Introduction
Osteosarcoma, a common primary bone tumor in
adolescents and young adults, is one of the leading causes of
tumor-related mortality in individuals <18 years old (1). Over the past few years, owing to the
combination of surgery with chemotherapy, the 5-year survival rate
for patients with osteosarcoma has remained at between 50 and 80%
(2). However, the prognosis of
osteosarcoma patients is still poor, and distant metastasis or
local recurrence after primary tumor resection remains a major
clinical problem (3). Osteosarcoma
tumorigenesis and malignant progression have previously been found
to be associated with genetic mechanisms, but the complicated
molecular mechanism of the disease has not been revealed (4–7).
Therefore, the molecular mechanism of osteosarcoma requires
investigation and it is important that effective therapeutic
targets are determined.
Aneuploidy is considered to be a major feature of
chromosomal instability and cancer development (8). Targeting protein for Xenopus
kinesin-like protein 2 (TPX2) is a microtubule-associated protein
critical for spindle morphogenesis that plays a key role in
chromosomal instability (9).
Upregulation of TPX2 can cause centrosome amplification and lead to
DNA polyploidy, thus regulating cell proliferation, apoptotic
processes and cell division (10).
Upregulation of TPX2 has been reported in multiple types of cancer,
including colon, lung, pancreatic, cervical and salivary gland
cancer (11–15). These observations indicate that
TPX2 plays a pivotal role in the oncogenesis of malignancies.
However, it is unclear whether TPX2 is involved in the occurrence
and development in osteosarcoma.
Therefore, the aim of the present study was to
evaluate the effect of TPX2 on the development of osteosarcoma. The
study proposed to systematically summarize the expression of TPX2
and clarify the molecular mechanisms of TPX2 in the promotion of
osteosarcoma proliferation.
Materials and methods
Bioinformatics analysis
TPX2 and microRNA (miRNA/miR)-29c-3p expression
levels were explored using the Gene Expression Profiling
Interactive Analysis (GEPIA) (http://gepia.cancer-pku.cn/index.html) and OncoLnc
(http://www.oncolnc.org/) databases, respectively.
In GEPIA, the term ‘TPX2’ was used in the ‘Gene’ field and ‘SARC’
was used in the ‘Datasets Selection’ field. Differential expression
of TPX2 was defined with the following significance cutoff levels:
|Log2FC|>1 and P<0.01. In OncoLnc, ‘SARC’ was selected in the
‘miRNA’ column, and the data was downloaded. The information for
miR-29c-3p was obtained. The Kaplan Meier plotter (http://kmplot.com/analysis/) was used to analyze the
overall survival of patients with different levels of TPX2 and
miR-29c-3p expression in sarcoma. TargetScan (http://www.targetscan.org/vert_71/) was used to
explore the potential association between TPX2 and miR-29c-3p.
Patients and specimens
Between January 2008 and December 2018 (age range,
2–54 years; average age, 19.47±3.30 years), a total of 52 pairs of
primary osteosarcoma and adjacent noncancerous tissues were
obtained from patients who had undergone a wide resection of
osteosarcoma at the First People's Hospital of Lianyungang
(Lianyungang, China). Inclusion criteria were as follows:
Osteosarcoma patients without radiotherapy or chemotherapy before
surgery. Exclusion criteria were as follows: i) Osteosarcoma
patients who received radiotherapy or chemotherapy before surgery;
and ii) patients who refused to sign the informed consent form.
Morphologically normal tissues that were <5 cm from the
cancerous tissues were used as adjacent noncancerous tissues. All
samples were frozen at −80°C until further use. The histological
diagnosis of osteosarcoma conformed to the World Health
Organization's histological criteria for osteosarcoma (16), as confirmed by two professional
pathologists.
Ethical statement
The Ethics Committee of the First People's Hospital
of Lianyungang approved all protocols, which complied with the
Declaration of Helsinki (December 2018; approval no. 20180203).
Written informed consent was obtained from all patients and/or
their legal guardians.
Cell culture
Human osteosarcoma MG63 and U2OS cell lines, and the
human normal osteoblastic hFOB cell line were purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. All cells were cultured in DMEM (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C in a humidified incubator containing
5% CO2.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
RNA isolation and RT-qPCR were performed as
described previously (17).
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to extract RNA from the three cell lines and the
tissues. Next, the EasyScript One Step gDNA Removal and cDNA
Synthesis SuperMix (Beijing TransGen Biotech Co., Ltd.) was used to
acquire the first strand cDNA according to the manufacturer's
instructions. Next, qPCR was performed using SYBR-Green Master Mix
kit (Roche Diagnostics GmbH) with an Applied Biosystems 7900
Real-Time PCR System (Thermo Fisher Scientific, Inc.). The reaction
conditions were as follows: Pre-denaturation at 95°C for 15 min,
followed by 35 cycles of denaturation at 95°C for 15 sec, annealing
at 58°C for 30 sec and extension at 72°C for 5 min, and then a
final extension at 72°C for 15 min. Expression levels of RNA were
calculated based on the comparative 2−ΔΔCq method
(18). U6 was used as an
endogenous control for miR-29c-3p, and β-actin was used as an
endogenous control for TPX2. All experiments were performed in
triplicate. The primers used are listed in Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers.
| Primer | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| TPX2 |
ACCTTGCCCTACTAAGATT |
AATGTGGCACAGGTTGAGC |
| miR-29c-3p |
TAACCGATTTCAAATGGTGCTA |
TGGTGTCGTGGAGTCG |
| β-actin |
TCACCCACACTGTGCCCATCTACGA |
CAGCGGAACCGCTCATTGCCAATGG |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
siRNA transfection
Knockdown experiments for TPX2 were performed using
siRNA oligonucleotides purchased from Shanghai GenePharma Co., Ltd.
The TPX2 siRNA sequences were as follows: Forward,
5′-CCAUUAACCUGCCAGAGAAT-3′ and reverse, 5′-UUCUCUGGCAGGUUAAUGGT-3′.
The negative control for siRNA silencing was a non-targeting
(scramble) siRNA sequence, with the sequence
5′-UUCUCCGAACGUGUCACGUTT-3′. MG63 and U2OS cells were plated in
6-well plates at a density of 2×105 cells/well and
cultured in growth medium until cell confluence reached 70%.
Transfection of siRNA (20 nM) was then performed using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Transfection was performed at 37°C for 6 h before replacing the
transfection medium with full culture medium. At 48 h
post-transfection, cells were harvested for RT-qPCR or western blot
analysis.
Cell proliferation assay
The MG63 and U2OS cells were seeded in 96-well
plates at a density of 1,000 cells per well with 5 replicate wells
at 48 h post-siRNA transfection. Cell proliferation was measured
using an MTT assay at 0, 24, 48 and 72 h after seeding. Next, 150
µl dimethylsulfoxide was added per well to dissolve the purple
formazan. The absorbance was measured at a wavelength of 490 nm
using a microplate reader. All proliferation experiments were
performed in triplicate.
Luciferase reporter assay
MG63 and U2OS cells were seeded into 24-well plates.
