Introduction
Capacitive-resistive electrothermal therapy (CRET)
is based on the non-invasive application of 0.45–0.60 MHz
radiofrequency (RF) currents that, when circulating through the
treated tissues, causes a temperature increase due to ion
reorientation and friction (1). This
therapy has been applied successfully for skin, muscle and
osteoarticular tissue repair (2,3), as well
as for the treatment of arthritis (4), Peyronie's disease (5) and ailments involving poor blood flow
(6,7).
There are indications that the induction of cellular
responses of a subthermal or microthermal nature could also play a
role in the therapeutic effects of these RF currents. Indeed, our
previous studies have revealed that electrical currents used in
CRET therapies, when applied in vitro at subthermal
densities, could induce significant effects on the proliferation
(8), differentiation (9,10) or
viability of different human cell types (1). These effects, which differ between
distinct cell types and are non-linearly dependent on RF signaling
parameters, such as frequency, modulation or current density, were
found to be mediated by electrically-induced changes in the
regulation of proteins involved in the aforementioned processes
(11,12). In cancer cells, CRET currents have
been shown to induce anti-proliferative and/or cytotoxic responses
(11,13–15).
The Ras/Raf/RAF/MEK/ERK signaling pathway plays an
essential role in the regulation of liver cell proliferation
(16), and alterations in this
pathway have been reported to be involved in the promotion of
hepatocellular carcinoma (HCC) (17). Thus, inhibition of the Ras/ERK
signaling pathway is considered a plausible approach for HCC
treatment. In this regard, the multikinase inhibitor, sorafenib, is
the only systemically applicable chemotherapeutic drug approved by
the United States Food and Drug Administration for the standardized
treatment of HCC (18,19). Sorafenib acts predominantly through
the inhibition of pathways involved in cell survival and
angiogenesis, such as the VEGFR or the platelet-derived growth
factor receptor (PDGFR) pathways (20), as well as the Raf kinases pathway, in
which sorafenib inhibits MEK and ERK phosphorylation (16). In the human cell line HepG2, CRET
exerts anti-proliferative and differentiating effects through
changes in the regulation of various proteins, including cyclins,
cyclin-dependent kinase inhibitors or kinases of MAPK pathways such
as ERK1/2 (1,9,21).
Thus, both CRET and sorafenib have been found to
inhibit HepG2 cell proliferation through their effects on members
of the Ras/ERK signaling pathway. This crosstalk between the
electrical and chemical response pathways leads to the possibility
of competitive, neutralizing or blocking interactions between both
types of treatment. The potential existence of interactions of this
nature raises doubts about the advisability of patients with cancer
being treated with CRET for analgesic, anti-inflammatory,
cicatrization or other purposes, while undergoing chemotherapy. The
present study aimed to analyze the in vitro effects of
simultaneous treatment with sorafenib and 448-kHz CRET electrical
current on the proliferation and viability of the cell line HepG2.
The obtained results revealed no evidence to suggest that the
electrical treatment counteracted or neutralized the cellular
response to sorafenib in the different conditions tested. On the
contrary, under certain conditions, the combined treatment produced
a significantly stronger anti-proliferative response than that
induced by each of the treatments when applied individually.
Materials and methods
Cell culture
The human liver cancer cell line, HepG2, was
obtained from the European Collection of Authenticated Cell
Cultures (cat. no. 85011430). The cells were seeded into
75-cm2 T-flasks (Falcon; Corning Life Sciences) in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
(vol/vol) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 4 mM L-glutamine (Gibco; Thermo Fisher Scientific, Inc.) and
100 U/ml penicillin-streptomycin with fungizone (Gibco; Thermo
Fisher Scientific, Inc.), and incubated in a 5% CO2
atmosphere at 37°C. The cultures were trypsinized and sub-cultured
once a week.
Treatment procedures
The procedure and materials for CRET exposure have
been described in previous studies (1,13).
Briefly, the cells were seeded in 60-mm Petri dishes (Nunc; Thermo
Fisher Scientific, Inc.) at a density of 105 cells/ml.
The plates were divided into four groups: Sham-treated control
cells incubated in the presence of unenergized electrodes, cells
treated with CRET only, cells treated with sorafenib only and cells
simultaneous treated with sorafenib + CRET. On day 4 after seeding,
when 70–80% confluence was reached, the cultures were subjected to
electrical or chemical treatment or both. Based on previously
reported data on the response of HepG2 cells to sorafenib (22,23),
cells were treated with sorafenib for 48 hours at concentrations of
3 µM, 5 µM or 7 µM, using DMSO (Gibco; Thermo Fisher Scientific,
Inc.) at a 1:1,000 dilution in DMEM with 10% FBS as a vehicle.
For simultaneous electrical and chemical treatment,
on day 4 post-seeding, after adding sorafenib, stainless steel
electrodes designed ad hoc for CRET treatment were inserted in all
plates, both treated and controls. Treatment with sinewave current
at 448 kHz and 50 µA/mm2 was applied cyclically (5 min
on/235 min off) for a total of 24 h, using a CRET system Indiba
Activ HCR 902 power supply (Indiba S.A.). All cultures were then
incubated for an additional 24 h in the absence of electrical
stimulation. For the western blotting experiments, the samples
received a short, 4-h exposure to the combined treatment. At the
end of the chemical and/or electrical treatments, the samples were
processed for the corresponding assays.
