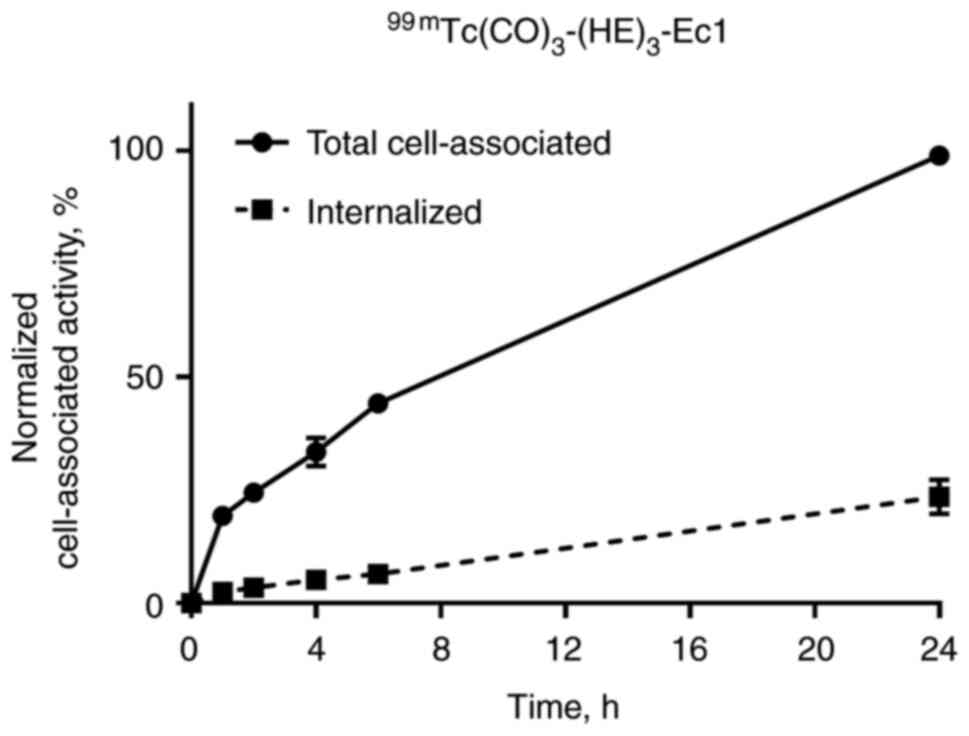
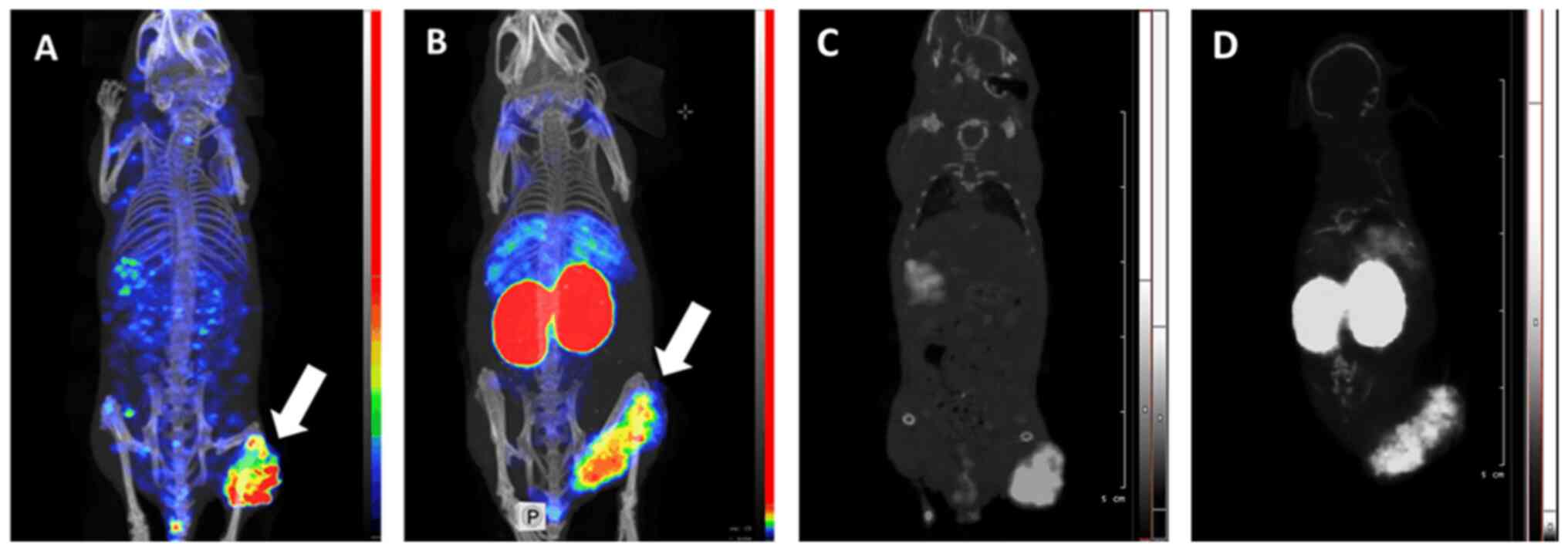
|
1
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryan CW, Vogelzang NJ and Stadler WM: A
phase II trial of intravenous gemcitabine and 5-fluorouracil with
subcutaneous interleukin-2 and interferon-alpha in patients with
metastatic renal cell carcinoma. Cancer. 94:2602–2609. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Went P, Dirnhofer S, Salvisberg T, Amin
MB, Lim SD, Diener PA and Moch H: Expression of epithelial cell
adhesion molecule (EpCam) in renal epithelial tumors. Am J Surg
Pathol. 29:83–88. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart GD, O'Mahony FC, Powles T, Riddick
AC, Harrison DJ and Faratian D: What can molecular pathology
contribute to the management of renal cell carcinoma? Nat Rev Urol.
8:255–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tran J and Ornstein MC: Clinical review on
the management of metastatic renal cell carcinoma. JCO Oncol Pract.
18:187–196. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osawa T, Takeuchi A, Kojima T, Shinohara
N, Eto M and Nishiyama H: Overview of current and future systemic
therapy for metastatic renal cell carcinoma. Jpn J Clin Oncol.
49:395–403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tung I and Sahu A: Immune checkpoint
inhibitor in first-line treatment of metastatic renal cell
carcinoma: A review of current evidence and future directions.
Front Oncol. 11:7072142021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elias DJ, Kline LE, Robbins BA, Johnson HC
Jr, Pekny K, Benz M, Robb JA, Walker LE, Kosty M and Dillman RO:
Monoclonal antibody KS1/4-methotrexate immunoconjugate studies in
non-small cell lung carcinoma. Am J Respir Crit Care Med.
150:1114–1122. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Braun S, Hepp F, Kentenich CR, Janni W,
Pantel K, Riethmüller G, Willgeroth F and Sommer HL: Monoclonal
