Introduction
Prostate cancer is a slowly developing disease with
a high mortality rate in men, especially in Western countries
(1). Castrate-resistant prostate
cancer (CRPC) is resistant to androgen ablation and cancer
metastases are often observed in patients with CRPC (2). Cancer metastasis is a complex
mechanism and cascade of events that allows tumor cells to travel
to other organs. Epithelial-mesenchymal transition (EMT) is as an
important event in the initial steps of cancer cell metastasis
(3). The loss of epithelial cell
characteristics leads to the transformation of epithelial cells to
mesenchymal cells with a stem cell-like phenotype. Notably, EMT can
result in increasing resistance to apoptosis and chemotherapy
(4,5). Previous studies have reported that
lipopolysaccharide (LPS), a component of gram-negative bacteria,
can trigger EMT, which induces the migration and invasion of cancer
cells (6–8).
Autophagy regulates cell damage and degradation and
processes the recycling of cell constituents. It is an adaptive
process and a form of cell death that occurs in response to stress,
including elevated levels of reactive oxygen species (ROS) and
anticancer agents (9,10). Autophagy may therefore serve a
pivotal role during chemotherapy. Phytochemicals or
chemotherapeutic agents can overcome drug resistance and induce
apoptosis in cancer cells (11–13).
Ferroptosis is a form of cell death, which has
characteristics that are different from apoptosis and autophagy.
The accumulation of intracellular iron and ROS, and the depletion
of glutathione (GSH) are characteristic of ferroptosis (14). Ferroptosis inducers can inhibit
cancer cell proliferation and may be a novel target for potential
cancer therapeutics (15,16).
Dietary natural products contain numerous bioactive
phytochemicals with a wide spectrum of pharmacological activities.
Ginger (Zingiber officinale) is commonly used as a spice and
a traditional medicine (17). One
component of ginger extract, 6-Gingerol, has anti-inflammatory,
anticancer and antioxidant effects (18–21).
In addition, 6-Gingerol has been reported to exhibit synergistic
effects on PC3 cells by inducing apoptosis (22) and to inhibit testosterone-induced
proliferation of LNCaP cells (23). However, to the best of our
knowledge, whether 6-Gingerol also inhibits EMT, and induces
autophagy or ferroptosis in prostate cancer cells is unknown.
The present study aimed to determine the
pharmacological effects of 6-Gingerol against LPS-induced migration
and invasion, and the potential of 6-Gingerol to inhibit
LPS-induced EMT in prostate cancer cells. It can therefore be
hypothesized that 6-Gingerol may be used as an effective
chemotherapeutic agent to treat prostate cancer.
Materials and methods
Chemicals and reagents
6-Gingerol (95–99% purity, determined by
high-performance liquid chromatography) was purchased from Chengdu
Biopurify Phytochemicals, Ltd. PI3K inhibitor (LY294002) and MTT
reagent were purchased from Beyotime Institute of Biotechnology.
LPS (from Escherichia coli 026:B6) and β-actin primary
antibodies (cat. no. A5441) were obtained from MilliporeSigma.
Ferrostatin-1 was purchased from Shanghai Aladdin Biochemical
Technology Co., Ltd. Primary antibodies against Beclin-1 (cat. no.
AB3219), LC3B (cat. no. CY5992), nuclear factor erythroid 2-related
factor 2 (NRF2; cat. no. CY1851) and GSH peroxidase (GPX) 4 (cat.
no. CY6959) were purchased from Shanghai Abways Biotechnology Co.,
Ltd. Primary antibodies against E-cadherin (cat. no. 3195),
N-cadherin (cat. no. 13116), Vimentin (cat. no. 5741) and zonula
occludens-1 (ZO-1; cat. no. 8193) were purchased from Cell
Signaling Technology, Inc. Anti-rabbit IgG horseradish peroxidase
HRP-linked antibody (cat. no. 7074) and anti-mouse IgG HRP-linked
antibody (cat. no. 7076) were purchased from Cell Signaling
Technology, Inc.
Cell culture
The human prostate cancer LNCaP, DU145 and PC3 cell
lines were purchased from Shanghai Fuheng Biotechnology Co., Ltd.
Cells were cultured at 37°C in a humidified atmosphere with 5%
CO2. LNCaP cells were grown in RPMI-1640 medium, DU145
and PC3 cells were grown in DMEM/Ham's F12 Kaighn's (K) medium
(both LONSERA ShangHai ShuangRu Biotech Co., Ltd). The media were
supplemented with 10% fetal bovine serum (FBS; LONSERA ShangHai
ShuangRu Biotech Co., Ltd.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Beyotime Institute of Biotechnology). In each
experiment, the control group was untreated cells.
Cell viability assay
LNCaP, DU145 and PC3 cells were seeded at a density
of 1×104 cells/well in 96-well plates. When cells
reached 80% confluency, cells were treated with 6-Gingerol (1–500
µM), with or without LPS (1 µg/ml), ferrostatin-1 (5 µM) and
LY294002 (10 µM), at 37°C for 24, 48 or 72 h. After incubation,
cell viability was determined using an MTT assay. The medium was
replaced with fresh medium, 10 µl MTT (5 mg/ml) was added to each
well contain 100 µl fresh medium and cells were incubated at 37°C
for 4 h. The supernatant was subsequently discarded and 100 µl DMSO
was used to dissolve the MTT-formazan crystals. Absorbance was then
quantified using a microplate reader at a wavelength of 570 nm.
Colony formation
LNCaP, DU145 and PC3 cells were seeded into a 6-well
plate at a density of 5×102 cells/well. Cells were
incubated at 37°C for 4 h and were subsequently treated with
different concentrations (1, 10, 100 and 500 µM) of 6-Gingerol.
After incubation at 37°C for 7 days without changing the medium, 4%
formaldehyde was applied for fixing cells for 20 min at room
temperature and stained with 0.2% crystal violet for 20 min at room
temperature. Colonies were defined as groups of >50 cells and
manually counted under an inverted light microscope (Nikon
TI-DH).