Analysis of cell viability by
quantification with Trypan Blue
The cells were trypsinized with a 0.25% solution of
trypsine (Gibco; Thermo Fisher Scientific, Inc.) and diluted in 1
ml of supplemented DMEM culture medium. Aliquots of this solution
were stained with 0.4% Trypan Blue (Sigma-Aldrich; Merck KGaA)
diluted 1:4 in PBS (Gibco) and counted in a Neubauer chamber for
assessment of cell viability.
XTT viability assay
Cell viability was determined by XTT assay (Roche
Diagnostics GmbH). After sham, electrical, chemical or simultaneous
treatments, the cells were incubated for 3 h with the tetrazolium
salt XTT in a 37°C and 6% CO2 atmosphere, as recommended
by the manufacturer. The metabolically active cells reduced XTT
into coloured formazan compounds that were quantified with a
microplate reader (Tecan) at a 492 nm wavelength.
Immunofluorescence evaluation of the
proliferation marker Ki67
An immunofluorescence assay for Ki67 was carried out
on cells cultured on coverslips. At the end of the 48-h treatment
period, the cells were fixed with 4% paraformaldehyde and
permeabilized with 95/5 (vol/vol) ethanol/acetic acid. The cells
were incubated overnight at 4°C with the monoclonal primary
antibody, anti-Ki67 (SP6; 1:250, cat. no. ab16667; Abcam). Next,
the secondary antibody, Alexa Fluor® 488-conjugated goat
anti-rabbit IgG (1:500; cat. no. A11034; Thermo Fisher Scientific,
Inc.) was added, and the samples were incubated at room temperature
for 1 h. The preparations were counterstained, mounted in ProLong™
Gold antifade reagent with DAPI (cat. no. P36941; Thermo Fisher
Scientific, Inc.), and observed through an inverted fluorescence
microscope (Nikon Eclipse Ts2R; Nikon Corporation) attached to a
digital camera DS-Ri2 (Nikon Corporation). Images from three
experimental replicates were recorded, and Ki67+ cells
as well as total cells were counted with NIS-Elements Br image
software (version 4.40; Nikon Corporation). Ki67+ cell
identification was based on fixed thresholds of fluorescence
determined and automated at the beginning of the analysis. In each
experimental repeat, 15 microscope fields were analyzed per
experimental condition.
Cell cycle analysis by flow cytometry
using propidium iodide
After trypsinization, the cells were centrifuged,
resuspended in 70% ethanol and fixed at 4°C for ≥24 h. To detect
apoptotic cells that could be suspended in the culture medium, the
media from all plates were collected and centrifuged. The resulting
pellets were processed together with the rest of the cells on the
plates. For propidium iodide labeling, the cells were resuspended
and stained with a solution of 3.4 mM sodium citrate (PanReac
Quimica SLU), 100 µg/ml RNAse A (Roche Diagnostics GmbH) and 20
µg/ml propidium iodide (Roche Diagnostics GmbH), and incubated in
the dark at room temperature for 1 h.
For data acquisition, a total of 20,000 events were
counted using FACScan Mod and FACScalibur flow cytometer (BD
Biosciences). The obtained data were analyzed using CellQuest 3.2
software (BD Immunocytometry Systems).
Assessment of proliferating cell
nuclear antigen (PCNA), cyclin D1, phosphorylated p-ERK1/2, ERK1/2,
p-EGFR and EGFR expression by western blotting
At the end of each experimental replicate, the cell
samples were centrifuged and lysed in lysis buffer containing 10 mM
Tris HCl (Merck KGaA) pH 7.6, 10 mM KCl (Merk KGaA), 1 mM
dithiothreitol (Sigma-Aldrich; Merck KGaA), 1 mM EDTA (Bio-Rad
Laboratories, Inc.), 1 mM PMSF (Sigma-Aldrich; Merck KGaA), 10
µg/ml leupeptin (Sigma-Aldrich; Merck KGaA), 5 µg/ml pepstatin
(Sigma-Aldrich; Merck KGaA), 100 mM NaF (Sigma-Aldrich; Merck
KGaA), 20 mM β-glycerophosphate (Calbiochem; Merck KGaA), 20 mM
sodium molybdate (Sigma-Aldrich; Merck KGaA), 0.5% Triton X-100
(ICN Biomedicals, Inc.) and 0.1% SDS (Bio-Rad Laboratories, Inc.).
Protein concentration was determined using a Pierce BCA Protein
assay (Thermo Fisher Scientific, Inc.). Next, protein samples
(100-µg protein aliquots) were separated in 8–10% SDS-PAGE and
electrophoretically transferred onto nitrocellulose membranes
(Amersham; Cytiva) using a semi-dry system (Trans-Blot®
SD semi-dry transfer cell; Bio-Rad Laboratories, Inc.).