After 24 h of incubation, 6 ng pmirGLO report vector (Shanghai
GenePharma Co., Ltd.) carrying the wild-type (wt) or mutant (mut)
3′-untranslated region (3′-UTR) of TPX2 was cotransfected with
miR-29c-3p mimics (100 nmol/l) or miR-29c-3p inhibitors (100
nmol/l) into the osteosarcoma cells using a Lipofectamine 2000 kit
(Invitrogen; Thermo Fisher Scientific, Inc.). The negative controls
for the miRNA mimics and inhibitors were non-targeting sequences.
The sequences of the mimics, inhibitors and negative controls were
as follows: miR-29c-3p mimic sense, 5′-UAGCACCAUUUGAAAUCGGUUA-3′
and antisense, 5′-UAACCGAUUUCAAAUGGUGCUA-3′; negative control mimic
sense, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′ and anti-sense,
5′-UCUACUCUUUCUAGGAGGUUGUGA-3′; miR-29c-3p inhibitor,
5′-UCUACUCUUUCUAGGAGGUUGUGA-3′; and negative control inhibitor,
5′-UAACCGAUUUCAAAUGGUGCUA-3′ (Shanghai GenePharma Co., Ltd.). The
amplified TPX2 wt or mut 3′UTR containing the miR-29c-3p target
sites was inserted into the pMIR-GLO luciferase reporter vectors
(Shanghai GenePharma Co., Ltd.). At 48 h post-transfection,
luciferase activities were examined with a dual-luciferase reporter
system (Shanghai GenePharma Co., Ltd.) and normalized with Renilla
luciferase activity using SpectraMax i3 (Molecular Devices
LLC).
Western blot analysis
Western blotting analysis was performed as
previously reported (17). MG63
and U20S cell lines were analyzed. Primary antibodies involved in
this assay were as follows: Anti-TPX2 (1:1,000; catalog no.
ab71816), anti-HSP90 (1:5,000; catalog no. ab203085), anti-AKT
(1:500; catalog no. ab8805), anti-p-AKT (1:1,000; catalog no.
ab18206), anti-BRCA1 (1:1,000; catalog no. ab245330) and anti-GAPDH
(1:1,000; catalog no. ab9485) (all Abcam). The secondary antibody
was anti-rabbit IgG (1:4,000; catalog no. ab150077; Abcam). Primary
antibodies were incubated at 4°C overnight, and secondary antibody
was incubated for 1 h at room temperature. The protein bands were
detected by a chemiluminescence detection system (Beyotime
Institute of Biotechnology). Pierce ECL Western Blotting Substrate
(cat. no. 32109; Thermo Fisher Scientific, Inc.) was used to
visualize the bound antibodies. Image analysis was performed using
ImageJ software (version 1.42; National Institutes of Health).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 8.0 (GraphPad Software, Inc.). Values are presented as the
mean ± standard deviation. The significance of differences between
two groups was assessed by paired or unpaired Student's t-test.
Pearson's χ2 and Fisher's exact tests were performed to
assess the categorical variables. Prognostic significance was
determined by Kaplan-Meier and log-rank analyses. A receiver
operating characteristic (ROC) curve was calculated to determine
the area under the curve (AUC) for biomarkers. Pearson's
correlation was used to evaluate the strength of linear correlation
between two continuous variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
TPX2 expression is upregulated and
miR-29c-3p expression is decreased in sarcoma
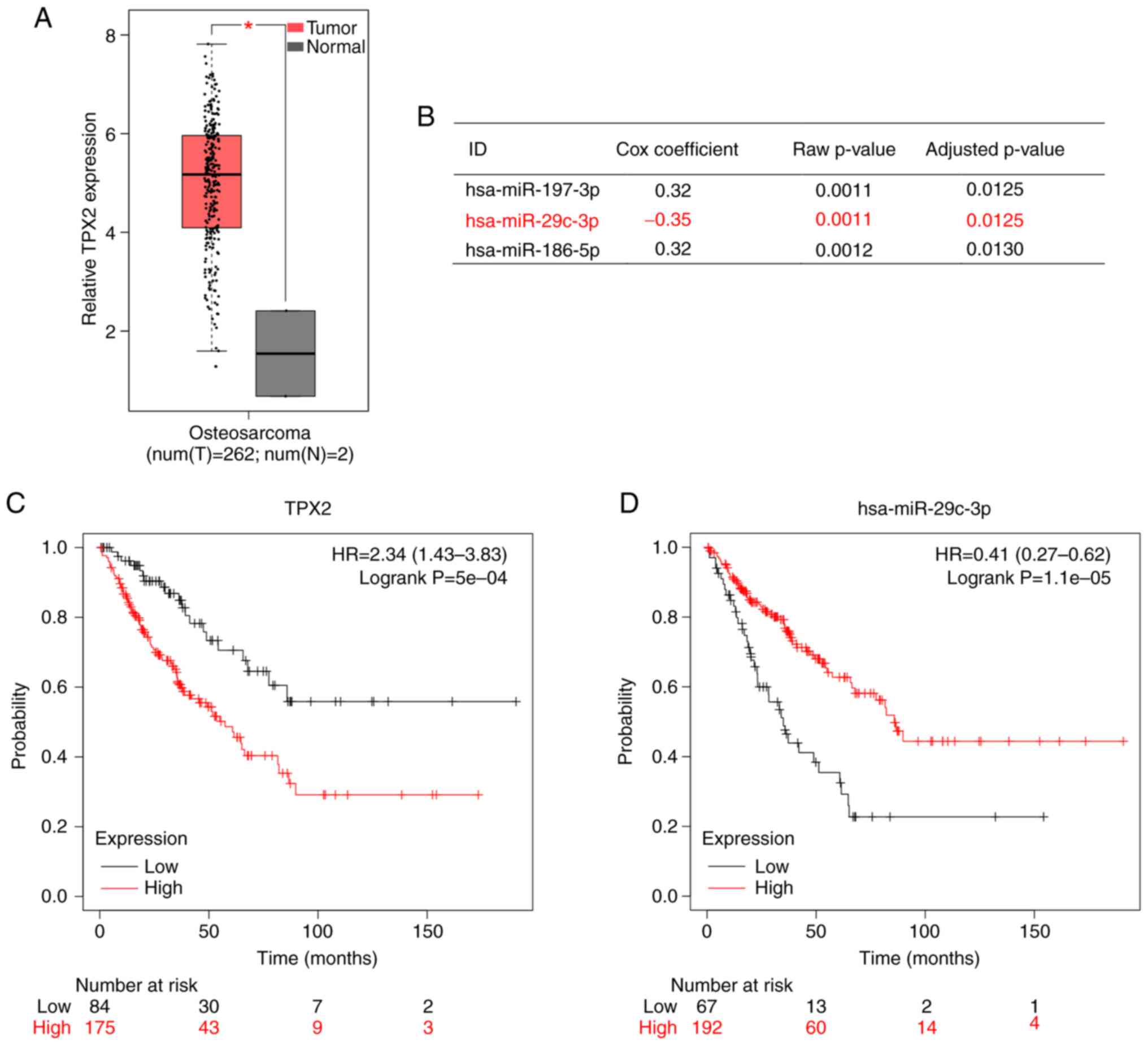
By analysis of the GEPIA public database, the
relative expression of TPX2 was found to be significantly higher in
sarcoma tissues (n=262) compared with that in normal tissues (n=2)
at the mRNA level (Fig. 1A). The
OncoLnc database showed that miR-29c-3p was expressed at lower
levels in tumor tissues compared with that in normal tissues
(Fig. 1B). Kaplan-Meier plotter
indicated that patients with high TPX2 expression experienced poor
overall survival (Fig. 1C), while
those with high miR-29c-3p expression experienced favorable overall
survival (Fig. 1D). In 52 pairs of
osteosarcoma tissues and adjacent normal tissues, the expression of
TPX2 was significantly upregulated in tumor tissues compared with
that in paired adjacent normal tissues (Fig. 2A). Additionally, miR-29c-3p
expression was downregulated in osteosarcoma (Fig. 2B). Furthermore, the TPX2 expression
level was measured in MG63 and U2OS cell lines. The RT-qPCR
analysis showed significantly higher mRNA levels of TPX2 in these
tumor cells compared with that in normal hFOB cells (Fig. 2C). By contrast, miR-29c-3p
expression was downregulated in the osteosarcoma cell lines
(Fig. 2D). Pearson's correlation
analysis disclosed a significant negative correlation between TPX2
and miR-29c-3p expression in osteosarcoma (P=0.002, r=−0.42)
(Fig. 2E).