antibody therapy with edrecolomab in breast cancer patients:
Monitoring of elimination of disseminated cytokeratin-positive
tumor cells in bone marrow. Clin Cancer Res. 5:3999–4004.
1999.PubMed/NCBI
|
|
10
|
Punt CJ, Nagy A, Douillard JY, Figer A,
Skovsgaard T, Monson J, Barone C, Fountzilas G, Riess H, Moylan E,
et al: Edrecolomab alone or in combination with fluorouracil and
folinic acid in the adjuvant treatment of stage III colon cancer: A
randomised study. Lancet. 360:671–677. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Paolo C, Willuda J, Kubetzko S, Lauffer
I, Tschudi D, Waibel R, Plückthun A, Stahel RA and
Zangemeister-Wittke U: A recombinant immunotoxin derived from a
humanized epithelial cell adhesion molecule-specific single-chain
antibody fragment has potent and selective antitumor activity. Clin
Cancer Res. 9:2837–2848. 2003.PubMed/NCBI
|
|
12
|
Andersson Y, Inderberg EM, Kvalheim G,
Herud TM, Engebraaten O, Flatmark K, Dueland S and Fodstad Ø:
Immune stimulatory effect of anti-EpCAM immunotoxin-improved
overall survival of metastatic colorectal cancer patients. Acta
Oncol. 59:404–409. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seimetz D, Lindhofer H and Bokemeyer C:
Development and approval of the trifunctional antibody catumaxomab
(anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer
Treat Rev. 36:458–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martin-Killias P, Stefan N, Rothschild S,
Plückthun A and Zangemeister-Wittke U: A novel fusion toxin derived
from an EpCAM-specific designed ankyrin repeat protein has potent
antitumor activity. Clin Cancer Res. 17:100–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu T, Vorobyeva A, Schulga A, Konovalova
E, Vorontsova O, Ding H, Gräslund T, Tashireva LA, Orlova A,
Tolmachev V and Deyev SM: Imaging-Guided therapy simultaneously
targeting HER2 and EpCAM with trastuzumab and EpCAM-Directed toxin
provides additive effect in ovarian cancer model. Cancers (Basel).