The blots were incubated at 4°C overnight in the
presence of the following antibodies: Mouse monoclonal primary
anti-cyclin D1 (1:1,000; cat. no. P2D11F11; Novocastra Laboratories
Ltd.), mouse monoclonal anti-PCNA (1:1,000; cat. no. sc-56; Santa
Cruz Biotechnology, Inc.), rabbit polyclonal primary antibody
anti-p-EGFR (1:1,000; cat. no. 3777; Cell Signaling Technology,
Inc.), mouse monoclonal primary antibody anti-EGFR (1:1,000; cat.
no. MA5-13877; Thermo Fisher Scientific, Inc.), rabbit polyclonal
primary anti-ERK1/2 (1:1,000; cat. no. 9102S; Thermo Fisher
Scientific, Inc.) and rabbit polyclonal primary antibody p-ERK1/2
(1:1,000; cat. no. 44-680G; Thermo Fisher Scientific, Inc.).
Monoclonal mouse anti-β-actin antibody (1:5,000; A5441;
Sigma-Aldrich; Merck KGaA) was used as the loading control. To
detect non-phosphorylated forms of ERK1/2, the membranes were
stripped with 25 mM glycine at pH 2.0 for 30 min. The proteins of
interest were detected using horseradish peroxidase-conjugated
secondary antibodies during 1 h at room temperature (ECL donkey
anti-rabbit; 1:3,000; cat. no. NA934; sheep anti-mouse; 1:5,000;
cat. no. NA931; or IgG horseradish peroxidase-linked
species-specific whole antibody; all from Cytiva) or a
fluorescently labeled secondary antibody (IRDye® 800 CW
goat anti-rabbit polyclonal secondary antibody; 1:10,000; cat. no.
926-32211; LI-COR Biosciences) in same incubation conditions.
ChemiDoc Imaging system (Bio-Rad Laboratories, Inc.) was used to
detect ECL in the blots, while detection of phosphorylated forms of
EGFR (p-EGFR) was carried out through immunofluorescence
development with Odyssey system (LI-COR Biosciences). All images
were analyzed with Quantity One software (version 4.6.7; Bio-Rad
Laboratories, Inc.).
Statistical analysis
Statistical differences between groups were
determined by one-way ANOVA followed by Bonferroni post-hoc tests
or via an unpaired Student's t-test, using GraphPad Prism 6.01
software (GraphPad Software, Inc.). At least three independent
replicates were conducted per experiment and exposure interval.
Results were expressed as the mean ± SD or SEM. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of sorafenib on cell viability
when administered at different concentrations
The preselection of sorafenib concentrations
suitable for the combined treatment with CRET was carried out using
the Trypan Blue exclusion assay for cell viability. After 48 h of
treatment, sorafenib inhibited cell viability and increased cell
death in a dose-dependent manner (Fig.
1A and B). The half-maximal inhibitory concentration
(IC50) of sorafenib, as determined by non-lineal
regression analysis using Excel software (Microsoft Excel 2013,
15.0.4945.1000, 32 bits), was 7 µM (Fig.
1C).
Effects of CRET and sorafenib on cell
viability
The effects of the chemical and electrical
treatments, administered individually or in combination, were
quantified with Trypan Blue 48 h after initiation of the treatment.
The results of the Trypan Blue assay (Fig. 1D) showed that, when applied
individually, both CRET exposure and chemical treatment at
different concentrations significantly reduced cell viability
compared with that of the corresponding controls. In the combined
treatment, CRET did not significantly alter the reduction in cell
viability induced by 3 or 7 µM sorafenib. However, at the
intermediate concentration of 5 µM, which, when applied alone,
induced a decrease in viability equivalent to that elicited by CRET
alone (~85% of the value in the controls), combined exposure
resulted in a decrease in viability significantly greater than that
induced by chemical treatment (~70% of the value in the
controls).
XTT results showed in supplementary data (Fig. S1) revealed that this technique is
less sensitive than Trypan Blue for detecting the effects of CRET
on the viability of HepG2 cells. This poor sensitivity of the XTT
assay has been previously reported when describing the effects of
anticancer drugs such as sorafenib (which is capable of inducing
the generation of reactive oxygen species) on the viability of
HepG2 cells (24). Based on these
results, the use of XTT and other assays that evaluate cellular
metabolic function through reduction of tetrazolium salts to
formazan was discarded in the present study. The subsequent
experiments were focused on the response of HepG2 cells to CRET
exposure in the presence of 5 µM sorafenib (IC15).
Effects of CRET and sorafenib on the
expression of proteins involved in cell proliferation
The effects of CRET and sorafenib on cell
proliferation were assessed by PCNA and Ki67 protein expression
analysis. PCNA expression was analyzed at 4 and 48 h of treatment.
At 4 h, only the samples subjected to the combined treatment showed
a non-significant decrease in PCNA expression compared with that of
the controls. At 48 h, the samples treated with sorafenib, either
in the presence or absence of CRET stimulation, showed equivalent,
statistically significant reductions in PCNA expression. When
applied alone, the electrical treatment did not significantly
affect the expression of PCNA after 4 or 48 h of exposure (Fig. 2A and B). After 2 days of treatment,
the number of cells expressing the proliferation marker, Ki67, was
significantly decreased in the samples subjected to the combined
treatment and in those exposed separately to the chemical or
physical treatment compared with that in the controls. The effect
of combined treatment was significantly stronger than that of CRET
only (Fig. 2C and D).