Wound healing assay
DU145 and PC3 cells at a density of 1×106
cells/well were cultured on a 6-well plate with medium containing
10% FBS. After reaching 100% confluency, the medium was replaced
with serum-free medium. A scratch was created on the cell
monolayers using a sterile 200-µl pipette tip and cells were then
treated with 6-Gingerol (10 µM), with or without LPS (1 µg/ml) at
37°C for 24 or 48 h. The images were observed and captured by image
device (NIS Elements version 4.30, Nikon) and inverted light
microscope (Nikon TI-DH). Wound healing was semi-quantified using
ImageJ 1.52a software (National Institutes of Health). The wound
area was calculated as the follows: (Initial wound width-final
wound width)/initial wound width ×100 (%).
Cell adhesion assay
Fibronectin (Beijing Solarbio Science &
Technology Co., Ltd.) was dissolved in PBS and used for coating.
Then, 0.1 ml of fibronectin (5 µg/ml) was added per well in a
96-well plate at 4°C overnight. After incubation, the wells were
washed with PBS twice and incubated with serum-free medium at 37°C
for 30 min. LNCaP, DU145 and PC3 cells (1×104) were
added to each well in fresh medium containing 6-Gingerol (100 and
500 µM), with or without LPS (1 µg/ml) incubated at 37°C at 1 and 2
h for adhesion. After incubation, the adhered cells were gently
washed twice with PBS and measured using MTT assay, as
aforementioned.
Migration and invasion assays
The migratory and invasive abilities of DU145 and
PC3 cells were determined using 8-µm Transwell filter membranes
(Costar; Corning, Inc.). For the migration assay, 1×104
cells were seeded into the upper chamber with DMEM/Ham's F12K
serum-free medium containing 6-Gingerol (10 µM), whereas the bottom
chamber was loaded with DMEM/Ham's F12K medium containing 10% FBS
with or without LPS (1 µg/ml) as a chemoattractant. After
incubation at 37°C for 48 h, cells in the upper chamber were gently
scraped off and the migrating cells that had accumulated in the
bottom chamber were fixed with 4% formaldehyde for 20 min at room
temperature and stained with 0.2% crystal violet for 20 min at room
temperature. The migrated cells on the bottom surface of the
membrane were captured (NIS Elements version 4.30, Nikon) and
counted manually under an inverted light microscope (Nikon TI-DH).
For the invasion assay, each Transwell plate was coated with
Matrigel (1 mg/ml, Corning, Inc.) with serum free medium at 37°C
for 1 h. The subsequent procedure was the same as that of migration
assay.
Western blotting
To examine the mechanism of underling the
anti-cancer effects of 6-Gingerol on prostate cancer cells, LNCaP,
DU145 and PC3 cells were treated with 6-Gingerol (1–100 µM), with
or without LPS (1 µg/ml) and ferrostatin-1 (5 µM) at 37°C for 24 or
48 h. After incubation, total protein was extracted by M-PER
mammalian protein extraction reagent (Thermo Fisher Scientific,
Inc.; cat. no. 78505). The concentration of protein was determined
by Pierce Coomassie (Bradford) Protein Assay Kit (Thermo
Scientific, cat. no. 23200) and was separated by 7.5, 10.0 or 12.0%
SDS-PAGE (20 µg total protein/lane). Separated proteins were
subsequently transferred onto a PVDF membrane. The membranes were
blocked with 5% non-fat dried milk 1X TBST buffer (20 mM Tris, 150
mM NaCl, 0.1% Tween 20) at room temperature for 1 h. Membranes were
incubated at 4°C overnight with the following primary antibodies:
Beclin-1 (1:1,000), LC3B (1:1,000), NRF2 (1:1,000), GPX4 (1:1,000),
E-cadherin (1:1,000), N-cadherin (1:1,000), Vimentin (1:1,000),
β-actin (1:8,000) and ZO-1 (1:1,000). Subsequently, membranes were
incubated for 1 h at room temperature with the secondary
antibodies, anti-rabbit IgG HRP-linked antibody (1:1,000) and
anti-mouse IgG HRP-linked antibody (1:1,000). Protein bands were
subsequently visualized using an enhanced chemiluminescent kit to
determine protein expression (Shanghai Epizyme Biomedical
Technology Co., Ltd). The bands were detected using a ChemiScope
3300 Mini (Clinx Science Instruments Co., Ltd.). β-actin was used
as the internal control for Western Blots. The densitometry of
protein expression was determined using ImageJ 1.52a software
(National Institutes of Health, USA).
Determination of intracellular ROS and
GSH
Intracellular ROS levels were determined using
reactive oxygen species assay kit (Biosharp; cat. no. BL714A).
according to the manufacturer's protocol. Briefly, the LNCaP, DU145
and PC3 cells were cultured in 6-well plates at density of
1×105 cells. Cells were treated with 6-Gingerol (100 µM)
with or without ferrostatin-1 (5 µM) at 37°C for 24 h. After the
incubation, the cells were collected, stained with H2DCFH-DA (10
µM) at 37°C for 30 min in the dark and then washed twice with serum
free medium. For each experiment, the fluorescence intensity of ROS
was quantified using flow cytometry (NovoCyte Flow Cytometer;
Agilent Technologies, Inc.). Data were analyzed using NovoExpress
1.2.5 software (2016 ACEA Biosciences, Inc.). GSH levels were
determined using a Glutathione Assay Kit (Nanjing Jiancheng
Bioengineering Institute; cat. no. A006-2-1). LNCaP, DU145 and PC3
cells at the density of 1×104 were seeded into a 24-well
plate and incubated overnight at 37°C. Cells were treated with
6-Gingerol (10, 100 µM) with or without ferrostatin-1 (5 µM) at
37°C for 24 h. Cells were then collected and homogenized. After
centrifugation at 14,000 g for 10 min at 4°C, the supernatant was
collected and GSH levels quantified according to the manufacturer's
instructions. The absorbance was measured using a microplate reader
at the wavelength of 405 nm. The content of GSH levels were
determined by the standard curve.