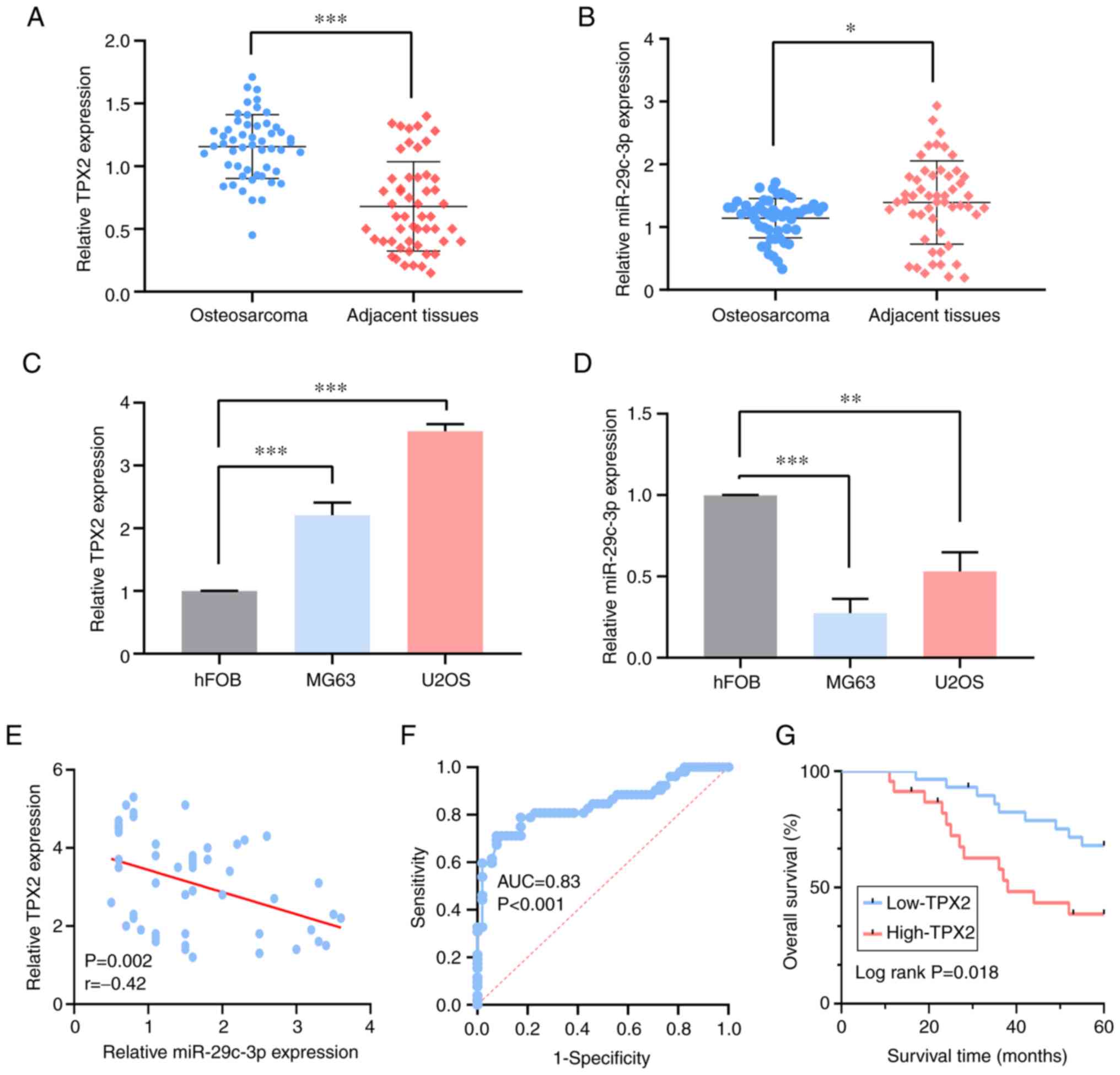 | Figure 2.TPX2 is upregulated in osteosarcoma
cell lines and tissues, and is associated with a poor prognosis in
patients with osteosarcoma. (A) The expression level of TPX2 in the
osteosarcoma samples was higher than that in the pair-matched
adjacent tissues, as determined by RT-qPCR. (B) The expression
level of miR-29c-3p in the osteosarcoma samples was lower than that
in the pair-matched adjacent tissues, as determined by RT-qPCR. (C)
TPX2 expression at the mRNA level was high in osteosarcoma cell
lines vs. the human osteoblast cells, as determined by RT-qPCR. (D)
miR-29c-3p expression level was low in the osteosarcoma cell lines
vs. the human osteoblast cells, as determined by RT-qPCR. (E)
Regression analysis of the correlation between miR-29c-3p and TPX2
expression. (F) The cut-off value of TPX2 was evaluated in the
patients with osteosarcoma. (G) Survival curves for patients with
osteosarcoma with regard to TPX2 expression. *P<0.05,
**P<0.01 and ***P<0.001. miR, microRNA; TPX2, targeting
protein for Xenopus kinesin-like protein 2; NC, negative
control; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; AUC, area under the curve. |
Association between TPX2 expression
and the clinicopathological characteristics of the osteosarcoma
patients
The optimal cut-off value of TPX2 was determined by
ROC analysis based on its expression level (P<0.001, AUC=0.83)
(Fig. 2F). The 52 patients were
consequently divided into two groups: TPX2 high expression (n=28)
and TPX2 low expression (n=24). The associations between TPX2
expression and various clinicopathological characteristics are
shown in Table II. The results
indicated that high TPX2 expression level was significantly
associated with tumor size (P=0.019), clinical stage (P=0.002) and
distant metastasis (P=0.018), compared with low expression level.
However, no association was found with other clinical parameters,
such as age, sex and anatomical location. Kaplan-Meier and log-rank
test analysis revealed that high TPX2 expression was significantly
associated with a poor prognosis (P=0.018) (Fig. 2G).
 | Table II.Association of TPX2 expression with
clinicopathological features of osteosarcoma. |
Table II.