Effects of CRET and sorafenib on
apoptosis and cell cycle
In order to identify processes underlying the
observed decrease in cell viability, the potential effects of the
aforementioned treatments on cell cycle or apoptosis were analyzed
by flow cytometry using propidium iodide staining. The data
summarized in Fig. 3 revealed that
the apoptosis rate (phase sub-G0/G1) was not
significantly increased in response to separate treatments with
CRET or 5 µM sorafenib, as well as to combined treatment. However,
the biological relevance of these potential proapoptotic effects is
expected to be rather limited, since the typical rates of
spontaneous apoptosis in the HepG2 cell line are markedly low (1.3%
in the controls). Cell cycle analysis revealed that the electrical
treatment reduced, although not significantly, the proportion of
cells in the S phase. Sorafenib when administered alone or in
combination with CRET, significantly reduced the rate of cells in
the S phase compared with the findings in the controls and in the
samples treated with CRET alone (Fig.
3A, B and S2).
Based on these results, the expression of cyclin D1,
a regulatory protein that participates in the progression of the
cell cycle from the G1 to S phase, was analyzed. At 4 h,
treatment with sorafenib applied alone or in combination with CRET
significantly reduced the expression of cyclin D1 compared with
that of the controls, and the effect of the combined treatment was
significantly stronger than that of CRET only. At the end of the
48-h treatment, only electrical treatment provoked a significant
reduction in cyclin D1 expression (Fig.
3C and D).
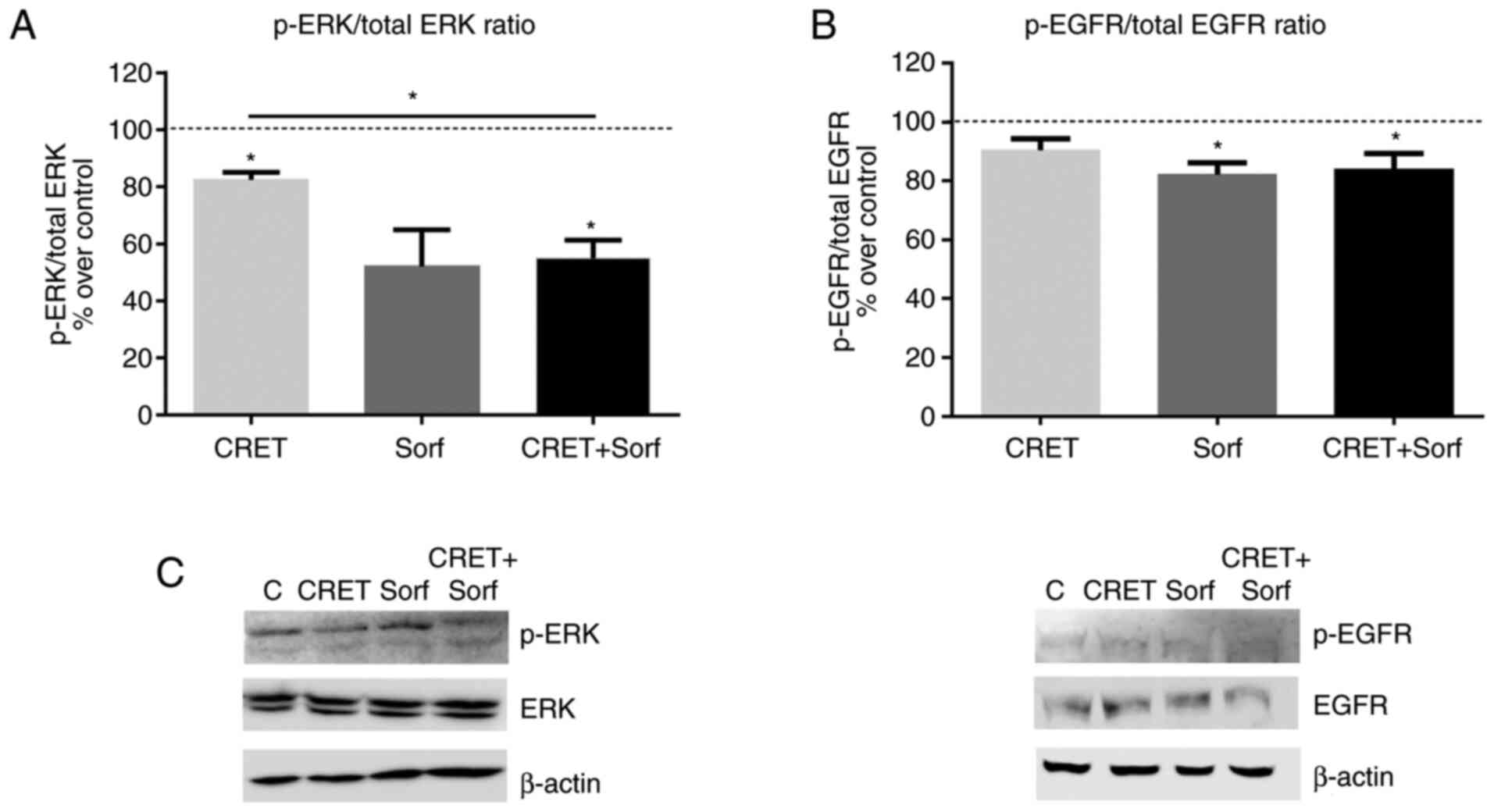
Effects of sorafenib and CRET on
ERK1/2 and EGFR
To study the potential early involvement of the
MAPK/ERK1/2 signaling pathway in the effects observed on cell
viability, the expression of the non-phosphorylated and
phosphorylated forms of ERK1/2 and EGFR in response to short-term
treatments, were analyzed by western blotting. After 4 h of
exposure, electrical treatment decreased ERK1/2 phosphorylation
(p-ERK1/2 over total ERK1/2 protein). This decrease was
significantly higher in the combined treatment (Fig. 4A and C). On the other hand, the
expression of the phosphorylated receptor (p-EGFR over total EGFR
protein) was not significantly affected by the electrical
treatment. In contrast, sorafenib and the combined treatment
significantly reduced the expression of the phosphorylated receptor
over controls (Fig. 4B and C)
Discussion
Electrothermal CRET therapies are currently used due
to their reported analgesic, anti-inflammatory and tissue
regenerative effects. The fact that these therapies are often
recommended for patients with cancer who are also undergoing
chemotherapy highlights the need to expand the currently
insufficient knowledge about potential interactions between
electrical and chemical therapies. The present study investigated
the effects of simultaneous treatment with the chemotherapeutic
drug, sorafenib, and a 448-kHz subthermal current on the
proliferation and viability of the liver cancer cell line, HepG2.
Our previous studies have shown that RF currents used in CRET
therapies can induce, by themselves and under subthermal
conditions, potential therapeutic responses, such as cell
proliferation and differentiation in human adipose-derived stem
cells (8–10).
Subthermal CRET treatments can also exert