Statistical analysis
The experiments were performed at three times
independently and the data analysis were done by Excel (Microsoft
365MSO, 16.0.14931.20118). Statistical comparisons among more than
two groups were performed using one-way ANOVA followed by Tukey's
post hoc test. All data are presented as the mean ± SEM. P<0.05
was considered to indicate a statistically significant
difference.
Results
6-Gingerol suppresses cell viability
and colony formation in prostate cancer cells
LNCaP, PC3 and DU145 cells were treated with
6-Gingerol (1–500 µM) for 24, 48 or 72 h. The viability of LNCaP,
PC3 and DU145 cells was inhibited by the different 6-Gingerol
(1–500 µM) treatments. The cell survival rate with 6-Gingerol (500
µM) at 72 h was 46.08±4.29, 47.20±5.90 and 50.59±4.20% in LNCaP,
PC3 and DU145 cells, respectively (Fig. 1A). Colony formation in the presence
of 6-Gingerol was also investigated. The colony number determined
for each treatment group (1–500 µM, 6-Gingerol) was significantly
reduced compared with the control group in LNCaP, PC3 and DU145
cells (Figs. 1B and S1), which suggested that 6-Gingerol
inhibited cell viability and colony formation in prostate cancer
cells. Furthermore, the cell survival rate of LNCaP, PC3 and DU145
cells treated with LPS was assessed (Fig. 1C). Several studies reported that
LPS can enhance the metastasis and invasion in prostate and breast
cancer cells (6–8). LPS (1 µg/ml) was not cytotoxic to any
of the cell lines; this concentration was therefore selected to
assess the adhesion, invasion, migration and EMT effects on
prostate cancer cells. 6-Gingerol (100 µM) can significantly
inhibit LPS-induced cell growth at 48 and 72 h (Fig. 1C). Overall, these results indicated
that 6-Gingerol may exhibit cytotoxicity in a dose-dependent manner
in LNCaP, PC3 and DU145 cells.
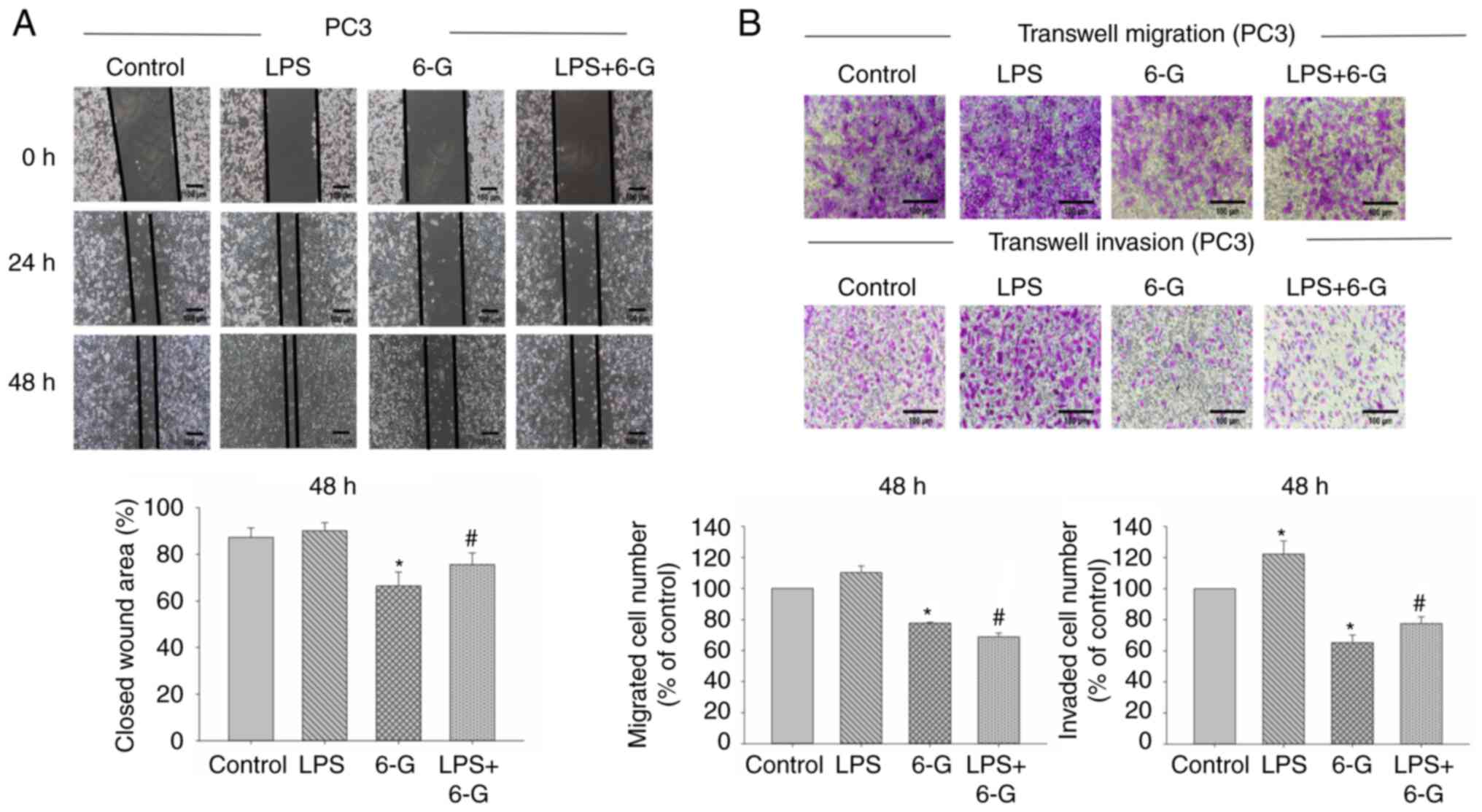
6-Gingerol attenuates migration,
invasion and adhesion in prostate cancer cells
CRPC is an aggressive disease, and it is not
sensitive to medical castration with higher potential of invasion
and metastasis (2). PC3 and DU145
cells are CRPC cells (7).
Therefore, we selected PC3 and DU145 cells for migration and
invasion assay. To investigate the mechanism of 6-Gingerol in cell
migration and invasion, the wound healing and Transwell assays were
performed. The results demonstrated that cell migration and
invasion were significantly enhanced in LPS-induced DU145 cells.
