Introduction
Src homology-2 domain-containing protein tyrosine
phosphatase (SHP2) is a member of the protein tyrosine phosphatase
(PTP) family (1). Among them,
SHP2/PTP non-receptor type 11 (PTPN11) is currently the only
proto-oncogene in the PTP family to be confirmed and is expressed
in a variety of human tissues (2).
SHP2/PTPN11 is expressed in numerous types of
tissues, where it serves a regulatory role in cell signaling events
important for an array of cellular processes, such as mitogenic
activation, metabolic control, transcription regulation and cell
migration (3). In a variety of
types of cancer, aberrant activation of SHP2/PTPN11 has been
previously documented to serve a significant pathogenic role. Wei
(4) found that SHP2 is highly
expressed in 60.78% (31/51) of gastric cancer tissues tested. In
another study, Tang et al (5) demonstrated that SHP2/PTPN11 is
significantly overexpressed in non-small cell lung cancer (NSCLC;
70%; 56/80) compared with that in adjacent normal tissues. In
addition, Han et al (6)
reported that SHP2/PTPN11 is significantly overexpressed in liver
cancer tissues (78.36%; 105/134). However, Jiang et al
(7) found that the expression
levels of SHP2 are reduced in 70.6% patients with liver cancer,
which were in turn associated with poorer prognosis.
Therefore, given the apparent close association
between SHP2/PTPN11 and a number of human malignancies
aforementioned, in addition to its presence in these malignant
tissues (7,8), a comprehensive analysis of
SHP2/PTPN11 would be of clinical significance. Therefore, the
present study comprehensively explored the association between the
expression levels of SHP2/PTPN11 and the risk of cancer.
Materials and methods
Article retrieval
In total, three major electronic databases, namely
PubMed, China National Knowledge Infrastructure and Cochrane
Library, were searched for the present study. The search terms used
include the following: ‘Src homology 2 domain containing protein
tyrosine phosphatase’ or ‘SHP2’ or ‘protein tyrosine phosphatase
non-receptor type 11’ or ‘PTPN11’ and ‘Neoplasm*’ or ‘Cancer*’ or
‘tumor*’ or ‘carcinoma*’. The search deadline was May 2022.
Eligibility criteria
Studies were eligible for inclusion if they met the
following criteria: i) The study having similar purposes and
statistical methods with complete data; ii) the eligible study
assessed the relationship between SHP2 or PTPN11 and risk of
cancer; iii) the study had clear experimental grouping methods and
SHP2 or PTPN11 detection methods; and iv) the nationality, race and
age of all patients were not restricted. The language of the
literature was not restricted.
By contrast, the study would be excluded if it met
the following criteria: i) The study had incomplete information;
ii) abstracts only and case reports; iii) animal studies, cellular
studies, systematic reviews and other non-original studies; iv) the
study did not have clear grouping methods and SHP2 or PTPN11
detection methods; and v) there was no control group.
Screening and data extraction
In total, two researchers independently read and
screened the retrieved studies. If there were any dispute, then a
third researcher would make a comprehensive judgment. EndNote X7
(Thomson Corporation) software was first used to automatically
exclude any duplicate literature, before the researchers browsed
all the remaining literature to manually remove further duplicated
studies. Subsequently, studies that did not meet the inclusion
criteria were excluded by reading their titles and abstracts. The
remaining studies that did not meet the inclusion criteria was then
also excluded by reading the full text, before relevant data were
extracted from the studies (Fig.
1). The information extracted were as follows: i) The different
detection methods may be used to detect the expression level of
SHP2/PTPN11 in different studies. Therefore, in order to have
comparable results, this meta-analysis used binary variables to
extract data, and extracted the number of occurrence and
non-occurrence in the experimental group and control group; ii)
SHP2 or PTPN11 detection method; iii) study grouping method, iv)
the name of the first author; v) the year of publication; vi) the
geographical location of the patient; and vii) patient age.
Statistical methods
The Newcastle-Ottawa Scale (NOS) was used to
independently evaluate the quality of articles that met the
inclusion criteria. Each article was then assigned a quality score
of ≤8 stars. Articles with a score of <5 represent low quality,
whereas articles with a score of >6 were classified as high
quality. Low-quality articles were subsequently excluded.
Statistical analysis was performed using the STATA 12.0 software
(StataCorp LLC). If there were ≥2 articles that examined the
relationship between the expression of SHP2/PTPN11 and the risk of
cancers, statistical analysis would then be performed. Otherwise,
descriptive analyses would be performed instead. Heterogeneity
among studies was assessed using the Chi-square test and
I2 test. The random-effect model was used to pool the
data. Begg and Egger tests would be used to assess any potential
publication bias, with P<0.05 considered to indicate a
statistically significant publication bias. In this manuscript,
since there are larger quantity of studies on lung cancer, gastric
cancer, cervical cancer and liver cancer, the Egger's linear
regression model and Begg's funnel plot were used to test for
publication bias. However, the number of studies investigating
breast cancer, ovarian cancer, thyroid cancer, colorectal cancer,
glioma, pancreatic cancer, nasopharyngeal cancer and prostate
cancer was ≤2, rendering them not suitable for performing
publication bias analysis. The pooled results were presented as the
odds ratio (OR) and 95% CI. If OR >1 and P<0.05 were
satisfied, this would indicate that SHP2/PTPN11 expression is
statistically associated with the risk of cancer. P<0.05 was
considered to indicate a statistically significant difference
throughout.