anti-proliferative and/or cytotoxic effects in neuroblastoma and
HCC cell lines through altering the expression of proteins involved
in the MAPK/ERK1/2 signaling pathway (11,21).
This pathway, when inhibited by sorafenib, restrains cell
proliferation and induces apoptosis in HCC cells (17,25).
Thus, it is conceivable that, by exhibiting a mutual molecular
target, synergistic, additive, enhancing, neutralizing or blocking
interactions could occur between chemotherapeutic drugs and RF CRET
currents. This possibility raises questions about the convenience
of simultaneously exposing patients with cancer undergoing
chemotherapy to CRET therapies for analgesic, anti-inflammatory or
other purposes.
The present results support previously reported
observations that, when applied separately, subthermal CRET
(1,21,26) and
sorafenib (27,28) can significantly reduce liver cancer
cell viability. Besides, the present data showed that, at a
concentration of 5 µM sorafenib, the decrease in HepG2 viability
after combined treatment with CRET + sorafenib was significantly
larger than that induced when CRET or sorafenib were applied
individually. This was suggestive of a cooperative effect of both
treatments when combined, which could not be detected at lower or
higher doses of the chemotherapeutic drug.
The analysis of apoptosis as a phenomenon
potentially involved in the observed effects on the viability of
HepG2 cells, did not reveal any significant differences in
apoptosis rates, neither between the treated samples and their
respective controls, nor between responses to different treatments.
The lack of pro-apoptotic effects was consistent with previous
results that reported that CRET induces cytostatic, but not
cytotoxic, effects in HepG2 cells (26). Regarding sorafenib, the present
results also reinforced previous observations about its lack of
pro-apoptotic effects on HepG2 cells when administered at a
concentration of 5 µM (17).
The potential anti-proliferative effects of CRET and
sorafenib, applied together or separately, were assessed by
quantifying the expression of PCNA and Ki67, two specific
proliferation-related antigens that are currently used as
biomarkers for clinical prognosis in patients with HCC (29,30).
PCNA is a nuclear antigen involved in DNA replication, molecular
synthesis, mismatch repair and chromatin assembly (31). Ki67 is a nuclear protein that is only
present in proliferating cells (32). During interphase, Ki67 is involved in
the intracellular distribution of heterochromatin antigens and the
nucleolar association of heterochromatin. During mitosis, Ki67
intervenes in the formation of the perichromosomal layer, thus
preventing mitotic chromosome aggregation (33). The present results showed a
significant reduction in the expression of both markers after 48 h
of treatment with CRET only, with sorafenib only or with the
combined treatment. Since preclinical tumor models demonstrated
that silencing Ki67 or PCNA blocked cell cycle and proliferation
(32), these results indicated that
the three applied treatments may exert anti-proliferative effects
on HepG2 cells. Furthermore, the decrease induced by the combined
treatment in the expression of both proteins was significantly
stronger than that elicited by CRET when administered alone. This
may be indicative of a cooperative or synergistic action between
the anti-proliferative effects of CRET and sorafenib when applied
simultaneously.