However, only cell invasion was significantly enhanced in
LPS-induced PC3 cells (Figs. 2 and
3). Moreover, 6-Gingerol (10 µM)
significantly inhibited migration and invasion in LPS-treated or
LPS-untreated PC3 and DU145 cells at 48 h, compared with the LPS or
control groups, respectively.
Cell attachment to the extracellular matrix is
important for cell metastasis in distant organs (24); therefore, the effect of 6-Gingerol
on prostate cancer cell adhesion to extracellular matrix proteins
was investigated. Fibronectin (5 µg/ml) significantly induced
adhesion in DU145 and LNCaP cells at 2 h (Fig. 4A). 6-Gingerol (100 and 500 µM)
significantly inhibited fibronectin-treated attachment at 2 h in
LNCaP, DU145 and PC3 cells (Fig.
4A). The results demonstrated that LPS significantly enhanced
the binding affinity of PC3, DU145 and LNCaP cells to fibronectin
compared with the group treated with LPS alone at 2 h (Fig. 4B). 6-Gingerol (100, 500 µM)
significantly decreased the binding affinity of LNCaP, PC3 and
DU145 cells to fibronectin with or without LPS treatment compared
with the LPS + fibronectin or fibronectin group, respectively at 2
h (Fig. 4). These results
indicated that 6-Gingerol may have anti-invasion, anti-migration
and anti-adhesion properties in prostate cancer cells.
6-Gingerol induces autophagy in
prostate cancer cells
Subsequently it was determined if 6-Gingerol could
induce autophagy in prostate cancer cells using western blotting to
analyze Beclin-1 and LC3B protein expression levels. LC3B-II is
important in autophagy and can be used as an autophagy marker
(25). The results demonstrated
that 6-Gingerol (10–100 µM) significantly induced LC3B-II protein
expression levels in LNCaP cancer cells compared with the control
(Fig. 5A). The LC3B-II protein
expression levels were significantly upregulated in
6-Gingerol-treated (1–10 µM) PC3 cells. However, this was not
observed in DU145 cells, due to the absence of the ATG5 protein,
which results in ATG12/ATG5 conjugate deficiency (26). 6-Gingerol (10–100 µM) significantly
upregulated Beclin-1 protein expression levels in LNCaP, PC3 and
DU145 cells compared with the control (Fig. 5A). LY294002, a known PI3K and
autophagy inhibitor, slightly enhanced 6-Gingerol cytotoxicity in
LNCaP and PC3 cells (Fig. S2);
however, this effect was significantly reversed in DU145 cells
compared with the 6-Gingerol group (Fig. 5B). These results therefore
indicated that 6-Gingerol potentially induced protective autophagy
in LNCaP and PC3 cells but promoted autophagic cell death in DU145
cells. The results suggested that 6-Gingerol induced autophagy by
regulating LC3B-II and Beclin-1 protein expression levels in LNCaP
and PC3 cells. Moreover, 6-Gingerol also induced autophagy by
inducing Beclin-1 and LC3B-I but without LC3B-II protein expression
in DU145 cells.
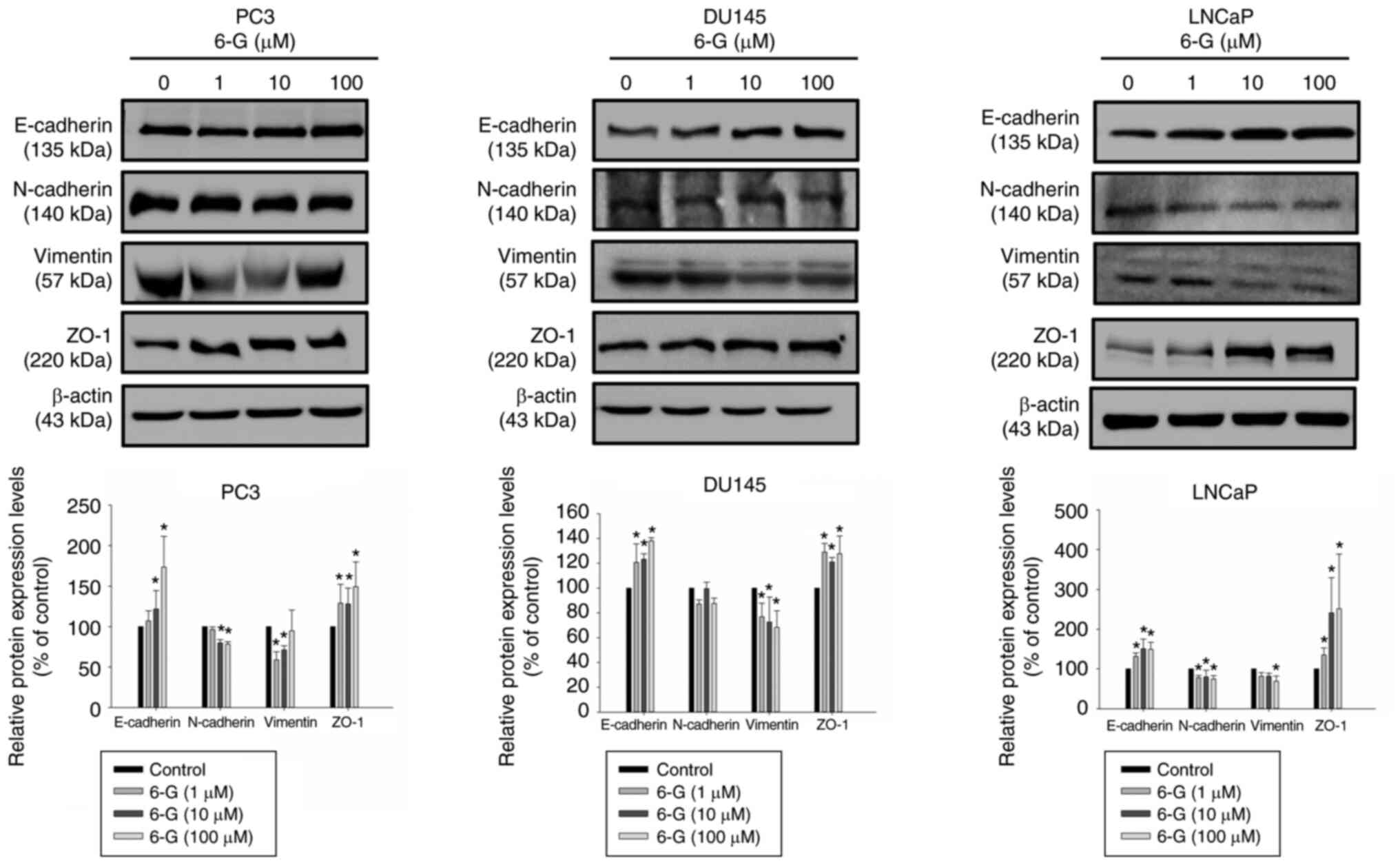
6-Gingerol suppresses EMT-related
protein expression in prostate cancer cells
EMT serves a significant role in cancer progression
and metastasis, a mechanism which LPS can trigger and enhance
(6–8). The protein expression levels of
E-cadherin, N-cadherin, Vimentin and ZO-1 were examined following
6-Gingerol (1–100 µM) treatment for 24 h in LNCaP, PC3 and DU145
cells. The results demonstrated that E-cadherin and ZO-1 were
significantly upregulated in 6-Gingerol-treated (10–100 µM)
prostate cancer cells compared with the control (Fig. 6); however, N-cadherin and Vimentin
were downregulated in 6-Gingerol-treated PC3 and LNCaP cells. The
protein expressions of N-cadherin were not significantly inhibited
by 6-Gingerol (1–100 µM) treatment for 24 h in DU145 cells. Cell
invasion and migration were significantly induced after LPS
treatment in DU145 cells. Therefore, DU145 cells were selected for
examining the underlying mechanism of action of EMT in LPS-treated
DU145 cells. Furthermore, the results indicated that LPS
significantly induced N-cadherin and Vimentin protein expression