Results
Characteristics of included
literature
A total of 1,217 articles were retrieved. After
excluding duplicated studies and filtering the studies using the
aforementioned criteria, 128 studies remained. A total of 23
articles were found to explore the relationship between the
expression levels of SHP2/PTPN11 and 12 types of cancer (Fig. 1; Table
I).
 | Table I.Basic characteristics of included
literature. |
Table I.
Basic characteristics of included
literature.
|
|
| Experience
group | Control group |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First author,
year | Country | + | - | + | - | Age | Method | Cancer | (Refs.) |
|---|
| Xiaolin et
al, 2016 | China | 117 | 218 | 130 | 205 | 10-79 | IHC | Liver cancer | (21) |
| Han et al,
2015 | China | 105 | 29 | 29 | 105 | - | Q-PCR | Liver cancer | (6) |
| Han et al,
2015 | China | 25 | 16 | 16 | 25 | - | WB | Liver cancer | (6) |
| Han et al,
2015 | China | 395 | 203 | 203 | 395 | - | IHC | Liver cancer | (6) |
| Jiang et al,
2012 | China | 220 | 113 | 322 | 11 | 51.8±10.5 | IHC | Liver cancer | (7) |
| Zhan et al,
2010 | China | 41 | 7 | 12 | 3 | - | IHC | NSCLC | (9) |
| Zhan et al,
2010 | China | 5 | 0 | 12 | 3 | - | IHC | SCLC | (9) |
| Tang et al,
2013 | China | 56 | 24 | 0 | 10 | - | IHC | NSCLC | (5) |
| Sansan, 2011 | China | 7 | 8 | 4 | 11 | - | WB | Lung cancer | (10) |
| He et al,
2019 | China | 14 | 6 | 7 | 13 | 33-76 | WB | NSCLC | (8) |
| Sun et al,
2017 | China | 5 | 18 | 3 | 20 | - | IHC | NSCLC | (11) |
| Meng et al,
2012 | China | 40 | 5 | 9 | 11 | 30-70 | IHC | Cervical
cancer | (18) |
| Zhang et al,
2015 | China | 45 | 24 | 9 | 15 | - | IHC | Cervical
cancer | (19) |
| Cao et al,
2019 | China | 82 | 3 | 8 | 14 | 15-76 | IHC | Cervical
cancer | (3) |
| Tao et al,
2008 | China | 18 | 2 | 6 | 14 | 32-61 | IHC | Cervical
cancer | (20) |
| Jiang et al,
2013 | China | 62 | 21 | 27 | 56 | 32-87 | IHC | Gastric cancer | (15) |
| Wei, 2016 | China | 31 | 20 | 0 | 51 | 36-78 | WB | Gastric cancer | (4) |
| Dong et al,
2012 | China | 29 | 11 | 7 | 25 | - | IHC | Gastric cancer | (16) |
| Zhou et al,
