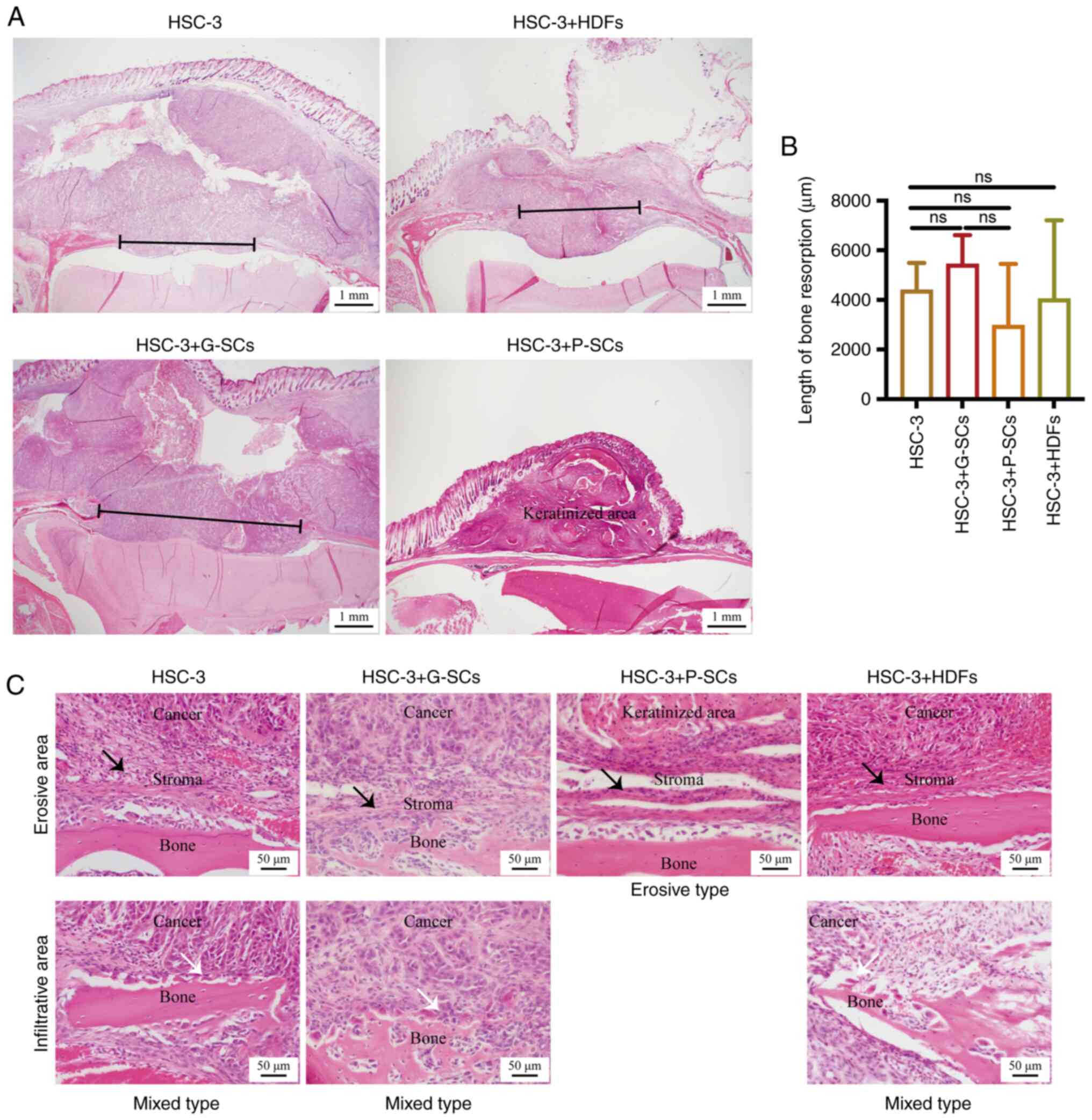
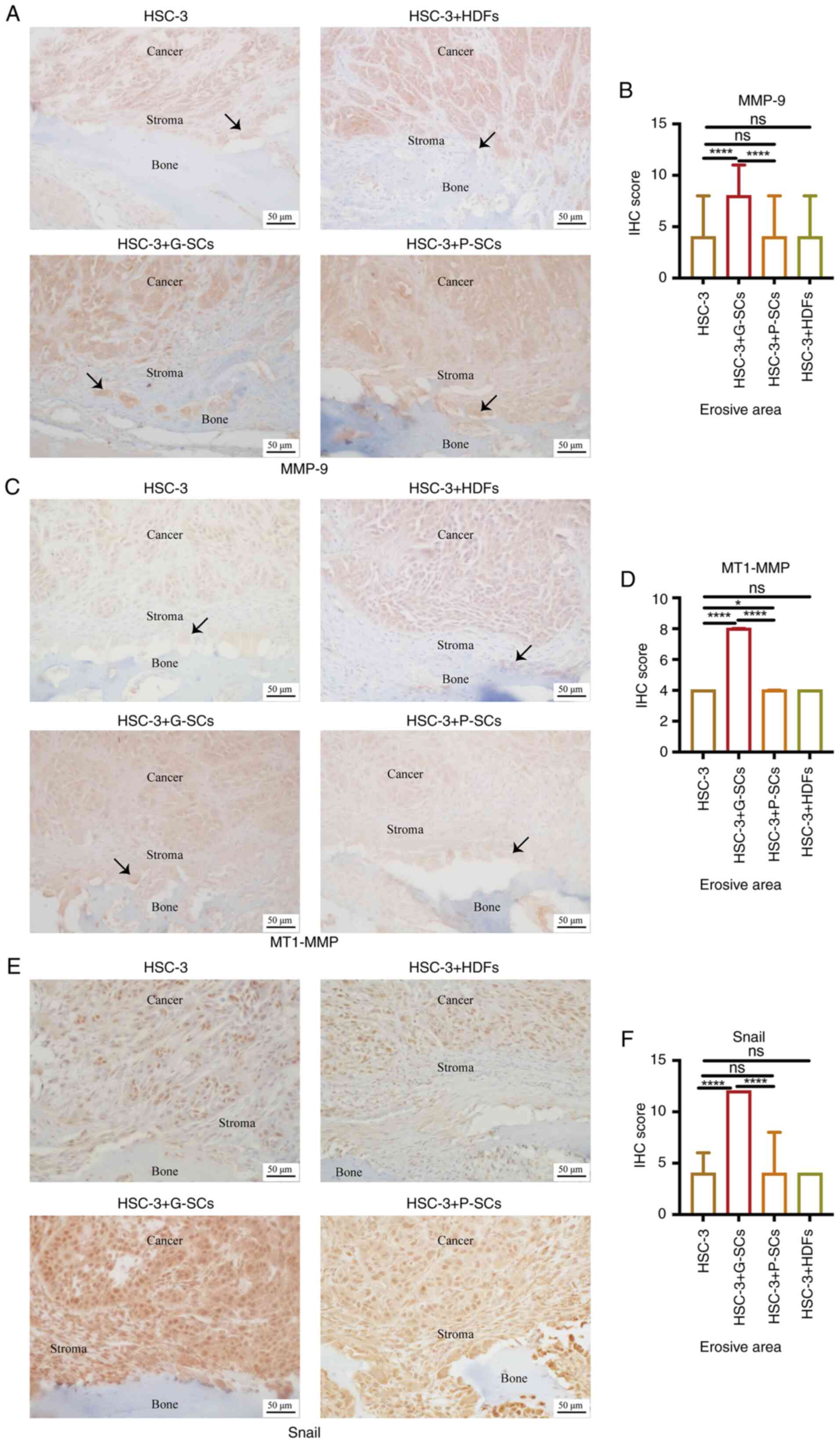
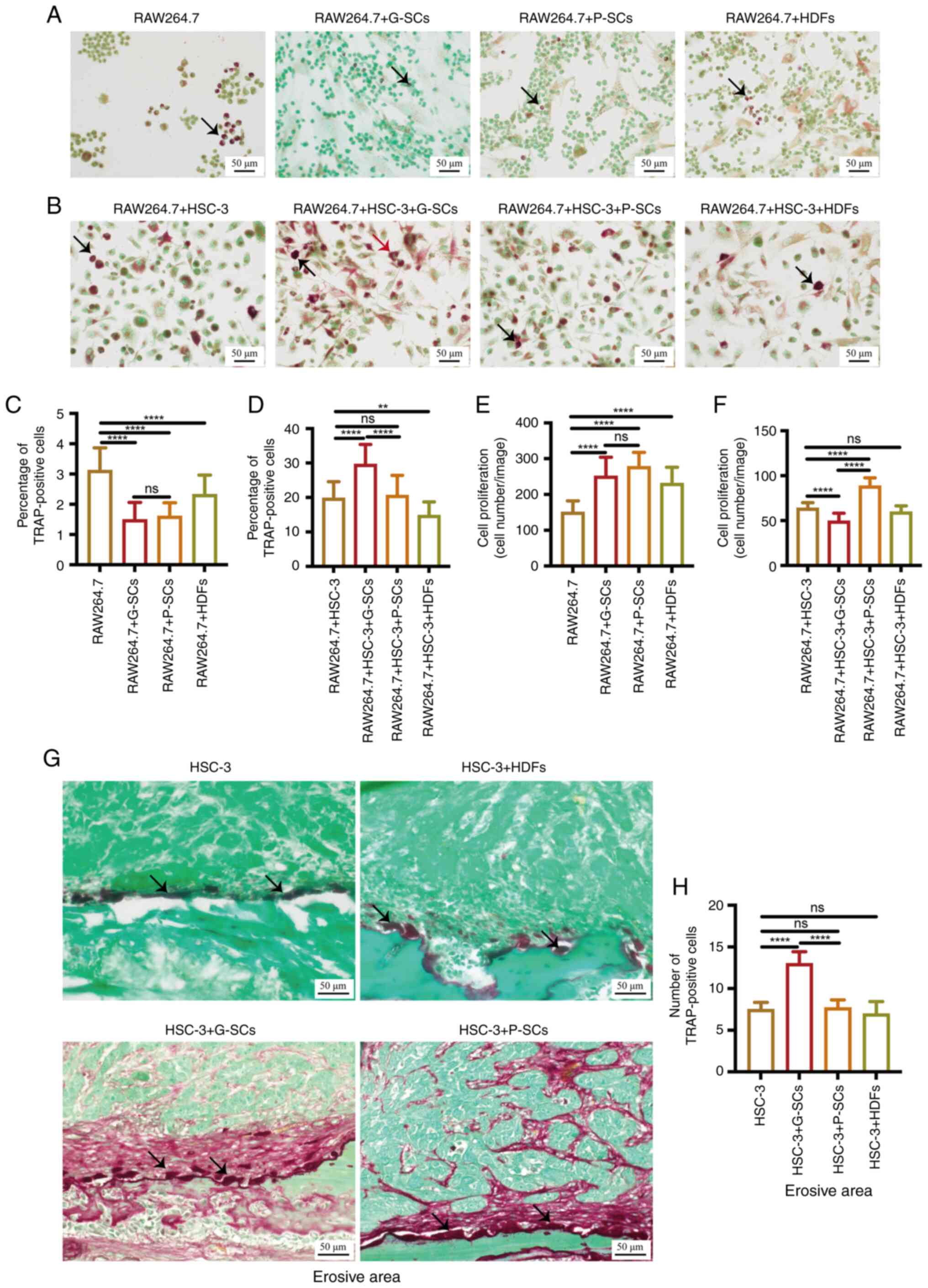
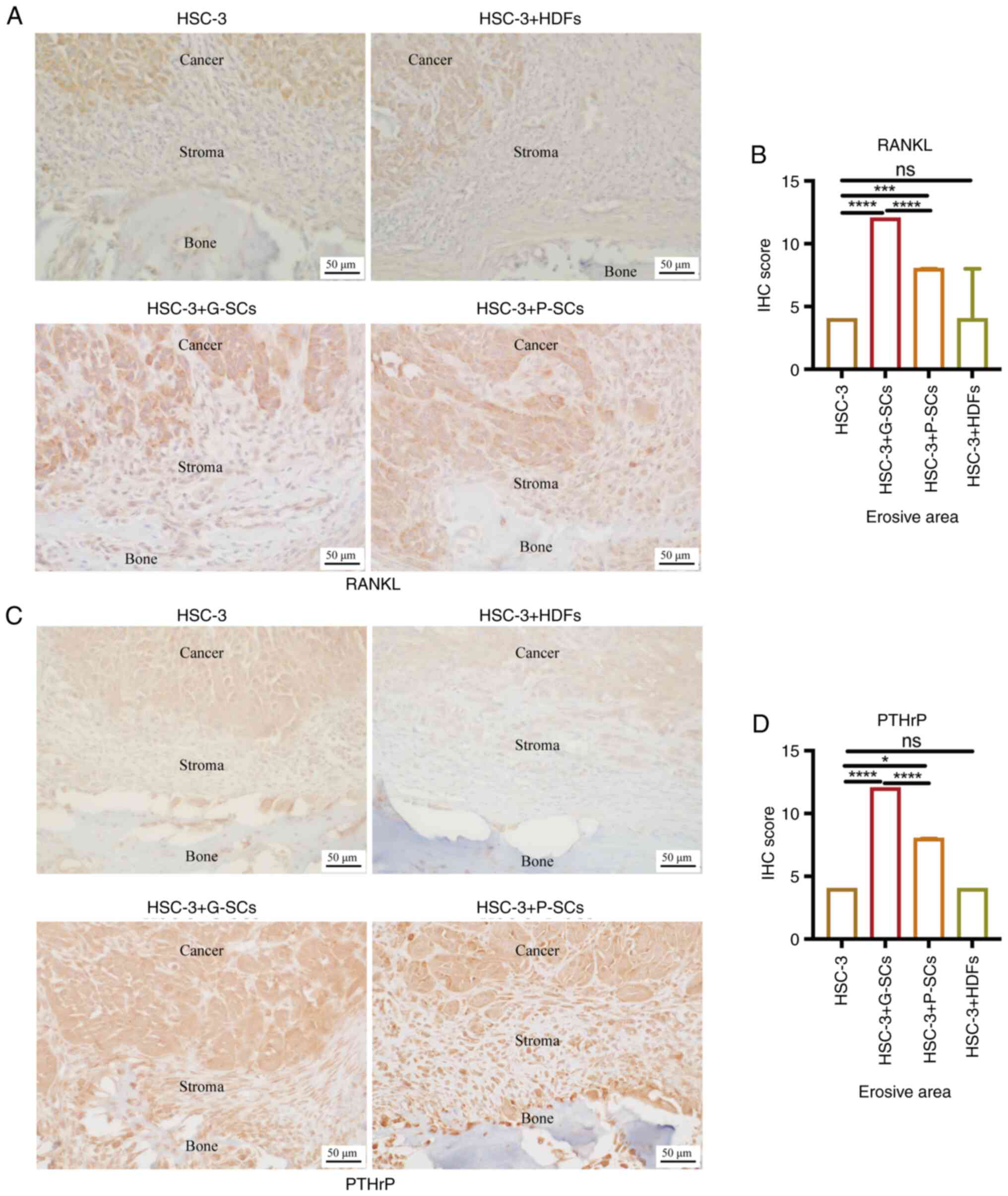
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
2
|
Lee KC, Chuang SK, Philipone EM and Peters
SM: Which clinicopathologic factors affect the prognosis of
gingival squamous cell carcinoma: A population analysis of 4,345
cases. J Oral Maxillofac Surg. 77:986–993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuk SK, Yoon HJ, Hong SD, Hong SP and Lee