levels in DU145 cells at 48 h compared with the control (Fig. 7A and C). In addition, 6-Gingerol
did not markedly increased E-cadherin protein expression levels,
whereas it significantly downregulated N-cadherin and Vimentin
protein expression levels in the LPS + 6-Gingerol group compared
with the LPS group. The results also demonstrated that the protein
expression levels of LC3B-I were significantly decreased in
LPS-stimulated DU145 cells compared with the control. 6-Gingerol
(100 µM) reversed the protein expression levels of LC3B-I in
LPS-stimulated DU145 cells. These data indicated that LPS
potentially stimulated EMT and that 6-Gingerol may reverse these
effects on EMT in LPS-treated prostate cancer cells.
6-Gingerol treatment induces
ferroptosis
Ferroptosis is associated with ROS production, which
leads to decreased cellular GSH levels (27). GPX4 is an enzyme that belongs to
the family of GPXs and GPX4 inactivation can promote ferroptosis
(28). Therefore, the role of ROS,
GSH, GPX4 and NRF2 protein expression in prostate cancer cells was
determined. GPX4 and NRF2 protein expression levels were
significantly downregulated after 24 h of 6-Gingerol (100 µM)
treatment in LNCaP, PC3 and DU145 cells (Fig. 7B and D). NRF2 protein expression
levels were increased after 6-Gingerol (1–10 µM) treatment in PC3
and DU145 cells, but this was not observed in LNCaP cells. PC3 and
DU145 are castration-resistant prostate cancer cells, and LNCaP is
androgen-dependent prostate cancer cell line (18,29).
This might slightly increase NRF2 levels after low concentration of
6-Gingerol treatment in PC3 and DU145 cells because of
castration-resistant prostate cancer cells. Furthermore, ROS levels
were significantly increased following 6-Gingerol treatment in
LNCaP, PC3 and DU145 cells compared with the control. Notably, this
effect was significantly attenuated by pre-treatment with
ferrostatin-1, compared with the 6-Gingerol only group (Figs. 8A and S3).
To further determine the effect of 6-Gingerol on
cell death, ferrostatin-1, an effective ferroptosis inhibitor, was
used. The results demonstrated that ferrostatin-1 significantly
alleviated a decrease in cell viability in LNCaP, PC3 and DU145
cells at 48 h in cells treated with 6-Gingerol (100 µM) compared
with the 6-Gingerol group (Fig.
8B). GSH levels were also significantly reduced after
6-Gingerol treatment (10–100 µM) compared with the control;
however, this effect was significantly attenuated by pre-treatment
with ferrostatin-1 (5 µM) compared with the 6-Gingerol group (100
µM) (Fig. 8C). GPX4 protein
expression levels were attenuated following 6-Gingerol (100 µM)
treatment for 24 h in DU145 cell. The expression was significantly
increased in ferrostatin-1 pre-treatment 6-Gingerol-treated (100
µM) DU145 cells compared with the 6-Gingerol group (Fig. 8D). These results indicated that
cell death may be mediated by a ferroptosis mechanism. Furthermore,
these data indicated that 6-Gingerol may induce ROS accumulation
and ferroptosis; therefore, ferroptosis may be a potential
mechanism, induced by 6-Gingerol, against prostate cancer cell
proliferation.
Discussion
6-Gingerol has been reported to induce apoptosis in
numerous types of cancer cells, including breast cancer, colon
cancer, prostate cancer and cervical cancer cells (21,30–32).
In addition, it may regulate both multidrug resistance-associated
protein 1 and glutathione S-transferase in docetaxel-resistant
prostate cancer cells (21). To
the best of our knowledge, no study has focused on the
anti-migratory and anti-invasive activity of 6-Gingerol in prostate
cancer cells. In the present study, it was reported that 6-Gingerol
affected human androgen-dependent (LNCaP) and castrate-resistant
(DU145 and PC3) prostate cancer cells by inducing autophagy and
ferroptosis. The results also demonstrated that 6-Gingerol
significantly inhibited cell migration and invasion via the
regulation of EMT-related proteins in prostate cancer cells.