2016 | China | 255 | 175 | 455 | 505 | 62.75±11.40 | Q-PCR | Gastric cancer | (17) |
| Sansan, 2011 | China | 10 | 5 | 7 | 8 | - | WB | Gastric cancer | (10) |
| Lei, 2010 | China | 28 | 2 | 2 | 28 | - | WB | Breast cancer | (22) |
| Sansan, 2011 | China | 14 | 15 | 1 | 28 | - | WB | Breast cancer | (10) |
| Hu et al,
2017 | China | 49 | 11 | 0 | 60 | 23-70 | WB | Ovarian cancer | (23) |
| Yajuan et
al, 2014 | China | 35 | 10 | 0 | 10 | - | IHC | Ovarian cancer | (24) |
| Hu et al,
2018 | China | 62 | 3 | 6 | 34 | - | IHC | Thyroid Cancer | (27) |
| Cao et al,
2018 | China | 6 | 24 | 180 | 133 | - | IHC | Thyroid Cancer | (26) |
| Sansan, 2011 | China | 9 | 5 | 6 | 8 | - | WB | Colorectal
cancer | (10) |
| Sansan, 2011 | China | 4 | 2 | 3 | 3 | - | WB | Glioma | (10) |
| Sansan, 2011 | China | 5 | 7 | 5 | 7 | - | WB | Nasopharyngeal
cancer | (10) |
| Zhang et al,
2016 | China | 10 | 4 | 6 | 8 | - | WB | Prostate
cancer | (28) |
| Zheng et al,
2016 | China | 44 | 35 | 8 | 71 | - | IHC | Pancreatic
cancer | (25) |
Higher expression levels of
SHP2/PTPN11 can increase the risk of NSCLC and reduce the overall
survival (OS) of patients
The relationship between the expression level of
SHP2/PTPN11 and the risk of lung cancer was analyzed in six
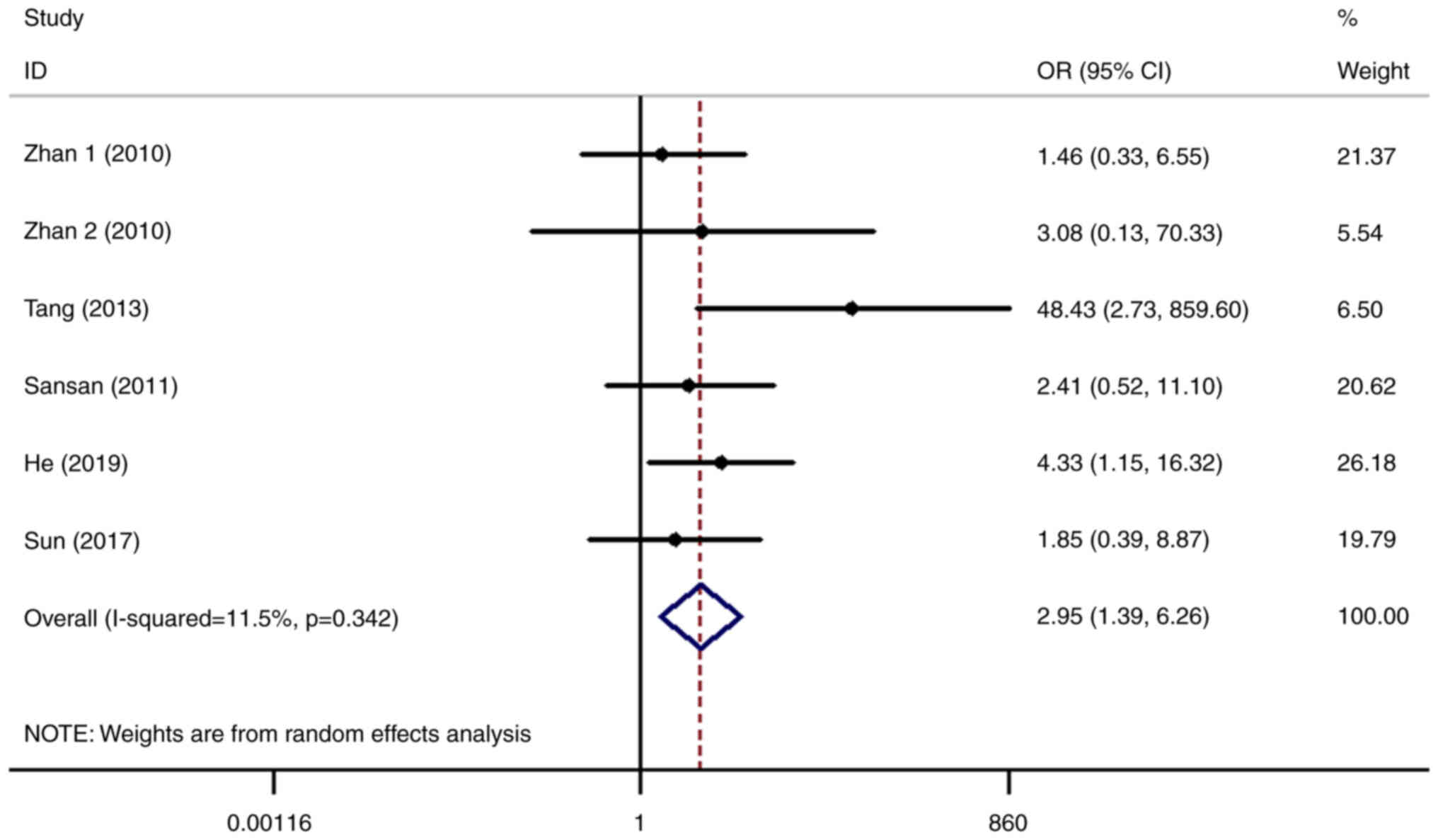
datasets (5,8–11).
After statistical analysis, it was found that the risk of lung
cancer in patients with higher expression levels of SHP2/PTPN11 was
2.95-fold higher compared with that in the normal control group
(OR=2.95, 95% CI=1.39-6.26; Fig.
2), with the difference of which being statistically
significant (P=0.005). Following further stratified analysis, it
was found that higher expression levels of SHP2/PTPN11 mainly
increased the risk of NSCLC (OR=3.41, 95% CI=1.07-10.80; P=0.037;
Fig. 2) but not small cell lung
cancer (SCLC). However, this may also be due to the relatively few
studies on the role of SHP2/PTPN11 in SCLC, resulting in false
negative results. Therefore, further scientific and clinical
research are required to investigate the relationship between
SHP2/PTPN11 and SCLC.