JI: Staging significance of bone invasion in small-sized (4cm or
less) oral squamous cell carcinoma as defined by the American joint
committee on cancer. Oral Oncol. 55:31–36. 2016. View Article : Google Scholar
|
|
4
|
Russmueller G, Moser D, Würger T, Wrba F,
Christopoulos P, Kostakis G, Seemann R, Stadler V, Wimmer G, Kornek
G, et al: Upregulation of osteoprotegerin expression correlates
with bone invasion and predicts poor clinical outcome in oral
cancer. Oral Oncol. 51:247–253. 2015. View Article : Google Scholar
|
|
5
|
Omori H, Shan Q, Takabatake K, Nakano K,
Kawai H, Sukegawa S, Tsujigiwa H and Nagatsuka H: The origin of
stroma influences the biological characteristics of oral squamous
cell carcinoma. Cancers (Basel). 13:34912021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin CK, Dirksen WP, Shu ST, Werbeck JL,
Thudi NK, Yamaguchi M, Wolfe TD, Heller KN and Rosol TJ:
Characterization of bone resorption in novel in vitro and in vivo
models of oral squamous cell carcinoma. Oral Oncol. 48:491–499.
2012. View Article : Google Scholar
|
|
7
|
Wong RJ, Keel SB, Glynn RJ and Varvares
MA: Histological pattern of mandibular invasion by oral squamous
cell carcinoma. Laryngoscope. 110:65–72. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishikuro M, Sakamoto K, Kayamori K, Akashi
T, Kanda H, Izumo T and Yamaguchi A: Significance of the fibrous
stroma in bone invasion by human gingival squamous cell carcinomas.
