizutani
Y, Iida T, Ushida K, Shiraki Y, Mii S and Takahashi M: The
Daple-CK1epsilon complex regulates Dvl2 phosphorylation and
canonical Wnt signaling. Biochem Biophys Res Commun. 532:406–413.
2020. View Article : Google Scholar : PubMed/NCBI
|