The relationship between the expression levels of
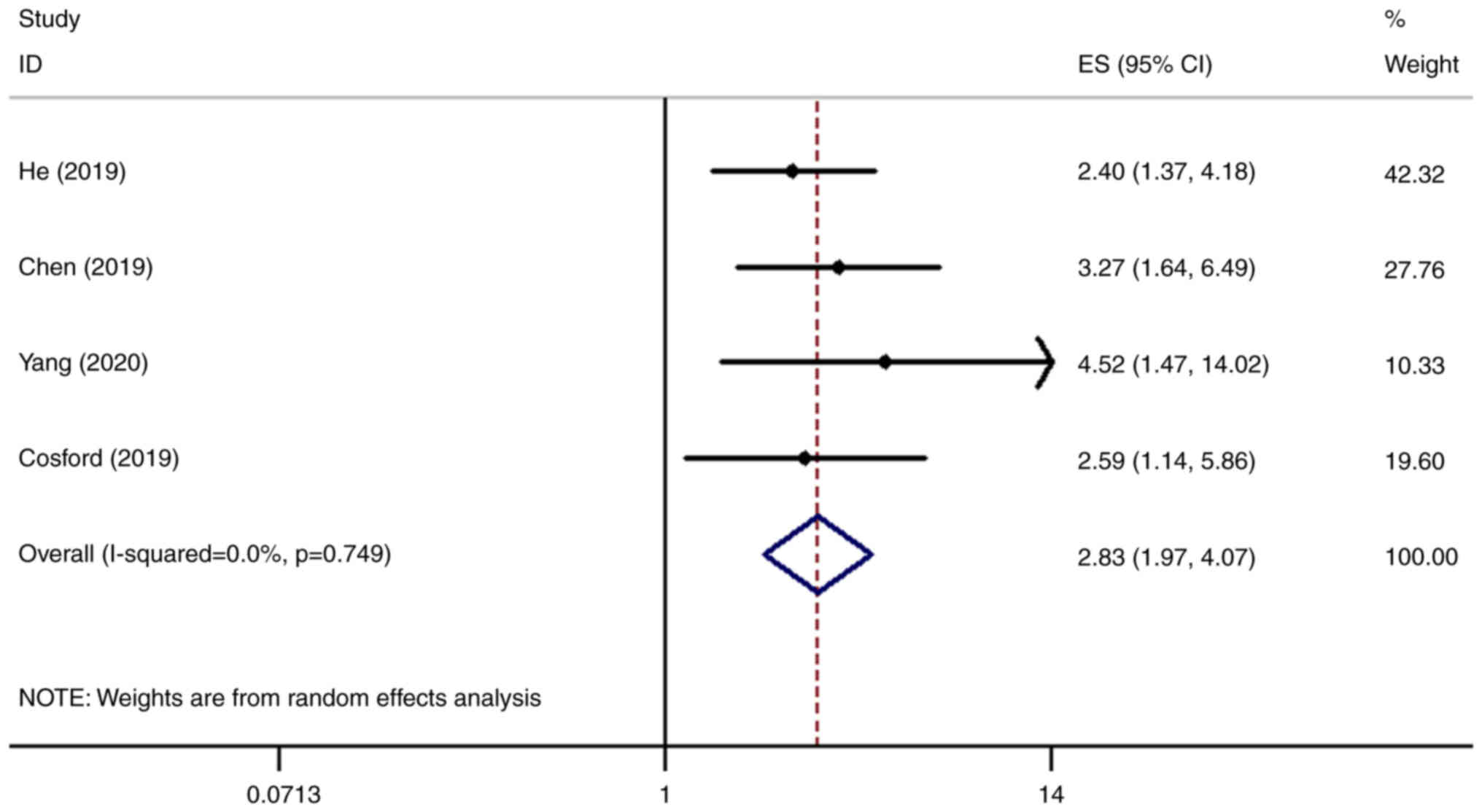
SHP2/PTPN11 and the OS of patients with NSCLC was analyzed in four
datasets (8,12–14).
It was found that the OS of patients with higher expression levels
of SHP2/PTPN11 was significantly lower compared with that of the
normal control group [hazards ratio (HR)=2.83, 95% CI=1.97-4.07;
P<0.001; Fig. 3].