Bone. 43:621–627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View
Article : Google Scholar
|
|
10
|
Takayanagi H: Osteoimmunology: Shared
mechanisms and crosstalk between the immune and bone systems. Nat
Rev Immunol. 7:292–304. 2007. View
Article : Google Scholar
|
|
11
|
Teitelbaum SL: Osteoclasts: What do they
do and how do they do it? Am J Pathol. 170:427–435. 2007.
View Article : Google Scholar
|
|
12
|
Suzuki T, Hayakawa T and Gomi K: GM-CSF
stimulates mouse macrophages and causes inflammatory effects in
vitro. J Hard Tissue Biol. 28:37–42. 2019. View Article : Google Scholar
|
|
13
|
Shibuya I, Takami M, Kawamoto M, Karakawa
A, Nakamura S and Kamijo R: Immunohistochemical analysis of the
distribution of RANKL-expressing cells and the expression of
osteoclast-related markers in giant cell tumor of bone. J Hard
Tissue Biol. 29:137–146. 2020. View Article : Google Scholar
|
|
14
|
Okamoto M, Hiura K, Ohe G, Ohba Y, Terai
K, Oshikawa T, Furuichi S, Nishikawa H, Moriyama K, Yoshida H and
Sato M: Mechanism for bone invasion of oral cancer cells mediated
by interleukin-6 in vitro and in vivo. Cancer. 89:1966–1975. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jimi E, Furuta H, Matsuo K, Tominaga K,
Takahashi T and Nakanishi O: The cellular and molecular mechanisms
of bone invasion by oral squamous cell carcinoma. Oral Dis.
17:462–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ono K, Akatsu T, Kugai N, Pilbeam CC and
Raisz LG: The effect of deletion of cyclooxygenase-2, prostaglandin
receptor EP2, or EP4 in bone marrow cells on osteoclasts induced by
mouse mammary cancer cell lines. Bone. 33:798–804. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kayamori K, Sakamoto K, Nakashima T,
Takayanagi H, Morita K, Omura K, Nguyen ST, Miki Y, Iimura T,
Himeno A, et al: Roles of interleukin-6 and parathyroid
hormone-related peptide in osteoclast formation associated with
oral cancers: Significance of interleukin-6 synthesized by stromal
cells in response to cancer cells. Am J Pathol. 176:968–980. 2010.
View Article : Google Scholar
|
|
18
|
Valkenburg KC, de Groot AE and Pienta KJ:
Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin
Oncol. 15:366–381. 2018. View Article : Google Scholar
|
|
19
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takabatake K, Kawai H, Omori H, Qiusheng
S, Oo MW, Sukegawa S, Nakano K, Tsujigiwa H and Nagatsuka H: Impact
of the stroma on the biological characteristics of the parenchyma
in oral squamous cell carcinoma. Int J Mol Sci. 21:77142020.
View Article : Google Scholar
|
|
21
|
Shan Q, Takabatake K, Omori H, Kawai H, Oo
MW, Nakano K, Ibaragi S, Sasaki A and Nagatsuka H: Stromal cells in
the tumor microenvironment promote the progression of oral squamous
cell carcinoma. Int J Oncol. 59:722021. View Article : Google Scholar
|
|
22
|
Shan Q, Takabatake K, Kawai H, Oo MW,
Inada Y, Sukegawa S, Fushimi S, Nakano K and Nagatsuka H:
Significance of cancer stroma for bone destruction in oral squamous
cell carcinoma using different cancer stroma subtypes. Oncol Rep.
47:812022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan Q, Takabatake K, Kawai H, Oo MW,
Sukegawa S, Fujii M, Nakano K and Nagatsuka H: Crosstalk between
cancer and different cancer stroma subtypes promotes the
infiltration of tumor-associated macrophages into the tumor
microenvironment of oral squamous cell carcinoma. Int J Oncol.
60:782022. View Article : Google Scholar
|
|
24
|
Quan J, Elhousiny M, Johnson NW and Gao J:
Transforming growth factor-β1 treatment of oral cancer induces
epithelial-mesenchymal transition and promotes bone invasion via
enhanced activity of osteoclasts. Clin Exp Metastasis. 30:659–670.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flecknell PA: Laboratory animal
anesthesia. 3rd edition. Academic Press; San Diego, CA: pp.