The potential anti-proliferative effects were also
investigated by analyzing the percentage of cells present in the
different phases of the cell cycle. The data revealed a slight,
non-significant increase in the fraction of cells in the
G0/G1 phase after treatment with sorafenib
only, which is consistent with previously reported results
(34). In the absence of sorafenib,
CRET induced a non-significant decrease in the percentage of cells
in the S phase which is also consistent with previously reported
data (1). By contrast, a significant
decrease in the fraction of cells in the S phase was observed in
samples subjected to combined treatment, which could be the result
of an enhancing effect of weaker cytostatic responses potentially
induced by each of both stimuli.
The cell cycle is regulated by a variety of
proteins, including cyclin D1, which is involved in the progression
of the cell cycle from the G1 to S phase. This cyclin forms active
complexes with the cyclin-dependent kinases, CDK4 and CDK6, which
promote cell cycle progression through phosphorylation and
inactivation of the retinoblastoma protein (35). Since cyclin D1 is upregulated in
hepatocarcinomas (36), its
inhibition may be a useful chemo-preventative strategy for the
treatment of this type of cancer. The decreased expression of
cyclin D1 induced by the three treatments evaluated in the present
study is consistent with the corresponding reductions observed in
the rate of cells in S-phase. Taken together, these effects are
consistent with the aforementioned anti-proliferative response. In
line with these results, a previous study showed that CRET in
combination with chemical drugs could induce anti-proliferative
effects mediated by cell cycle arrest. Saitoh et al
(15) reported that CRET and
ascorbic acid synergistically inhibited the proliferation of
Ehrlich ascites tumor cells through the generation of reactive
oxygen species and inducing cell cycle arrest in the
G2/M phase.
The possibility that the Ras/ERK1/2 signaling
pathway may be involved in the anti-proliferative responses
observed in the present study has been investigated. The
Ras/Raf/MEK/ERK signaling cascade is an important MAPK signaling
pathway. Various stimuli capable of activating cell surface
receptors in turn activate this cascade, leading to the expression
of a number of genes that regulate cell proliferation,
differentiation and apoptosis. ERK regulates cell cycle progression
from the G1 to S phase through activation of cyclin D1, and ERK
phosphorylation has been shown to activate a variety of target
molecules involved in liver cancer. Since the Raf kinase of the
Ras/ERK signaling pathway is also known to be a molecular target of
sorafenib (25), the present study
investigated the involvement of the Ras/ERK signaling pathway in
the effects of CRET, sorafenib and the aforementioned combined
treatment, as well as the potential interactions between the
chemical and electrical treatments via such pathway. For that
purpose, the present study analyzed the expression levels of the
active and non-active forms of ERK1/2 and EGFR, which is one of the
receptors that activates the Ras/ERK1/2 signaling pathway (35). The anti-proliferative and angiogenic
effects of sorafenib on HCC cells is mediated by the inhibition of
the Raf, BRAF, VEGFR2, VEGFR3 and PDGFR pathways. Although the EGFR
pathway would not be directly involved in these effects, it has
been shown that the activation of this receptor modulates cellular
sensitivity to sorafenib (36). The
results obtained in the present study after 4 h of exposure to
CRET, alone or in combination with sorafenib, revealed that the
ratios p-ERK1/2/total protein and p-EGFR/total protein were
downregulated, which indicated that these treatments induce a
significant reduction in the activation, but not in the expression,
of ERK1/2 and EGFR. These early effects, which could lead to a
slower kinetics of the MAPK/ERK signaling pathway (25), would result in the anti-proliferative
responses observed 44 h later. In addition, the decreased
activation of EGFR induced by sorafenib or in combination, could
promote a decrease in the resistance of HepG2 cells to
sorafenib.
In conclusion, at the concentrations evaluated in
the present study, no evidence was found to suggest that CRET
exposure could compromise the anti-proliferative efficacy of
sorafenib on HepG2 cells. Furthermore, at the standard
pharmacological concentration of 5 µM, simultaneous treatment with
CRET induced an anti-proliferative response in HepG2 cells that was
significantly greater than that induced by each of the treatments
when applied separately. Such increase could be due to a
deceleration of the cell cycle, mediated at least in part by the
decreased expression of cyclin D1, and a slowdown in the kinetics
of the Ras/ERK1/2 signaling pathway. Taken together, these data do
not support the hypothesis that CRET exposure may inhibit or
diminish the effects of a chemotherapeutic drug used for cancer
treatment.