13:39392021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang BL, Li D, Gong YL, Huang Y, Qin DY,
Jiang L, Liang X, Yang X, Gou HF, Wang YS, et al: Preclinical
evaluation of chimeric antigen receptor-modified T cells specific
to epithelial cell adhesion molecule for treating colorectal
cancer. Hum Gene Ther. 30:402–412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pilanc KN, Ordu Ç, Akpnar H, Balc C,
Başsülü N, Köksal Üİ, Elbüken F, Okutur K, Bülbül G, Sağlam S and
Demir G: Dramatic response to catumaxomab treatment for malign
ascites related to renal cell carcinoma with sarcomotoid
differentiation. Am J Ther. 23:e1078–e1081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmidt M, Scheulen ME, Dittrich C, Obrist
P, Marschner N, Dirix L, Schmidt M, Rüttinger D, Schuler M,
Reinhardt C and Awada A: An open-label, randomized phase II study
of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy
in patients with metastatic breast cancer. Ann Oncol. 21:275–282.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marschner N, Rüttinger D, Zugmaier G,
Nemere G, Lehmann J, Obrist P, Baeuerle PA, Wolf A, Schmidt M,
Abrahamsson PA, et al: Phase II study of the human anti-epithelial
cell adhesion molecule antibody adecatumumab in prostate cancer
patients with increasing serum levels of prostate-specific antigen
after radical prostatectomy. Urol Int. 85:386–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zimpfer A, Maruschke M, Rehn S, Kundt G,
Litzenberger A, Dammert F, Zettl H, Stephan C, Hakenberg OW and
Erbersdobler A: Prognostic and diagnostic implications of
epithelial cell adhesion/activating molecule (EpCAM) expression in
renal tumours: A retrospective clinicopathological study of 948
cases using tissue microarrays. BJU Int. 114:296–302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bensch F, Lamberts LE, Smeenk MM,
Jorritsma-Smit A, Lub-de Hooge MN, Terwisscha van Scheltinga AGT,
de Jong JR, Gietema JA, Schröder CP, Thomas M, et al:
89Zr-Lumretuzumab PET Imaging before and during HER3 antibody
lumretuzumab treatment in patients with solid tumors. Clin Cancer
Res. 23:6128–6137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jauw YW, Zijlstra JM, de Jong D, Vugts DJ,
Zweegman S, Hoekstra OS, van Dongen GA and Huisman MC: Performance
of 89Zr-Labeled-Rituximab-PET as an Imaging Biomarker to Assess
CD20 Targeting: A pilot study in patients with relapsed/refractory
diffuse large B cell lymphoma. PLoS One. 12:e01698282017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ulaner GA, Lyashchenko SK, Riedl C, Ruan
S, Zanzonico PB, Lake D, Jhaveri K, Zeglis B, Lewis JS and
O'Donoghue JA: First-in-Human human epidermal growth factor
receptor 2-Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry
and clinical application in patients with breast cancer. J Nucl
Med. 59:900–906. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garousi J, Orlova A, Frejd FY and
Tolmachev V: Imaging using radiolabelled targeted proteins:
Radioimmunodetection and beyond. EJNMMI Radiopharm Chem. 5:162020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tolmachev VM, Chernov VI and Deyev SM:
Targeted nuclear medicine. Seek and destroy. Rus Chem Rev.