191–192. 2009
|
|
26
|
Adams S and Pacharinsak C: Mouse
anesthesia and analgesia. Curr Protoc Mouse Biol. 5:51–63. 2015.
View Article : Google Scholar
|
|
27
|
An YZ, Cho E, Ling J and Zhang X: The
axin2-snail axis promotes bone invasion by activating
cancer-associated fibroblasts in oral squamous cell carcinoma. BMC
Cancer. 20:9872020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chuang FH, Hsue SS, Wu CW and Chen YK:
Immunohistochemical expression of RANKL, RANK, and OPG in human
oral squamous cell carcinoma. J Oral Pathol Med. 38:753–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quan J, Hou Y, Long W, Ye S and Wang Z:
Characterization of different osteoclast phenotypes in the
progression of bone invasion by oral squamous cell carcinoma. Oncol
Rep. 39:1043–1051. 2018.PubMed/NCBI
|
|
30
|
Quan J, Johnson NW, Zhou G, Parsons PG,
Boyle GM and Gao J: Potential molecular targets for inhibiting bone
invasion by oral squamous cell carcinoma: A review of mechanisms.
Cancer Metastasis Rev. 31:209–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brown JS, Lowe D, Kalavrezos N, D'Souza J,
Magennis P and Woolgar J: Patterns of invasion and routes of tumor
entry into the mandible by oral squamous cell carcinoma. Head Neck.
24:370–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shaw RJ, Brown JS, Woolgar JA, Lowe D,
Rogers SN and Vaughan ED: The influence of the pattern of
mandibular invasion on recurrence and survival in oral squamous
cell carcinoma. Head Neck. 26:861–869. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ito M, Izumi N, Cheng J, Sakai H, Shingaki
S, Nakajima T, Oda K and Saku T: Jaw bone remodeling at the
invasion front of gingival squamous cell carcinomas. J Oral Pathol
Med. 32:10–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vincent C, Kogawa M, Findlay DM and Atkins
GJ: The generation of osteoclasts from RAW 264.7 precursors in
defined, serum-free conditions. J Bone Miner Metab. 27:114–119.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim H, Lee JH, Lee SK, Song NY, Son SH,
Kim KR and Chung WY: Chemerin treatment inhibits the growth and
bone invasion of breast cancer cells. Int J Mol Sci. 21:28712020.
View Article : Google Scholar
|
|
36
|
Woodward JK, Holen I, Coleman RE and
Buttle DJ: The roles of proteolytic enzymes in the development of
tumour-induced bone disease in breast and prostate cancer. Bone.
41:912–927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van der Pluijm G: Epithelial plasticity,
cancer stem cells and bone metastasis formation. Bone. 48:37–43.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Raggatt LJ and Partridge NC: Cellular and
molecular mechanisms of bone remodeling. J Biol Chem.
285:25103–25108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elmusrati AA, Pilborough AE, Khurram SA
and Lambert DW: Cancer-associated fibroblasts promote bone invasion
in oral squamous cell carcinoma. Br J Cancer. 117:867–875. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ueda M, Goto T, Kuroishi KN, Gunjigake KK,
Ikeda E, Kataoka S, Nakatomi M, Toyono T, Seta Y and Kawamoto T:
Asporin in compressed periodontal ligament cells inhibits bone
formation. Arch Oral Biol. 62:86–92. 2016. View Article : Google Scholar
|
|
41
|
Nishigaki M, Yamamoto T, Ichioka H, Honjo
KI, Yamamoto K, Oseko F, Kita M, Mazda O and Kanamura N:
β-cryptoxanthin regulates bone resorption related-cytokine
production in human periodontal ligament cells. Arch Oral Biol.
58:880–886. 2013. View Article : Google Scholar
|
|
42
|
Kats A, Gerasimcik N, Näreoja T, Nederberg
J, Grenlöv S, Lagnöhed E, Desai S, Andersson G and Yucel-Lindberg
T: Aminothiazoles inhibit osteoclastogenesis and PGE2 production in
LPS-stimulated co-cultures of periodontal ligament and RAW 264.7
cells, and RANKL-mediated osteoclastogenesis and bone resorption in
PBMCs. J Cell Mol Med. 23:1152–1163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu R, Bal HS, Desta T, Krothapalli N,
Alyassi M, Luan Q and Graves DT: Diabetes enhances periodontal bone
loss through enhanced resorption and diminished bone formation. J
Dent Res. 85:510–514. 2006. View Article : Google Scholar : PubMed/NCBI
|