High expression levels of SHP2/PTPN11
increase the risk of gastric cancer and cervical cancer
The relationship between the expression levels of
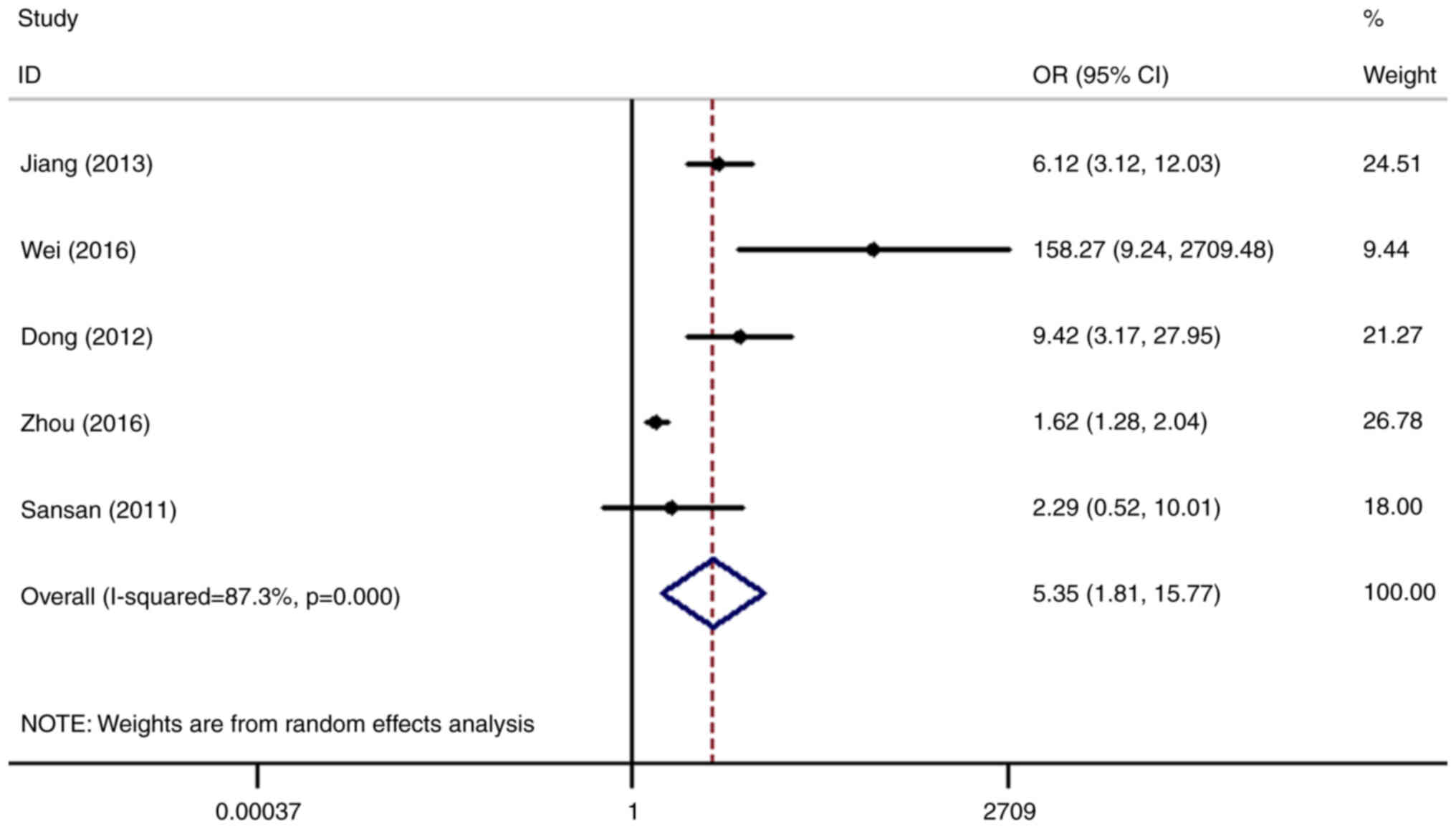
SHP2/PTPN11 and the risk of gastric cancer was analyzed in five
datasets (4,10,15–17).
The results showed that the risk of gastric cancer in patients with
higher expression levels of SHP2/PTPN11 was 5.35-fold higher
compared with that in the normal control group (OR=5.35, 95%
CI=1.81-15.77), with the difference being statistically significant
(P=0.002; Fig. 4).
Subsequently, the relationship between the
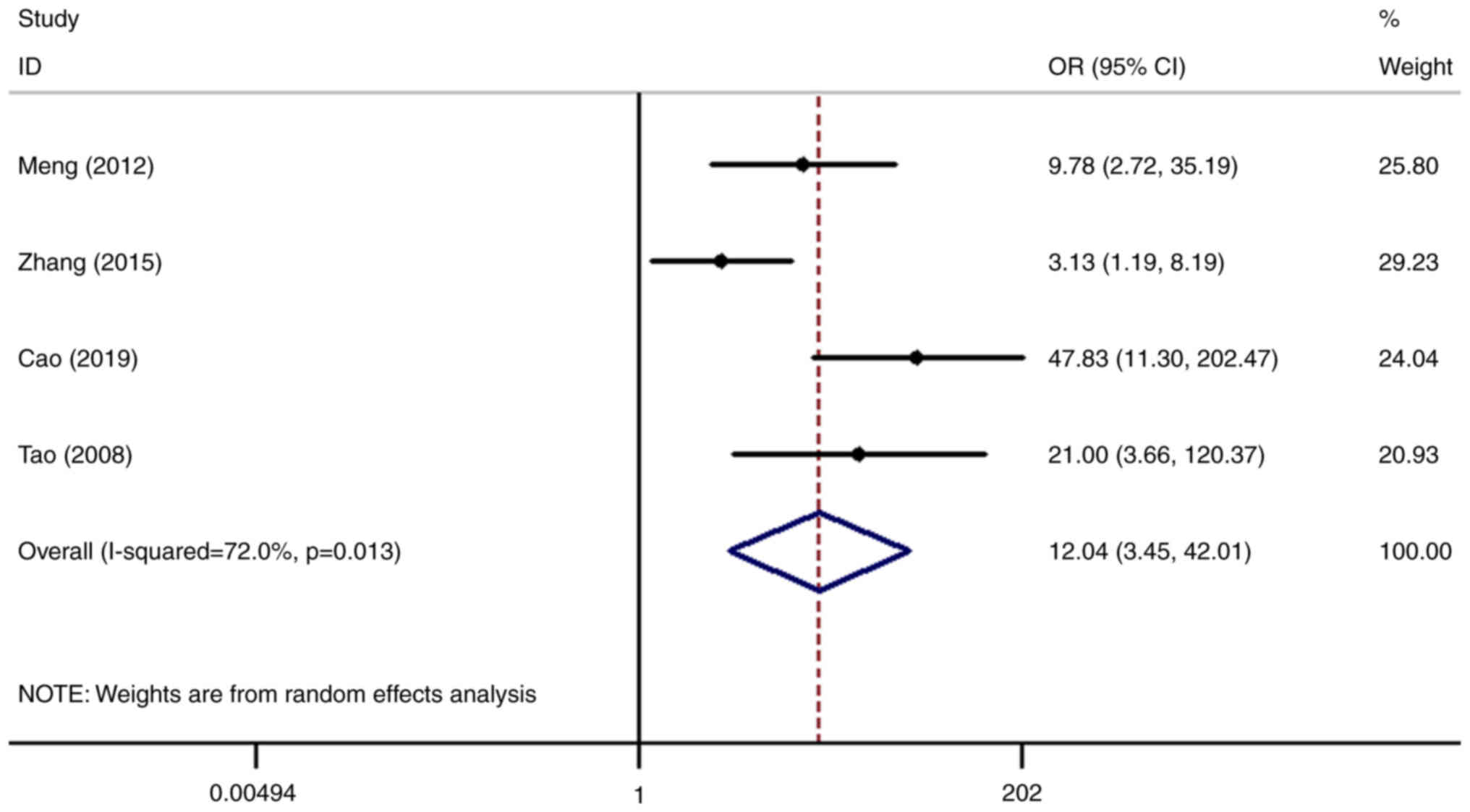
expression levels of SHP2/PTPN11 and the risk of cervical cancer
was analyzed in four datasets (3,18–20).
The risk of cervical cancer in patients with high SHP2/PTPN11
expression was 12.04-fold higher compared with that in the normal
control group (OR=12.04, 95% CI=3.45-42.01), with the difference
found to be statistically significant (P<0.001; Fig. 5).
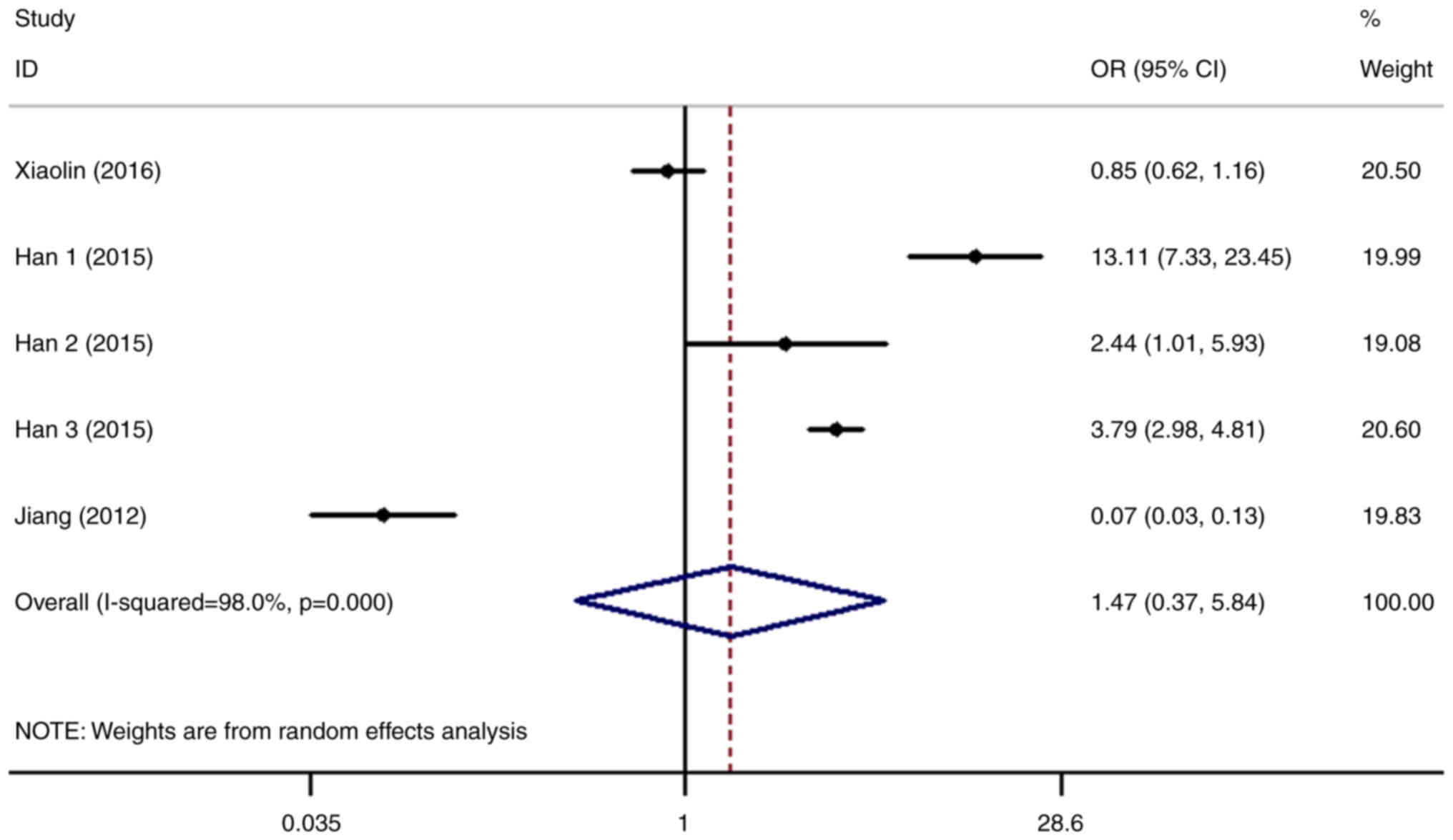
The relationship between the expression level of
SHP2/PTPN11 and the risk of liver cancer was next analyzed in five
datasets (6,7,21).
The results revealed that the risk of liver cancer in patients with
higher SHP2/PTPN11 expression levels was 1.47-fold higher compared
with that in the normal control group (OR=1.47, 95% CI=0.37-5.84).
However, the difference was not statistically significant (P=0.582;