EMT serves a significant role in cancer progression,
whereby epithelial cells lose cell polarity and are transformed
into cells with a mesenchymal phenotype, which exhibit increased
migratory and invasive abilities in combination with reduced
intracellular adhesion (33). EMT
is also associated with cancer stem cell-like properties and
chemotherapy drug resistance (4).
Therefore, a therapeutic agent that can effectively inhibit the EMT
process may be a potential anti-metastatic strategy. Cadherins,
named for ‘calcium-dependent adhesion’, serve a key role in
adherens junctions (34). A loss
in E-cadherin expression can result in the loss of contact
inhibition, and increase cell motility and invasion (35). Notably, N-cadherin is expressed in
mesenchymal cells and is overexpressed in cancer cells (36). Vimentin is an intermediate filament
protein, which is a cytoskeletal component in mesenchymal cells
(37). In the present study, it
was demonstrated that E-cadherin and ZO-1 protein expression levels
were significantly upregulated following 6-Gingerol treatment in
prostate cancer cells, whereas the mesenchymal markers, Vimentin
and N-cadherin were significantly decreased following 6-Gingerol
treatment in the PC3 and LNCaP cell lines. Our previous study
reported that LPS can enhance cell migration, invasion and
inflammation in prostate cancer cells (8). LPS is known to induce EMT in prostate
and breast cancer cells, which results in metastasis (7,38).
In the present study, the results demonstrated that LPS stimulated
EMT progression by significantly increasing Vimentin and N-cadherin
and did not markedly attenuate E-cadherin protein expression levels
in DU145 cells. Cell invasion and migration were significantly
induced following LPS treatment, whereas 6-Gingerol significantly
suppressed cell migration and invasion, and EMT by reversing this
pattern of EMT protein expression levels in LPS-treated DU145
cells.
Autophagy is a form of cell death that can remove
mis-folded proteins and maintain cellular homeostasis under
stressful conditions; notably, excess autophagy can also result in
cell death (39). Therefore, the
induction or inhibition of autophagy is considered to be a
potential novel strategy for the treatment of cancer (39). In the present study, 6-Gingerol
significantly induced LC3B conversion and Beclin-1 protein
expression in prostate cancer cells. However, autophagy inhibitor
LY294002 increased 6-Gingerol-induced cell death in PC3 and LNCaP
cells. Previous studies have reported that autophagy serves a
cytoprotective role against apoptosis (39,40).
These results revealed that autophagy induction of 6-Gingerol might
protect PC3 and LNCaP cells from cytotoxicity effects. However,
cell viability was increased following 6-Gingerol combined with
LY294002 treatment in DU145 cells. Protective autophagy (PC3 and
LNCaP) and autophagic cell death (DU145) were observed after
6-Gingerol treatment in prostate cancer cells.
Recent studies have demonstrated that ferroptosis is
important in the regulation of tumor cell proliferation, including
in breast, lung and prostate cancer (41–43).
Therefore, ferroptosis may be a potential novel strategy and
therapeutic target for the treatment of cancer. Ferroptosis results
from the depletion of GSH, GPX4 inactivation and intracellular ROS
accumulation (44). In the present
study, 6-Gingerol significantly decreased the levels of GPX4 and
GSH, and significantly elevated ROS accumulation in PC3, DU145 and
LNCaP cells. Previous studies have reported that 6-Gingerol-induced
ROS production is accompanied by apoptosis in gastric cancer, human
epidermoid carcinoma and myeloid leukemia cells (45–47).
The results of the present study demonstrated that
6-Gingerol may have significantly induced ROS production via a
ferroptosis mechanism in prostate cancer cells and that
pretreatment with the ferroptosis inhibitor, ferrostatin-1,
significantly reversed 6-Gingerol-induced ferroptosis. NRF2 is a
transcription factor that regulates signaling pathways in response
to oxidative stress. Inhibition or knockdown of the NRF2 gene has
been shown to enhance ferroptosis that results in decreased GSH
synthesis and GPX4 inhibition (48,49).
The present study demonstrated that 6-Gingerol (100 µM)
significantly decreased NRF2 protein expression levels in prostate
cancer cells. Taken together, these data suggested that 6-Gingerol
may promote ferroptosis, which could be beneficial for the
treatment of prostate cancer. Furthermore, these results indicated
that ferroptosis potentially serves an important role in mediating
cell death in DU145 cells treated with 6-Gingerol.
6-Gingerol is a flavonoid antioxidant that is
enriched in fresh ginger. Numerous studies have reported that
6-Gingerol has anticancer and anti-inflammatory effects (20,50–53).
The present study provided new evidence that 6-Gingerol may have
potential anti-metastatic and anticancer activities in prostate
cancer cells (Fig. 9). 6-Gingerol
significantly regulated EMT-related protein expression levels in
LPS-stimulated and LPS-unstimulated prostate cancer cells.
Furthermore, 6-Gingerol may trigger autophagy and ferroptosis,
which suggested that both mechanisms may serve pivotal roles in
regulating cell survival. In summary, 6-Gingerol may be considered
an important novel therapeutic agent for the prevention and
treatment of prostate cancer as a result of its numerous
pharmacological activities. Our study demonstrated that 6-Gingerol
can suppress migration, invasion and cell survival in CRPC, and
androgen-dependent prostate cancer cells. In vivo studies
are needed to verify these results in the future.
 | Figure 9.Diagram demonstrating the inhibition
of cell proliferation and EMT in prostate cancer cells following
6-Gingerol treatment. In the present study, 6-Gingerol induced
autophagy and ferroptosis. 6-Gingerol also reversed the EMT in
LPS-treated and LPS-untreated prostate cancer cells. EMT,
epithelial-mesenchymal transition; LPS, lipopolysaccharide; TLR4,
toll-like receptor 4; NRF2, nuclear factor erythroid 2-related
factor 2; GSH, glutathione; GPX4, glutathione peroxidase 4; ZO-1,
zonula occludens-1; HO-1, heme oxygenase-1; GS-SG, oxidized
glutathione. |
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Yichun University Local
Development Research Center (grant no. DF2019002) and the PhD
Research Foundation of Yichun University (grant no.