Association of TPX2 expression with
clinicopathological features of osteosarcoma.
|
|
| TPX2
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Number of
cases | High, n (%) | Low, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.514 |
|
<18 | 13 | 6 (46.15) | 7 (53.85) |
|
|
≥18 | 39 | 22 (56.41) | 17 (43.59) |
|
| Sex |
|
|
| 0.209 |
|
Male | 38 | 18 (47.37) | 20 (52.63) |
|
|
Female | 14 | 10 (71.43) | 4 (28.57) |
|
| Tumor size, cm |
|
|
| 0.019 |
|
<8 | 17 | 5 (29.41) | 12 (70.59) |
|
| ≥8 | 35 | 23 (65.71) | 12 (34.29) |
|
| Anatomical
location |
|
|
| 0.736 |
|
Tibia/femur | 42 | 22 (52.38) | 20 (47.62) |
|
|
Elsewhere | 10 | 6 (60.00) | 4 (40.00) |
|
| Serum level of
lactate dehydrogenase |
|
|
|
|
|
Elevated | 38 | 22 (57.89) | 16 (42.11) | 0.366 |
|
Normal | 14 | 6 (42.86) | 8 (57.14) |
|
| Serum level of
alkaline phosphatase |
|
|
|
|
|
Elevated | 31 | 18 (58.06) | 13 (41.94) | 0.573 |
|
Normal | 21 | 10 (47.62) | 11 (52.38) |
|
| Clinical
stagea |
|
|
| 0.002 |
| I | 12 | 2 (16.67) | 10 (83.33) |
|
| II | 20 | 10 (50.00) | 10 (50.00) |
|
|
III | 20 | 16 (80.00) | 4 (20.00) |
|
| Distant
metastasis |
|
|
| 0.018 |
|
Absent | 34 | 14 (41.18) | 20 (58.82) |
|
|
Present | 18 | 14 (77.78) | 4 (22.22) |
|
| Response to
chemotherapy |
|
|
| 0.229 |
|
Good | 36 | 17 (47.22) | 19 (52.78) |
|
|
Poor | 16 | 11 (68.75) | 5 (31.25) |
|
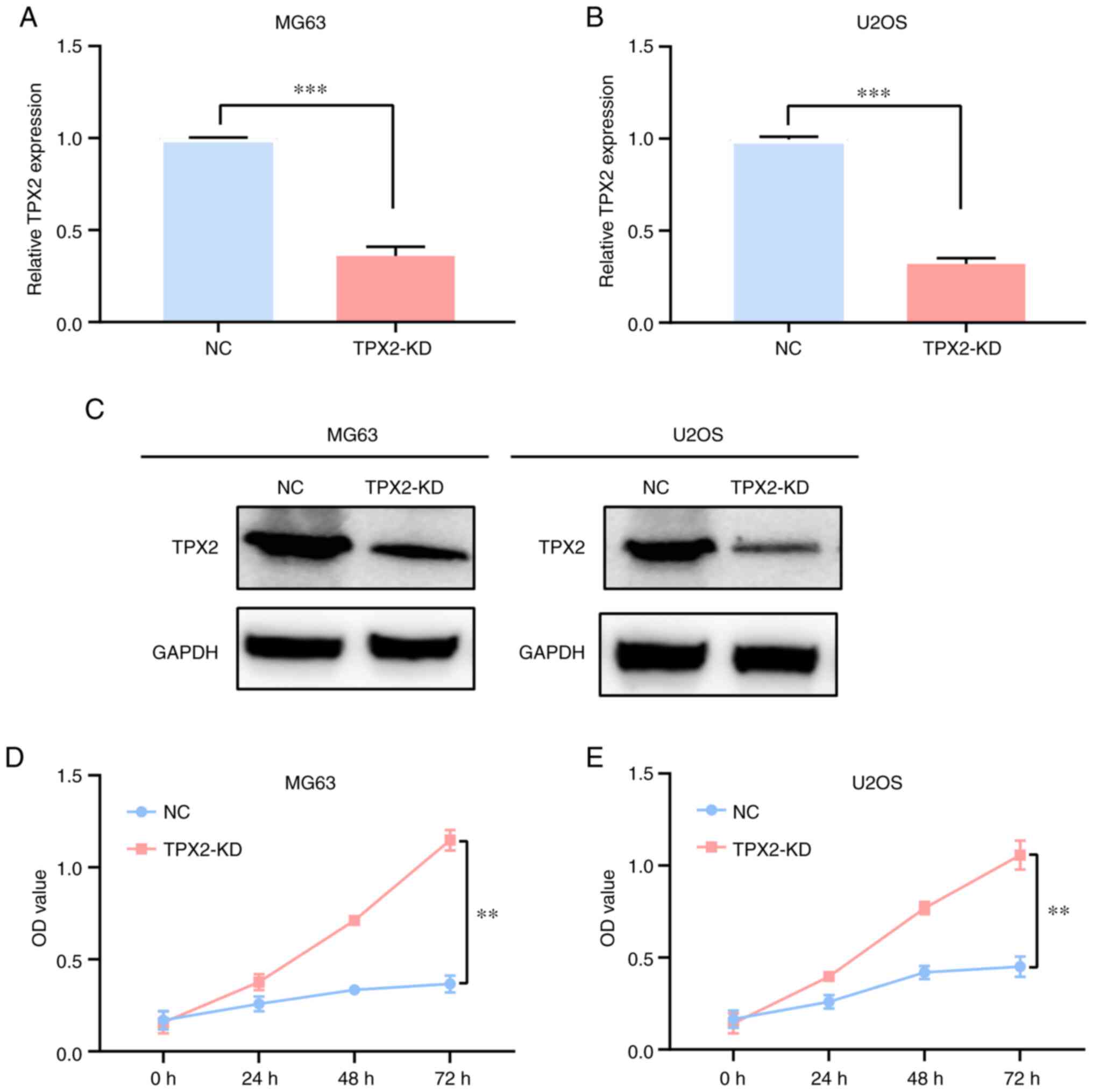
Knockdown of TPX2 inhibits
osteosarcoma cell proliferation
TPX2 siRNA plasmids were transfected into MG63 and
U2OS osteosarcoma cell lines to knockdown TPX2 expression. RT-qPCR
was then used to explore the efficiency of the knockdown (Fig. 3A and B). The transfection
efficiency was also confirmed by western blotting (Fig. 3C). MTT assays results indicated
that TPX2 knockdown inhibited osteosarcoma MG63 and U2OS cell line
proliferation (Fig. 3D and E).