91:RCR50342022. View Article : Google Scholar
|
|
26
|
Plückthun A: Designed ankyrin repeat
proteins (DARPins): Binding proteins for research, diagnostics, and
therapy. Annu Rev Pharmacol Toxicol. 55:489–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stefan N, Martin-Killias P, Wyss-Stoeckle
S, Honegger A, Zangemeister-Wittke U and Plückthun A: DARPins
recognizing the tumor-associated antigen EpCAM selected by phage
and ribosome display and engineered for multivalency. J Mol Biol.
413:826–843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deyev SM, Vorobyeva A, Schulga A,
Abouzayed A, Günther T, Garousi J, Konovalova E, Ding H, Gräslund
T, Orlova A and Tolmachev V: Effect of a radiolabel biochemical
nature on tumor-targeting properties of EpCAM-binding engineered
scaffold protein DARPin Ec1. Int J Biol Macromol. 145:216–225.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vorobyeva A, Bezverkhniaia E, Konovalova
E, Schulga A, Garousi J, Vorontsova O, Abouzayed A, Orlova A, Deyev
S and Tolmachev V: Radionuclide molecular imaging of EpCAM
expression in triple-negative breast cancer using the scaffold
protein DARPin Ec1. Molecules. 25:47192020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wållberg H and Orlova A: Slow
internalization of anti-HER2 synthetic affibody monomer
111In-DOTA-ZHER2:342-pep2: Implications for development of labeled
tracers. Cancer Biother Radiopharm. 23:435–442. 2008.PubMed/NCBI
|
|
31
|
Tolmachev V, Orlova A and Andersson K:
Methods for radiolabelling of monoclonal antibodies. Methods Mol
Biol. 1060:309–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guide for the Care and Use of Laboratory
Animals. The National Academies Press; Washington, DC: 1996,
https://doi.org/10.17226/5140
|
|
33
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tolmachev V and Orlova A: Affibody
molecules as targeting vectors for PET Imaging. Cancers (Basel).
12:6512020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hosseinimehr SJ, Tolmachev V and Orlova A:
Liver uptake of radiolabeled targeting proteins and peptides:
Considerations for targeting peptide conjugate design. Drug Discov
Today. 17:1224–1232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vorobyeva A, Schulga A, Konovalova E,
Güler R, Löfblom J, Sandström M, Garousi J, Chernov V, Bragina O,
Orlova A, et al: Optimal composition and position of
histidine-containing tags improves biodistribution of
99mTc-labeled DARPin G3. Sci Rep. 9:94052019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deyev SM, Xu T, Liu Y, Schulga A,
Konovalova E, Garousi J, Rinne SS, Larkina M, Ding H, Gräslund T,
et al: Influence of the position and composition of radiometals and
radioiodine labels on imaging of Epcam expression in prostate
cancer model using the DARPin Ec1. Cancers (Basel). 13:35892021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vorobyeva A, Sсhulga A, Konovalova E,
Güler R, Mitran B, Garousi J, Rinne S, Löfblom J, Orlova A, Deyev S
and Tolmachev V: Comparison of tumor-targeting properties of
directly and indirectly radioiodinated designed ankyrin repeat
protein (DARPin) G3 variants for molecular imaging of HER2. Int J
Oncol. 54:1209–1220. 2019.PubMed/NCBI
|
|
39
|
Wikman M, Steffen AC, Gunneriusson E,
Tolmachev V, Adams GP, Carlsson J and Ståhl S: Selection and
characterization of HER2/neu-binding affibody ligands. Protein Eng
Des Sel. 17:455–462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deyev S, Vorobyeva A, Schulga A, Proshkina
G, Güler R, Löfblom J, Mitran B, Garousi J, Altai M, Buijs J, et
al: Comparative evaluation of Two DARPin variants: Effect of
affinity, size, and label on tumor targeting properties. Mol Pharm.
16:995–1008. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vorobyeva A, Schulga A, Rinne SS, Günther
T, Orlova A, Deyev S and Tolmachev V: Indirect radioiodination of
DARPin G3 Using N-succinimidyl-Para-Iodobenzoate Improves the
Contrast of HER2 molecular imaging. Int J Mol Sci. 20:30472019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Altai M, Garousi J, Rinne SS, Schulga A,
Deyev S and Vorobyeva A: On the prevention of kidney uptake of
radiolabeled DARPins. EJNMMI Res. 10:72020. View Article : Google Scholar : PubMed/NCBI
|