211-3360118006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CML and LA designed the present study and performed
the experiments. MS, ZS, YL and XL helped to perform the
experiments. CML, ZW, AJO and YJ contributed to the conception of
the study and analyzed the data. CML and LA confirm the
authenticity of all the raw data. CML and LA wrote the manuscript.
CML approved the version to be published and provided funding. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schatten H: Brief overview of prostate
cancer statistics, grading, diagnosis and treatment strategies. Adv
Exp Med Biol. 1095:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Logothetis C, Morris MJ, Den R and Coleman
RE: Current perspectives on bone metastases in castrate-resistant
prostate cancer. Cancer Metastasis Rev. 37:189–196. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suarez-Carmona M, Lesage J, Cataldo D and
Gilles C: EMT and inflammation: Inseparable actors of cancer
progression. Mol Oncol. 11:805–823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21:9652016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saitoh M: Involvement of partial EMT in
cancer progression. J Biochem. 164:257–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang T, Chen Z and Fang L: Curcumin
inhibits LPS-induced EMT through downregulation of NF-kappaB-snail
signaling in breast cancer cells. Oncol Rep. 29:117–124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian QX, Zhang ZH, Ye QL, Xu S, Hong Q,
Xing WY, Chen L, Yu DX, Xu DX and Xie DD: Melatonin inhibits
migration and invasion in LPS-stimulated and -unstimulated prostate
cancer cells through blocking multiple EMT-relative pathways. J
Inflamm Res. 14:2253–2265. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Z, Chen CY, Kao CL, Jiang Y and Liu CM:
Docosahexaenoic acid inhibits lipopolysaccharide-induced metastatic
activities by decreasing inflammation on prostate cancer cell.
Pharmazie. 74:675–679. 2019.PubMed/NCBI
|
|
9
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer. 19:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim TW, Lee SY, Kim M, Cheon C and Ko SG:
Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway
and inhibition of G9a in gastric cancer cells. Cell Death Dis.
9:8752018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang G, He J, Ye X, Zhu J, Hu X, Shen M,
Ma Y, Mao Z, Song H and Chen F: β-Thujaplicin induces autophagic
cell death, apoptosis, and cell cycle arrest through ROS-mediated
Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma.
Cell Death Dis. 10:2552019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramirez JA, Romagnoli GG and Kaneno R:
Inhibiting autophagy to prevent drug resistance and improve
anti-tumor therapy. Life Sci. 265:1187452021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lou JS, Zhao LP, Huang ZH, Chen XY, Xu JT,
Tai WC, Tsim KWK, Chen YT and Xie T: Ginkgetin derived from Ginkgo
biloba leaves enhances the therapeutic effect of cisplatin via
ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR
wild-type non-small-cell lung cancer. Phytomedicine. 80:1533702021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Tan H, Daniels JD, Zandkarimi F,
Liu H, Brown LM, Uchida K, O'Connor OA and Stockwell BR: Imidazole
ketone erastin induces ferroptosis and slows tumor growth in a
mouse lymphoma model. Cell Chem Biol. 26:623–633. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gundala SR, Mukkavilli R, Yang C, Yadav P,
Tandon V, Vangala S, Prakash S and Aneja R: Enterohepatic
recirculation of bioactive ginger phytochemicals is associated with
enhanced tumor growth-inhibitory activity of ginger extract.
Carcinogenesis. 35:1320–1329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong MK, Hu LL, Zhang YX, Xu YL, Liu XY,
He PK and Jia YH: 6-Gingerol ameliorates sepsis-induced liver
injury through the Nrf2 pathway. Int Immunopharmacol.
80:1061962020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu S, Zhang H, Liu T, Wang Z, Yang W, Hou
T, Wang X, He D and Zheng P: 6-Gingerol suppresses tumor cell
metastasis by increasing YAP(ser127) phosphorylation in renal cell
carcinoma. J Biochem Mol Toxicol. 35:e226092021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen CY, Kao CL and Liu CM: The cancer
prevention, anti-inflammatory and anti-oxidation of bioactive
phytochemicals targeting the TLR4 signaling pathway. Int J Mol Sci.
19:27292018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu CM, Kao CL, Tseng YT, Lo YC and Chen
CY: Ginger phytochemicals inhibit cell growth and modulate drug
resistance factors in docetaxel resistant prostate cancer cell.
Molecules. 22:14772017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brahmbhatt M, Gundala SR, Asif G, Shamsi
SA and Aneja R: Ginger phytochemicals exhibit synergy to inhibit
prostate cancer cell proliferation. Nutr Cancer. 65:263–272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shukla Y, Prasad S, Tripathi C, Singh M,
George J and Kalra N: In vitro and in vivo modulation of
testosterone mediated alterations in apoptosis related proteins by
[6]-gingerol. Mol Nutr Food Res. 51:1492–1502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin SK, Kamelgarn M and Kyprianou N:
Cytoskeleton targeting value in prostate cancer treatment. Am J
Clin Exp Urol. 2:15–26. 2014.PubMed/NCBI
|
|
25
|
Dai SN, Hou AJ, Zhao SM, Chen XM, Huang
HT, Chen BH and Kong HL: Ginsenoside Rb1 ameliorates autophagy of
hypoxia cardiomyocytes from neonatal rats via AMP-activated protein
kinase pathway. Chin J Integr Med. 25:521–528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ouyang DY, Xu LH, He XH, Zhang YT, Zeng
LH, Cai JY and Ren S: Autophagy is differentially induced in
prostate cancer LNCaP, DU145 and PC-3 cells via distinct splicing
profiles of ATG5. Autophagy. 9:20–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi
AA and Lei P: Ferroptosis: Mechanisms and links with diseases.