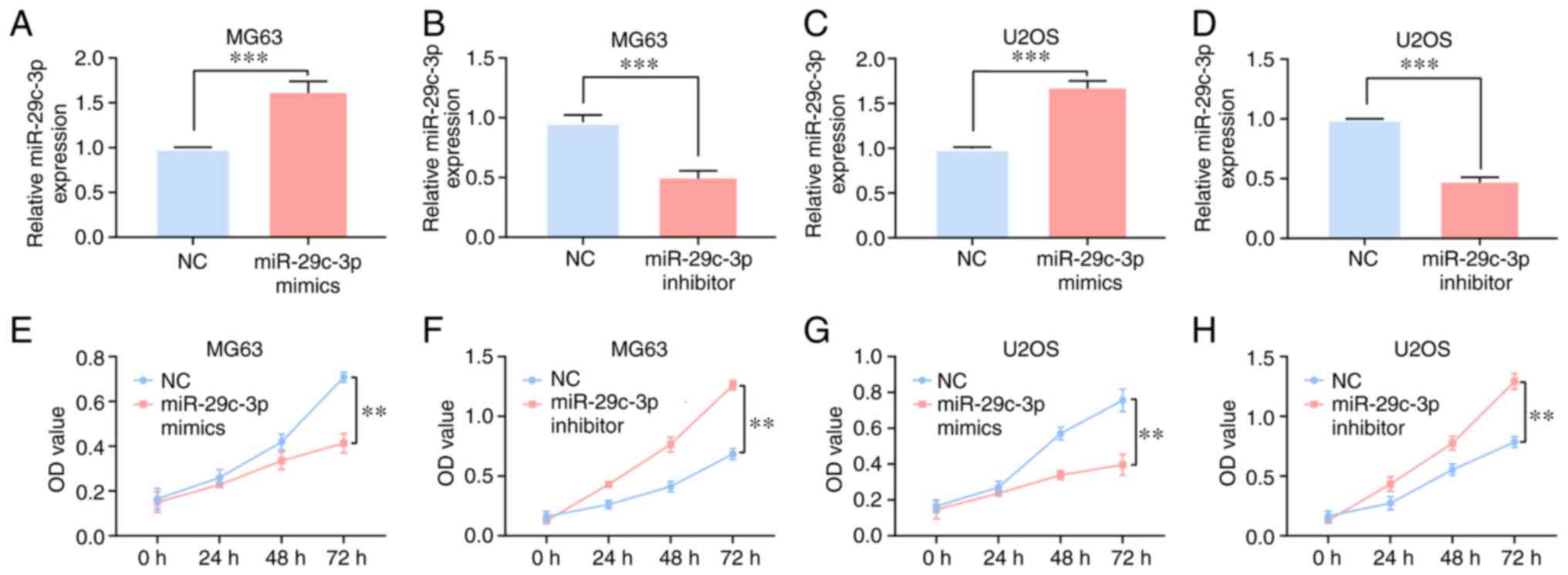
miR-29c-3p inhibits osteosarcoma cell
proliferation
In osteosarcoma MG63 and U2OS cell lines, after
transfection of miR-29c-3p mimics or inhibitors, the efficiency was
confirmed by RT-qPCR (Fig. 4A-D).
MTT assays results indicated that miR-29c-3p mimics could
significantly decrease the proliferation of the MG63 and U2OS cell
lines, while miR-29c-3p inhibitors could significantly promote MG63
and U2OS cell line proliferation (Fig.
4E-H).
miR-29c-3p targets TPX2 and decreases
TPX2 expression in osteosarcoma cell lines
Using the TargetScan database, it was determined
that the TPX2 3′UTR included a putative miR-29c-3p binding site
(Fig. 5A). This notion was
subsequently validated using a luciferase reporter assay. MG63 and
U2OS cell lines were cotransfected with luciferase reporter vectors
containing TPX2-3′UTR-wt or TPX2-3′UTR-mut, along with miR-29c-3p
mimics or inhibitors. Luciferase reporter gene assay revealed that
miR-29c-3p mimics significantly inhibited luciferase activity in
the TPX2-wt group (Fig. 5B and C)
and that miR-29c-3p inhibitors promoted luciferase activity in the
TPX2-wt group (Fig. 5D and E).
Additionally, TPX2 expression decreased in miR-29a-5p mimics, while
it increased in the miR-29c-3p inhibitors group compared with the
NC (Fig. 5F and G). Taken
together, these data show that TPX2 is a target gene of miR-29c-3p
and that a negative correlation exists between miR-29c-3p and TPX2
in osteosarcoma.
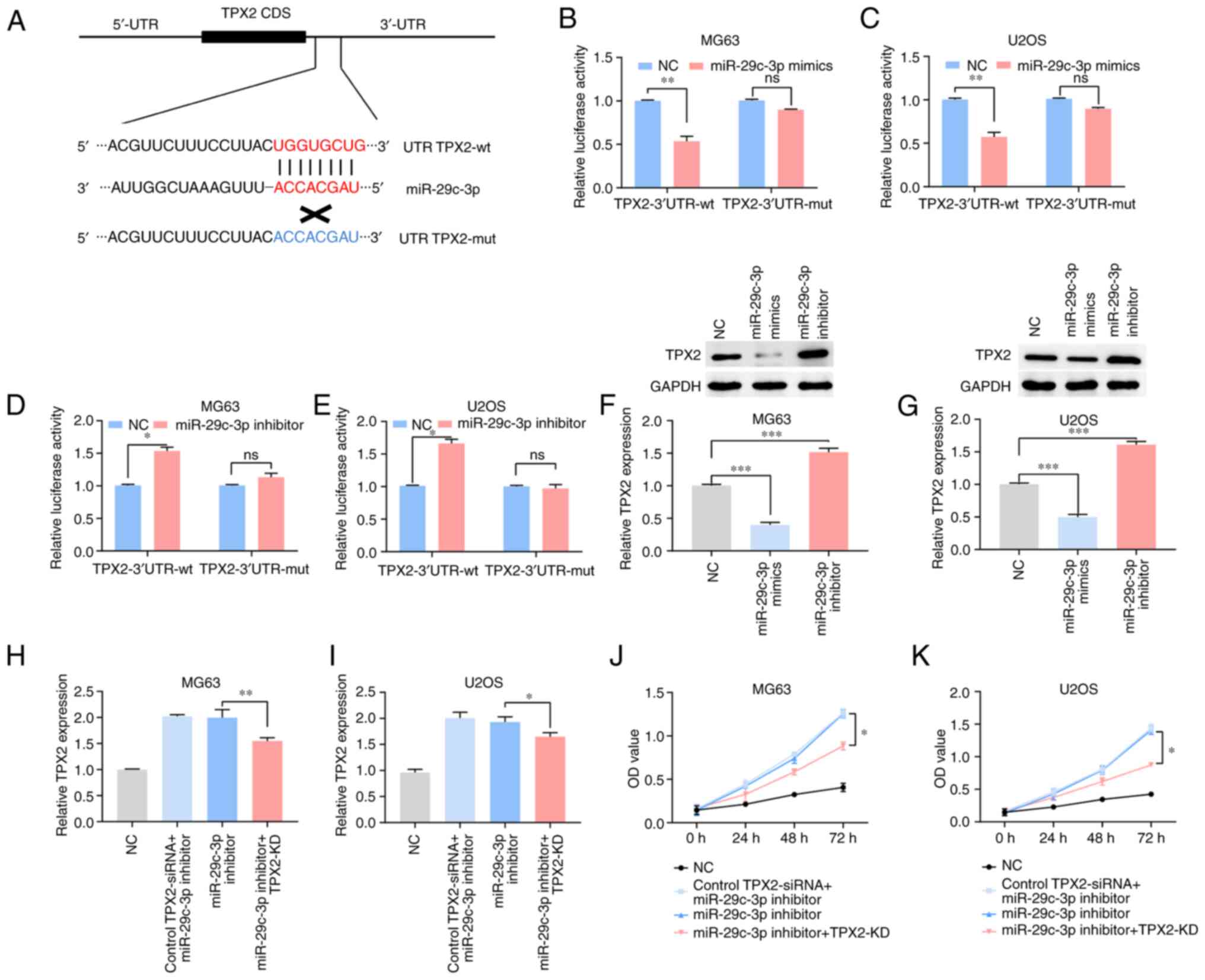 | Figure 5.miR-29c-3p downregulates TPX2
expression level by binding to its 3′UTR directly. (A) The target
site of miR-29c-3p is in the TPX2 3′UTR. (B-E) Luciferase reporter
gene assays were performed to detect the fluorescence activities of
the TPX2 3′UTR in MG63 and U2OS cells, which were co-transfected
with wt TPX2 3′UTR or mut TPX2 3′UTR and miR-29c-3p
mimics/inhibitors, respectively. TPX2 expression in transfected (F)
MG63 and (G) U2OS cells, as determined by RT-qPCR and western blot
analysis. TPX2 mRNA expression level was detected by RT-qPCR in (H)
MG63 cells and (I) U2OS cells co-transfected with TPX2 siRNA and
miR-29c-3p inhibitor. An MTT assay was performed to detect
proliferation abilities of (J) MG63 and (K) U2OS cells
co-transfected with TPX2 siRNA and miR-29c-3p inhibitor.