Fig. 6).
Relationship between SHP2/PTPN11
expression level and other types of cancer
In addition, it was also found that the higher
expression levels of SHP2/PTPN11 in breast cancer (10,22),
ovarian cancer (23,24) and pancreatic cancer (25) can promote the occurrence of tumors
compared with that in normal tissues. By contrast, the expression
levels of SHP2/PTPN11 in thyroid cancer (26,27),
colorectal cancer (10), glioma
(5), nasopharyngeal cancer
(10) and prostate cancer
(28) did not exhibit any
statistically significant associations (Table II). However, for these
malignancies, the quantity of relevant clinical information on the
role of SHP2/PTPN11 remain insufficient. Therefore, additional
research is required to explore the role of SHP2/PTPN11 in these
types of cancer.
 | Table II.The relationship between SHP2/PTPN11
expression level and tumorigenesis risk. |
Table II.
The relationship between SHP2/PTPN11
expression level and tumorigenesis risk.
| Cancer | OR | 95%CI | P-value | Cancer | OR | 95%CI | P-value |
|---|
| Breast cancer | 73.34 | 9.78-550.15 | <0.001 | Glioma | 2.00 | 0.19-20.61 | 0.560 |
| Ovarian cancer | 196.53 | 25.51-1513.92 | <0.001 | Pancreatic
cancer | 11.16 | 4.74-26.24 | <0.001 |
| Thyroid cancer | 4.54 | 0.001-2672.41 | 0.642 | Nasopharyngeal
cancer | 1.00 | 0.20-5.07 | 1.000 |
| Colorectal
cancer | 2.40 | 0.52-10.99 | 0.259 | Prostate
cancer | 3.33 | 0.69-16.02 | 0.133 |