Despite the demonstrated beneficial effects of
sorafenib as a treatment for HCC, a significant number of patients
experience recurrence (25,37,38). The
present results and our previously reported data on the
anti-proliferative response of human cancer cells to subtermal
electrical stimulation, applied alone or in combination with
chemical agents, are consistent with those from a number of
experimental studies on therapeutic applications in oncology
(39–41). Thus, innovative treatments, such as
CRET plus chemotherapy, may offer novel possibilities for improving
the survival of patients with cancer, and highlight the need for
further research into novel therapeutic approaches for the
treatment of cancer, such as those based on the combined action of
electrical and chemical treatments.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
Foundation for Biomedical Research of the Ramón y Cajal University
Hospital (FiBio-HRC Project; grant no. 2015/0050) and the European
Defense Agency/Spanish Ministry of Defense (Project RF Biological
Effects; grant no. MOU EUROPA ERG 101.013).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MLHB, MAM, MAT and AU conceived and designed the
experiments, analyzed and interpreted the data, and wrote the
manuscript. MLHB, MAM, MAT, LM and ETM performed the experiments,
and collected and analyzed data. MLHB, MAM and AU confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hernández-Bule ML, Trillo MA, Cid MA, Leal
J and Ubeda A: In vitro exposure to 0.57-MHz electric currents
exerts cytostatic effects in HepG2 human hepatocarcinoma cells. Int
J Oncol. 30:583–592. 2007.PubMed/NCBI
|
|
2
|
Naranjo P, Lopez-Estebaranz J, Shoaib T
and Pinto H: Non-ablative capacitive resistive 448 kHz
radiofrequency for wrinkle reduction pilot study. Aesthetic Med.
6:41–48. 2020.
|
|
3
|
Yokota Y, Tashiro Y and Suzuki Y: Effect
of capacitive and resistive electric transfer on tissue
temperature, muscle flexibility, and blood circulation. J Nov
Physiother. 7:325–331. 2017. View Article : Google Scholar
|
|
4
|
Coccetta CA, Sale P, Ferrara PE, Specchia
A, Maccauro G, Ferriero G and Ronconi G: Effects of capacitive and
resistive electric transfer therapy in patients with knee
osteoarthritis: A randomized controlled trial. Int J Rehabil Res.
42:106–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pavone C, Romeo S, D'Amato F, Usala M,
Letizia Mauro G and Caruana G: Does transfer capacitive resistive
energy has a therapeutic effect on Peyronie's disease? Randomized,
single-blind, sham-controlled study on 96 patients: Fast Pain
Relief. Urol Int. 99:77–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bito T, Tashiro Y, Suzuki Y, Kajiwara Y,
Zeidan H, Kawagoe M, Sonoda T, Nakayama Y, Yokota Y, Shimoura K, et
al: Acute effects of capacitive and resistive electric transfer
(CRet) on the Achilles tendon. Electromagn Biol Med. 38:48–54.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fousekis K, Chrysanthopoulos G, Tsekoura
M, Mandalidis D, Mylonas K, Angelopoulos P, Koumoundourou D, Billis
V and Tsepis E: Posterior thigh thermal skin adaptations to
radiofrequency treatment at 448 kHz applied with or without
Indiba® fascia treatment tools. J Phys Ther Sci.
32:292–296. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hernández-Bule ML, Paíno CL, Trillo MÁ and
Úbeda A: Electric stimulation at 448 kHz promotes proliferation of
human mesenchymal stem cells. Cell Physiol Biochem. 34:1741–1755.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hernandez Bule ML, Angeles Trillo M,
Martinez Garcia MA, Abilahoud C and Ubeda A: Chondrogenic
differentiation of adipose-derived stem cells by radiofrequency
Electric stimulation. J Stem Cell Res Ther. 7:72017. View Article : Google Scholar
|
|
10
|
Hernández-Bule ML, Martínez-Botas J,
Trillo MÁ, Paíno CL and Úbeda A: Antiadipogenic effects of
subthermal electric stimulation at 448 kHz on differentiating human
mesenchymal stem cells. Mol Med Rep. 13:3895–3903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hernández-Bule ML, Medel E, Colastra C,
Roldán R and Úbeda A: Response of neuroblastoma cells to RF
currents as a function of the signal frequency. BMC Cancer.
19:8892019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trillo MÁ, Martínez MA and Úbeda A:
Effects of the signal modulation on the response of human
fibroblasts to in vitro stimulation with subthermal RF currents.
Electromagn Biol Med. 40:201–209. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernández-Bule ML, Roldán E, Matilla J,
Trillo MA and Ubeda A: Radiofrequency currents exert cytotoxic
effects in NB69 human neuroblastoma cells but not in peripheral
blood mononuclear cells. Int J Oncol. 41:1251–1259. 2012.PubMed/NCBI
|
|
14
|
Kato S, Saitoh Y and Miwa N: Repressive
effects of a capacitive-resistive electric transfer (CRet)
hyperthermic apparatus combined with provitamin C on intracellular
lipid-droplets formation in adipocytes. Int J Hyperthermia.
29:30–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saitoh Y, Yoshimoto T, Kato S and Miwa N:
Synergic carcinostatic effects of ascorbic acid and hyperthermia on
Ehrlich ascites tumor cell. Exp Oncol. 37:94–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Méndez-Sánchez N, Vásquez-Fernández F,
Zamora-Valdés D and Uribe M: Sorafenib, a systemic therapy for
hepatocellular carcinoma. Ann Hepatol. 7:46–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ge S and Huang D: Systemic therapies for
hepatocellular carcinoma. Drug Discov Ther. 9:352–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al SHARP Investigators Study Group, : Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hernández-Bule ML, Trillo MÁ and Úbeda A:
Molecular mechanisms underlying antiproliferative and
differentiating responses of hepatocarcinoma cells to subthermal
electric stimulation. PLoS One. 9:e846362014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Omar HA, Tolba MF, Hung JH and Al-Tel TH:
OSU-2S/Sorafenib synergistic antitumor combination against
hepatocellular carcinoma: The Role of PKCδ/p53. Front Pharmacol.