*P<0.05, **P<0.01 and ***P<0.001. si/siRNA, small
interfering; KD, knockdown; TPX2, targeting protein for
Xenopus kinesin-like protein 2; UTR, untranslated region;
miR, microRNA; NC, negative control; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; OD, optical
density; wt, wild-type; mut, mutant; ns, not significant; CDS,
coding sequence. |
Knockdown of TPX2 markedly reverses
miR-29c-3p-mediated inhibition of cell proliferation in
osteosarcoma cell lines
The findings showed that, compared with that in the
NC group, TPX2 expression in MG63 and U2OS cells transfected with
miR-29c-3p inhibitors increased; however, when cotransfected with
TPX2 siRNA and miR-29c-3p inhibitors, the expression of TPX2 was
significantly decreased compared with that in the group without
siRNA (Fig. 5H and I). MTT assays
results indicated that the knockdown of TPX2 significantly reversed
miR-29c-3p inhibitor-mediated promotion of cell proliferation in
the osteosarcoma cell lines (Fig. 5J
and K).
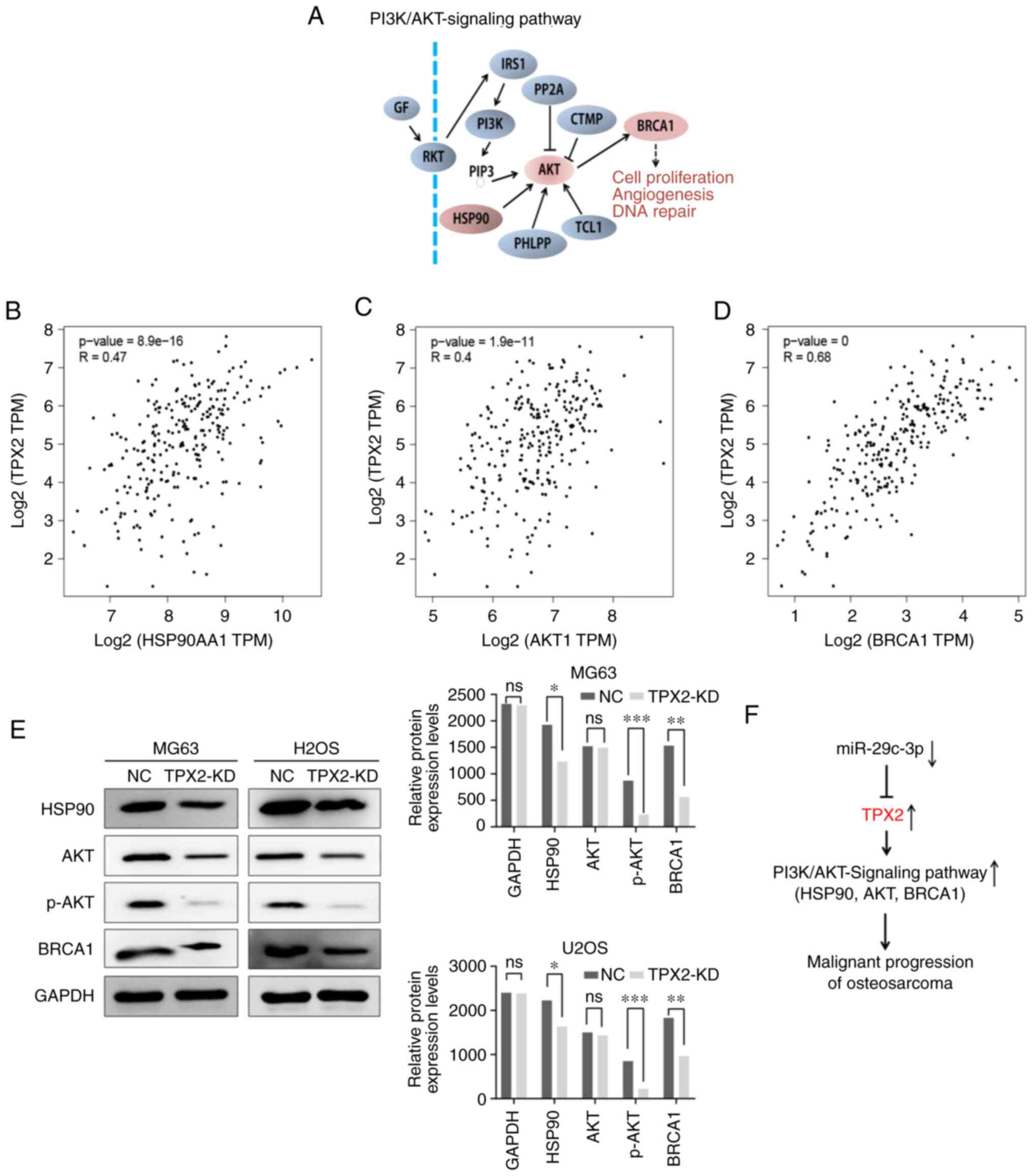
Silencing of TPX2 suppresses AKT
signaling pathway activity in osteosarcoma cells
The AKT signaling pathway is an important signaling
pathway that controls the proliferation of cancer cells (Fig. 6A). A positive correlation was found
between TPX2 and the three key genes in the AKT signaling pathway
using the GEPIA database (Fig.
6B-D). Western blotting was used to detect the protein
expression levels of HSP90, AKT and BRCA1 in osteosarcoma cells
after TPX2 silencing, in order to determine the effect of TPX2 on
the AKT pathway. It was found that the expression of the
aforementioned key genes was decreased when TPX2 was knocked down
in U2OS and MG63 cells (Fig. 6E).
Taken together, the results showed that TPX2 regulated by
miR-29c-3p induces cell proliferation in osteosarcoma via the AKT
signaling pathway (Fig. 6F).