Publication bias assessment
Since there was a large quantity of studies on lung
cancer, gastric cancer, cervical cancer and liver cancer, Egger's
linear regression model and Begg's funnel plot were used to assess
publication bias. However, the number of studies that assessed the
association between SHP2/PTPN11 and breast cancer, ovarian cancer,
thyroid cancer, colorectal cancer, glioma, pancreatic cancer,
nasopharyngeal cancer and prostate cancer were ≤2, rendering them
not suitable for performing publication bias analysis. The results
revealed that there was no publication bias among the studies of
lung cancer, gastric cancer, cervical cancer and liver cancer
(P>0.05; Table III).
 | Table III.Published bias analysis of lung
cancer, gastric cancer, cervical cancer and liver cancer. |
Table III.
Published bias analysis of lung
cancer, gastric cancer, cervical cancer and liver cancer.
|
| P-value of
Publication bias |
|---|
|
|
|
|---|
| Cancer | Begg's test | Egger's test |
|---|
| Lung cancer | 0.707 | 0.286 |
| Gastric cancer | 0.806 | 0.067 |
| Cervical
cancer | 0.308 | 0.140 |
| Liver cancer | 0.462 | 0.663 |
Discussion
PTP catalyzes the dephosphorylation of
phosphotyrosine and is a key control mechanism in mammalian signal
transduction (11). Aberrant
expression levels of SHP2/PTPN11 have been reported to promote a
number of diseases, including types of cancer, diabetes and
autoimmune diseases (29).