Signal Transduct Target Ther. 6:492021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yun DK, Lee J and Keum YS: Finasteride
increases the expression of hemoxygenase-1 (HO-1) and NF-E2-related
factor-2 (Nrf2) proteins in PC-3 cells: Implication of
finasteride-mediated high-grade prostate tumor occurrence. Biomol
Ther (Seoul). 21:49–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sp N, Kang DY, Lee JM, Bae SW and Jang KJ:
Potential antitumor effects of 6-gingerol in p53-dependent
mitochondrial apoptosis and inhibition of tumor sphere formation in
breast cancer cells. Int J Mol Sci. 22:46602021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Radhakrishnan EK, Bava SV, Narayanan SS,
Nath LR, Thulasidasan AK, Soniya EV and Anto RJ: [6]-Gingerol
induces caspase-dependent apoptosis and prevents PMA-induced
proliferation in colon cancer cells by inhibiting MAPK/AP-1
signaling. PLoS One. 9:e1044012014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kapoor V, Aggarwal S and Das SN:
6-gingerol mediates its anti tumor activities in human oral and
cervical cancer cell lines through apoptosis and cell cycle arrest.
Phytother Res. 30:588–595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Babaei G, Aziz SG and Jaghi NZZ: EMT,
cancer stem cells and autophagy; The three main axes of metastasis.
Biomed Pharmacother. 133:1109092021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Perez TD and Nelson WJ: Cadherin adhesion:
Mechanisms and molecular interactions. Handb Exp Pharmacol. 3–21.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mendonsa AM, Na TY and Gumbiner BM:
E-cadherin in contact inhibition and cancer. Oncogene.
37:4769–4780. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu W, Yang L, Li T and Zhang Y: Cadherin
signaling in cancer: Its functions and role as a therapeutic
target. Front Oncol. 9:9892019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leggett SE, Hruska AM, Guo M and Wong IY:
The epithelial-mesenchymal transition and the cytoskeleton in
bioengineered systems. Cell Commun Signal. 19:322021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo BP, Luo J, Hu YB, Yao XW and Wu FH:
Cyclin D1b splice variant promotes alphavbeta3-mediated EMT induced
by LPS in breast cancer cells. Curr Med Sci. 38:467–472. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu T, Zhang J, Li K, Deng L and Wang H:
Combination of an autophagy inducer and an autophagy inhibitor: A
smarter strategy emerging in cancer therapy. Front Pharmacol.
11:4082020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
El-Khattouti A, Selimovic D, Haikel Y and
Hassan M: Crosstalk between apoptosis and autophagy: Molecular
mechanisms and therapeutic strategies in cancer. J Cell Death.
6:37–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ding Y, Chen X, Liu C, Ge W, Wang Q, Hao
X, Wang M, Chen Y and Zhang Q: Identification of a small molecule
as inducer of ferroptosis and apoptosis through ubiquitination of
GPX4 in triple negative breast cancer cells. J Hematol Oncol.
14:192021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen P, Wu Q, Feng J, Yan L, Sun Y, Liu S,
Xiang Y, Zhang M, Pan T, Chen X, et al: Erianin, a novel dibenzyl
compound in Dendrobium extract, inhibits lung cancer cell growth
and migration via calcium/calmodulin-dependent ferroptosis. Signal
Transduct Target Ther. 5:512020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou X, Zou L, Chen W, Yang T, Luo J, Wu
K, Shu F, Tan X, Yang Y, Cen S, et al: Flubendazole, FDA-approved
anthelmintic, elicits valid antitumor effects by targeting P53 and
promoting ferroptosis in castration-resistant prostate cancer.
Pharmacol Res. 164:1053052021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun Y, Zheng Y, Wang C and Liu Y:
Glutathione depletion induces ferroptosis, autophagy, and premature
cell senescence in retinal pigment epithelial cells. Cell Death
Dis. 9:7532018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rastogi N, Gara RK, Trivedi R, Singh A,
Dixit P, Maurya R, Duggal S, Bhatt ML, Singh S and Mishra DP:
[6]-Gingerol induced myeloid leukemia cell death is initiated by
reactive oxygen species and activation of miR-27b expression. Free
Radic Biol Med. 68:288–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nigam N, Bhui K, Prasad S, George J and
Shukla Y: [6]-Gingerol induces reactive oxygen species regulated
mitochondrial cell death pathway in human epidermoid carcinoma A431
cells. Chem Biol Interact. 181:77–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mansingh DP, O JS, Sali VK and Vasanthi
HR: [6]-Gingerol-induced cell cycle arrest, reactive oxygen species
generation, and disruption of mitochondrial membrane potential are
associated with apoptosis in human gastric cancer (AGS) cells. J
Biochem Mol Toxicol. 32:e222062018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Salazar M, Rojo AI, Velasco D, de Sagarra
RM and Cuadrado A: Glycogen synthase kinase-3beta inhibits the
xenobiotic and antioxidant cell response by direct phosphorylation
and nuclear exclusion of the transcription factor Nrf2. J Biol
Chem. 281:14841–14851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abusarah J, Benabdoune H, Shi Q, Lussier
B, Martel-Pelletier J, Malo M, Fernandes JC, de Souza FP, Fahmi H
and Benderdour M: Elucidating the Role of protandim and
[6]-gingerol in protection against osteoarthritis. J Cell Biochem.
118:1003–1013. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang Q, Wei Q, Yang Q, Cao X, Li Q, Shi F,
Tong SS, Feng C, Yu Q, Yu J and Xu X: A novel formulation of
[6]-gingerol: Proliposomes with enhanced oral bioavailability and
antitumor effect. Int J Pharm. 535:308–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Adetuyi BO and Farombi EO: 6-Gingerol, an
active constituent of ginger, attenuates lipopolysaccharide-induced
oxidation, inflammation, cognitive deficits, neuroplasticity, and
amyloidogenesis in rat. J Food Biochem. 45:e136602021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
de Lima RMT, Dos Reis AC, de Menezes APM,
Santos JVO, Filho J, Ferreira JRO, de Alencar M, da Mata A, Khan
IN, Islam A, et al: Protective and therapeutic potential of ginger
(Zingiber officinale) extract and [6]-gingerol in cancer: A
comprehensive review. Phytother Res. 32:1885–1907. 2018. View Article : Google Scholar : PubMed/NCBI
|