Discussion
As the most frequently occurring malignant bone
tumor in adolescents and young adults, osteosarcoma accounts for
~20% of pediatric, malignant and solid tumors. Although
improvements have been made with regard to the diagnosis and
treatment of osteosarcoma, the overall survival rate has been
fairly constant for >20 years (19). Accumulating evidence has
demonstrated that osteosarcoma is a complex disease that involves
multiple genes (20). Therefore,
determining the underlying molecular mechanism for osteosarcoma
will aid the search for novel diagnostic biomarkers and therapeutic
targets for this disease.
Previously, numerous studies have analyzed the
association between TPX2 and human malignancy (12–14).
To the best of our knowledge, the present study is the first to
focus on TPX2 function in osteosarcoma. The clinical study of
patients with osteosarcoma demonstrated that TPX2 expression was
upregulated in human osteosarcoma tissues. TPX2 expression levels
in osteosarcoma were also closely associated with tumor size
(P=0.019), clinical stage (P=0.002) and distant metastasis
(P=0.018), and high TPX2 expression levels predicted a worse
prognosis for affected patients. Similar to the present study, Cai
et al (21) noted that TPX2
was a novel predictor of metastasis risk in breast cancer. A
previous study on esophageal cancer also found that high expression
of TPX2 was an independent prognostic factor, and that knocking
down TPX2 expression could significantly inhibit the proliferation
of esophageal cancer cells (22).
In the present study, the analysis based on the ROC curve showed
that the AUC was 0.83, meaning that TPX2 may be a highly sensitive
predictor for the diagnosis of osteosarcoma. Furthermore,
Kaplan-Meier analyses indicated that patients with high TPX2
expression experienced a markedly shorter overall survival time
compared with patients with low TPX2 expression (P=0.018). Overall,
TPX2 could be used as an effective biomarker of prognosis in
patients with osteosarcoma and may be a potential therapeutic
target of the disease.
miRNA serves an important role in the regulation of
numerous cellular processes, including proliferation,
differentiation and apoptosis. Mature miRNA binds to the
complementary sequence at the 3′UTR of its target mRNA, thus
inhibiting mRNA translation or inducing its degradation, and
exerting a negative regulatory effect on gene expression. In the
present study, using TargetScan, miR-29c-3p was assessed and found
to have a targeted binding site with TPX2. This miRNA was
downregulated in the patients with osteosarcoma. miR-29 has been
reported to restrain proliferation, migration and invasion in
breast cancer cells through the targeting of PDCD4 (23). In osteosarcoma, Liu et al
(24) demonstrated that miR-29
could target PTEN to inhibit cell proliferation and migration. Ma
et al (25) revealed that
miR-29c-3p was able to inhibit the malignant progression of
osteosarcoma by targeting PIK3R3. In 2018, one study of human
osteosarcoma revealed that hsa-circ-001564 was upregulated in tumor
tissues and that it aggravated the malignant process by the
negative targeting of miR-29c-3p in MG63 and HOS cell lines, as
measured by colony formation and cell apoptosis assays (26). Recently, it was found that
miR-29c-3p could modulate FOS expression to inhibit
epithelial-mesenchymal transition and cell proliferation, while
inducing apoptosis in TGF-β2-treated lens epithelial cells
regulated by lncRNA KCNQ1OT1 (27). The present study also verified that
miR-29c-3p could regulate the expression of TPX2 at the cellular
level, and that overexpression of miR-29c-3p could reverse the TPX2
inhibitory impact on the proliferation of osteosarcoma cell
lines.
The AKT signaling pathway is known to play an
important role in malignant tumor occurrence and development, and
AKT activation is associated with the proliferation of tumor cells
(28,29). Two different studies previously
reported that inhibiting TPX2 could result in tumor suppression by
inhibiting the AKT signaling pathway in hepatocellular carcinoma
(30,31). Similar findings were observed in
breast cancer (32). In the
present study, the knockdown of TPX2 was found to suppress the
proliferation of osteosarcoma cells. HSP90 serves a vital role in
the stability of a number of proteins that are crucial for cell
survival, and contributes to the functional stabilization of
PI3K/Akt signaling and cell survival (33). The activation of AKT also seems to
support BRCA1 nuclear localization, and co-expression of BRCA1 and
activated AKT decreases radiation sensitivity, indicating the
functional consequences of this interaction with regard to the role
of BRCA1 in DNA repair (34).
Additionally, TPX2 knockdown resulted in altered protein expression
levels of HSP90, AKT and BRCA1, indicating that TPX2 may promote
the activation of AKT signal transduction.
A number of studies have reported that the
activation of TPX2 is associated with a variety of different cancer
types (12–15), but the molecular mechanisms
underlying TPX2 function in osteosarcoma are not well
understood.
Overall, TPX2 may be used as a biomarker for the
diagnosis and prognosis of osteosarcoma, and is expected in future
to provide novel targets for the treatment of this disease.
However, there were some limitations to the present study. Firstly,
the clinical sample size used was relatively small. In addition,
the study was only conducted in vitro. The association between
miR-29c-3p expression and the clinicopathological characteristics
of patients with osteosarcoma needs further investigation. Lastly,
the study only analyzed the effects of TPX2 and miR-29c-3p on
osteosarcoma cancer cells in terms of cell proliferation.
Therefore, the other potential molecular mechanism, i.e., that TPX2
enhances the carcinogenesis of osteosarcoma, requires further
investigation. Future studies will use the miR-29c-3p/TPX2/AKT axis
as a target site for osteosarcoma molecular therapy, in order to
improve the therapeutic effects and prognosis of patients with
osteosarcoma.
In conclusion, the results of the present study
indicated that TPX2 could exert an effect on tumor progression by
activating the AKT signaling pathway in osteosarcoma, and that this
effect may be suppressed by miR-29c-3p. TPX2 could be used as a
potential target and biomarker for future novel therapeutic
development.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ wrote the manuscript. DZ and XX performed the
experiments. DZ, XX, MZ and TW collected the patients' samples. DZ,
XX, MZ and TW designed the experiments and analyzed the data. DZ,
XX, MZ and TW critically revised the manuscript for important
intellectual content. DZ helped with the statistical analysis. DZ,
XX, MZ and TW confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First People's Hospital of Lianyungang
(Lianyungang, China). Written informed consent was obtained from
all patients and/or their legal guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eaton BR, Schwarz R, Vatner R, Yeh B,
Claude L, Indelicato DJ and Laack N: Osteosarcoma. Pediatr Blood
Cancer. 68 (Suppl 2):e283522021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sadykova LR, Ntekim AI, Muyangwa-Semenova
M, Rutland CS, Jeyapalan JN, Blatt N and Rizvanov AA: Epidemiology
and risk factors of osteosarcoma. Cancer Invest. 38:259–269. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bishop MW, Janeway KA and Gorlick R:
Future directions in the treatment of osteosarcoma. Curr Opin
Pediatr. 28:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knott MM, Hölting TL, Ohmura S, Kirchner
T, Cidre-Aranaz F and Grünewald TG: Targeting the undruggable:
Exploiting neomorphic features of fusion oncoproteins in childhood
sarcomas for innovative therapies. Cancer Metastasis Rev.