7:4632016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang SS, Ni YH, Zhao CR, Qiao Z, Yu HX,
Wang LY, Sun JY, Du C, Zhang JH, Dong LY, et al: Capsaicin enhances
the antitumor activity of sorafenib in hepatocellular carcinoma
cells and mouse xenograft tumors through increased ERK signaling.
Acta Pharmacol Sin. 39:438–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nowak E, Kammerer S and Küpper JH:
ATP-based cell viability assay is superior to trypan blue exclusion
and XTT assay in measuring cytotoxicity of anticancer drugs Taxol
and Imatinib, and proteasome inhibitor MG-132 on human hepatoma
cell line HepG2. Clin Hemorheol Microcirc. 69:327–336. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marisi G, Cucchetti A, Ulivi P, Canale M,
Cabibbo G, Solaini L, Foschi FG, De Matteis S, Ercolani G,
Valgiusti M, et al: Ten years of sorafenib in hepatocellular
carcinoma: Are there any predictive and/or prognostic markers?
World J Gastroenterol. 24:4152–4163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hernández-Bule ML, Cid MA, Trillo MA, Leal
J and Ubeda A: Cytostatic response of HepG2 to 0.57 MHz electric
currents mediated by changes in cell cycle control proteins. Int J
Oncol. 37:1399–1405. 2010.PubMed/NCBI
|
|
27
|
Chai H, Luo AZ, Weerasinghe P and Brown
RE: Sorafenib downregulates ERK/Akt and STAT3 survival pathways and
induces apoptosis in a human neuroblastoma cell line. Int J Clin
Exp Pathol. 3:408–415. 2010.PubMed/NCBI
|
|
28
|
Yang Y, Qin S-K, Wu Q, Wang ZS, Zheng RS,
Tong XH, Liu H, Tao L and He XD: Connexin-dependent gap junction
enhancement is involved in the synergistic effect of sorafenib and
all-trans retinoic acid on HCC growth inhibition. Oncol Rep.
31:540–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tiniakos DG and Brunt EM: Proliferating
cell nuclear antigen and Ki-67 labeling in hepatocellular nodules:
A comparative study. Liver. 19:58–68. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma S, Yang J, Li J and Song J: The
clinical utility of the proliferating cell nuclear antigen
expression in patients with hepatocellular carcinoma. Tumour Biol.
37:7405–7412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boehm EM, Gildenberg MS and Washington MT:
The many roles of PCNA in eukaryotic DNA replication. Enzymes.
39:231–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burkhart RA, Ronnekleiv-Kelly SM and
Pawlik TM: Personalized therapy in hepatocellular carcinoma:
Molecular markers of prognosis and therapeutic response. Surg
Oncol. 26:138–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun X and Kaufman PD: Ki-67: More than a
proliferation marker. Chromosoma. 127:175–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duval AP, Troquier L, de Souza Silva O,
Demartines N and Dormond O: Diclofenac potentiates sorafenib-based
treatments of hepatocellular carcinoma by enhancing oxidative
stress. Cancers (Basel). 11:112019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji L, Lin Z, Wan Z, Xia S, Jiang S, Cen D,
Cai L, Xu J and Cai X: miR-486-3p mediates hepatocellular carcinoma
sorafenib resistance by targeting FGFR4 and EGFR. Cell Death Dis.
11:2502020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lei X-F, Ke Y, Bao T-H, Tang HR, Wu XS,
Shi ZT, Lin J, Zhang ZX, Gu H and Wang L: Effect and safety of
sorafenib in patients with intermediate hepatocellular carcinoma
who received transarterial chemoembolization: A retrospective
comparative study. World J Clin Cases. 6:74–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Falzone L, Salomone S and Libra M:
Evolution of cancer pharmacological treatments at the turn of the
third millennium. Front Pharmacol. 9:13002018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giladi M, Weinberg U, Schneiderman RS,
Porat Y, Munster M, Voloshin T, Blatt R, Cahal S, Itzhaki A, Onn A,
et al: Alternating electric fields (tumor-treating fields therapy)
can improve chemotherapy treatment efficacy in non-small cell lung
cancer both in vitro and in vivo. Semin Oncol. 41 (Suppl
6):S35–S41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang W-H, Xie J, Lai Z-Y, Yang MD, Zhang
GH, Li Y, Mu JB and Xu J: Radiofrequency deep hyperthermia combined
with chemotherapy in the treatment of advanced non-small cell lung
cancer. Chin Med J (Engl). 132:922–927. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wust P, Stein U and Ghadjar P: Non-thermal
membrane effects of electromagnetic fields and therapeutic
applications in oncology. Int J Hyperthermia. 38:715–731. 2021.
View Article : Google Scholar : PubMed/NCBI
|