38:625–642. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glover J, Man TK, Barkauskas DA, Hall D,
Tello T, Sullivan MB, Gorlick R, Janeway K, Grier H, Lau C, et al:
Osteosarcoma enters a post genomic era with in silico
opportunities: Generation of the High Dimensional Database for
facilitating sarcoma biology research: A report from the Children's
Oncology Group and the QuadW Foundation. PLoS One. 12:e01812042017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perry JA, Kiezun A, Tonzi P, Van Allen EM,
Carter SL, Baca SC, Cowley GS, Bhatt AS, Rheinbay E, Pedamallu CS,
et al: Complementary genomic approaches highlight the PI3K/mTOR
pathway as a common vulnerability in osteosarcoma. Proc Natl Acad
Sci USA. 111:E5564–E5573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lengauer C, Kinzler KW and Vogelstein B:
Genetic instabilities in human cancers. Nature. 396:643–649. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carter SL, Eklund AC, Kohane IS, Harris LN
and Szallasi Z: A signature of chromosomal instability inferred
from gene expression profiles predicts clinical outcome in multiple
human cancers. Nat Genet. 38:1043–1048. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wieczorek M, Bechstedt S, Chaaban S and
Brouhard GJ: Microtubule-associated proteins control the kinetics
of microtubule nucleation. Nat Cell Biol. 17:907–916. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taherdangkoo K, Kazemi Nezhad SR, Hajjari
MR and Tahmasebi Birgani M: miR-485-3p suppresses colorectal cancer
via targeting TPX2. Bratisl Lek Listy. 121:302–307. 2020.PubMed/NCBI
|
|
12
|
Huo C, Zhang MY, Li R, Zhou XJ, Liu TT, Li
JP, Liu X and Qu YQ: Comprehensive analysis of TPX2-related ceRNA
network as prognostic biomarkers in lung adenocarcinoma. Int J Med
Sci. 17:2427–2439. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomes-Filho SM, Dos Santos EO, Bertoldi
ER, Scalabrini LC, Heidrich V, Dazzani B, Levantini E, Reis EM and
Bassères DS: Aurora A kinase and its activator TPX2 are potential
therapeutic targets in KRAS-induced pancreatic cancer. Cell Oncol
(Dordr). 43:445–460. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang P, Shen K, Wang X, Song H, Yue Y and
Liu T: TPX2 regulates tumor growth in human cervical carcinoma
cells. Mol Med Rep. 9:2347–2351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shigeishi H, Ohta K, Hiraoka M, Fujimoto
S, Minami M, Higashikawa K and Kamata N: Expression of TPX2 in
salivary gland carcinomas. Oncol Rep. 21:341–344. 2009.PubMed/NCBI
|
|
16
|
Cates JMM: Simple staging system for
osteosarcoma performs equivalently to the AJCC and MSTS systems. J
Orthop. 36:2802–2808. 2018.PubMed/NCBI
|
|
17
|
Mu J, Fan L, Liu D and Zhu D:
Overexpression of shugoshin1 predicts a poor prognosis for prostate
cancer and promotes metastasis by affecting epithelial-mesenchymal
transition. Onco Targets Ther. 12:1111–1118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joko R, Yamada D, Nakamura M, Yoshida A,
Takihira S, Takao T, Lu M, Sato K, Ito T, Kunisada T, et al: PRRX1
promotes malignant properties in human osteosarcoma. Transl Oncol.
14:1009602021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian W, Li Y, Zhang J, Li J and Gao J:
Combined analysis of DNA methylation and gene expression profiles
of osteosarcoma identified several prognosis signatures. Gene.
650:7–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai Y, Mei J, Xiao Z, Xu B, Jiang X, Zhang
Y and Zhu Y: Identification of five hub genes as monitoring
biomarkers for breast cancer metastasis in silico. Hereditas.
156:202019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu PK, Chen HY, Yeh YC, Yen CC, Wu YC,
Hsu CP, Hsu WH and Chou TY: TPX2 expression is associated with cell
proliferation and patient outcome in esophageal squamous cell
carcinoma. J Gastroenterol. 49:1231–1240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao G, Liang X and Ma W: Sinomenine
restrains breast cancer cells proliferation, migration and invasion
via modulation of miR-29/PDCD-4 axis. Artif Cells Nanomed
Biotechnol. 47:3839–3846. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Q, Geng P, Shi L, Wang Q and Wang P:
miR-29 promotes osteosarcoma cell proliferation and migration by
targeting PTEN. Oncol Lett. 17:883–890. 2019.PubMed/NCBI
|
|
25
|
Ma HZ, Wang J, Shi J, Zhang W and Zhou DS:
MicroRNA-29c-3p inhibits osteosarcoma cell proliferation through
targeting PIK3R3. Eur Rev Med Pharmacol Sci. 24:2239–2247.
2020.PubMed/NCBI
|
|
26
|
Song YZ and Li JF: Circular RNA
hsa_circ_0001564 regulates osteosarcoma proliferation and apoptosis
by acting miRNA sponge. Biochem Biophys Res Commun. 495:2369–2375.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao L, Yang L, Song H, Liu T and Yan H:
MicroRNA miR-29c-3p modulates FOS expression to repress EMT and
cell proliferation while induces apoptosis in TGF-β2-treated lens
epithelial cells regulated by lncRNA KCNQ1OT1. Biomed Pharmacother.
129:1102902020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Zhang Q, Wu Q, Cui Y, Zhu H, Fang M,
Zhou X, Sun Z and Yu J: Interleukin-22 secreted by
cancer-associated fibroblasts regulates the proliferation and
metastasis of lung cancer cells via the PI3K-Akt-mTOR signaling
pathway. Am J Transl Res. 11:4077–4088. 2019.PubMed/NCBI
|
|
29
|
Yun WK, Hu YM, Zhao CB, Yu DY and Tang JB:
HCP5 promotes colon cancer development by activating AP1G1 via
PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 23:2786–2793.
2019.PubMed/NCBI
|
|
30
|
Huang DH, Jian J, Li S, Zhang Y and Liu
LZ: TPX2 silencing exerts anti-tumor effects on hepatocellular
carcinoma by regulating the PI3K/AKT signaling pathway. Int J Mol
Med. 44:2113–2122. 2019.PubMed/NCBI
|
|
31
|
Wang F, Zhao W, Gao Y, Zhou J, Li H, Zhang
G, Guo D, Xie C, Li J, Yin Z and Zhang J: CDK5-mediated
phosphorylation and stabilization of TPX2 promotes hepatocellular
tumorigenesis. J Exp Clin Cancer Res. 38:2862019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen M, Zhang H, Zhang G, Zhong A, Ma Q,
Kai J, Tong Y, Xie S, Wang Y, Zheng H, et al: Targeting TPX2
suppresses proliferation and promotes apoptosis via repression of
the PI3k/AKT/P21 signaling pathway and activation of p53 pathway in
breast cancer. Biochem Biophys Res Commun. 507:74–82. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Redlak MJ and Miller TA: Targeting
PI3K/Akt/HSP90 signaling sensitizes gastric cancer cells to
deoxycholate-induced apoptosis. Dig Dis Sci. 56:323–329. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nelson AC, Lyons TR, Young CD, Hansen KC,
Anderson SM and Holt JT: AKT regulates BRCA1 stability in response
to hormone signaling. Mol Cell Endocrinol. 319:129–142. 2010.
View Article : Google Scholar : PubMed/NCBI
|