SHP2/PTPN11 is involved in the regulation of various signaling
pathways, such as Ras/ERK, Janus kinase/STAT, PI3K/AKT and NF-κB.
As a result, they can regulate a variety of physiological
processes, such as cell proliferation, differentiation, cell cycle
progression and migration (30–32).
SHP2/PTPN11 mutations or their altered expression
levels has been demonstrated to lead to the development of leukemia
and a number of solid tumors, such as lung adenocarcinoma, colon
cancer, breast cancer, neuroblastoma and melanoma (33). For example, cBioPortal was used to
analyze the rate of SHP2/PTPN11 mutations in lung cancer. The
results showed that SHP2/PTPN11 had the highest mutation rate in
lung adenocarcinoma (7%) (Fig.
S1A) and lung squamous cell carcinoma (6%) (Fig. S1B), while the mutation rate in
small cell lung cancer was lower, only 1.7% (Fig. S1C). Mutations in lung
adenocarcinoma and squamous cell carcinoma are mainly high
expression of mRNA (Fig. S1). At
present, commonly mutated genes that have been used in clinical
practice for lung cancer include EGFR, KRAS, BRAF, HER2, MET, ALK,
ROS1, RET and NTRK. Mutations in EGFR, KRAS, BRAF, HER2, MET mainly
occur in lung adenocarcinoma, whereas mutations in ALK, ROS1 and
RET mainly occur in non-small cell lung cancer, and mutations in
NTRK can occur across various pathological types of lung cancer
(34,35). These observations suggested that
SHP2/PTPN11 also can serve as an ideal target for cancer
intervention (36).
In solid tumors, excessive activation of SHP2/PTPN11
has been reported to serve a vital pathogenic role. SHP2/PTPN11 was
found to be highly expressed in 70% of NSCLC tissue samples
compared with that in their corresponding normal lung tissue
samples. In addition, the expression levels of SHP2/PTPN11 and
HOOK1 in NSCLC are significantly positively correlated, such that
the higher expression levels of SHP2/PTPN11 can significantly
reduce the OS of patients with NSCLC (8). Previous reports also showed that
SHP2/PTPN11 can regulate KRASG12C signaling and the
tumor microenvironment (35,36).
Indeed, KRASG12C inhibitors and programmed death-ligand
1 (PD-L1)/programmed cell death protein 1 (PD-1) blocking is main
treatment method against lung cancer (37,38).
In addition. these studies reveal that the expression level of
SHP2/PTPN11 is increased in different KRAS mutant subtypes
(37,38). Treatment with immune checkpoint
inhibitors (ICI) tends to be more beneficial in patients with NSCLC
with high expression levels of SHP2/PTPN11 (37,38).
Therefore, high expression of SHP2/PTPN11 may increase the
therapeutic effects of ICI in patients with NSCLC with certain KRAS
genotypes, including KRASG12C (37,38).
SHP2/PTPN11 expression in NSCLC is also positively correlated with
that of PD-L1. PD-1 blockade by ICI may reduce the immunoreceptor
tyrosine-based inhibitory motif (ITIM)/immunoreceptor
tyrosine-based switch motif (ITSM) phosphorylation, downregulating
SHP2 signaling. This may in turn alleviate the inhibitory effects
of PD-1 on T cell activation, thereby promoting anti-tumor immune
responses (37,38). Jiang et al (15) reveal that there is a significantly
increased rate of SHP2/PTPN11-positive expression (74.6%) in
gastric cancer compared with that in the normal mucosa, but find no
correlation between Helicobacter pylori infection and the
positive staining rate of SHP2/PTPN11 expression. By contrast, the
role of SHP2/PTPN11 in liver cancer remains controversial. Jiang
et al (7) proposed that
SHP2/PTPN11 is a tumor suppressor in liver cancer. Compared with
that in normal tissues (96.7%), the positive expression rate of
SHP2/PTPN11 is significantly lower in liver cancer tissues (66.1%).
In addition, lower expression levels of SHP2/PTPN11 were found to
associate with longer OS time in patients. The present
meta-analysis revealed that higher expression levels of SHP2/PTPN11
could increase the risk of NSCLC, gastric cancer and cervical
cancer, whilst reducing the OS time of patients with NSCLC.
However, there was no significant association between the
expression of SHP2/PTPN11 and the risk of liver cancer. Therefore,
further clinical studies are required to verify the role of
SHP2/PTPN11 in liver cancer.
The present meta-analysis comprehensively analyzed
the role of SHP2/PTPN11 in tumors. However, a number of limitations
remain. In several malignancies, the quantity of studies for
SHP2/PTPN11 is insufficient, resulting in the lack of conclusive
evidence to explain the role of SHP2/PTPN11 in these particular
tumors. In lung cancer, there remains a lack of clinical evidence
on the association between the expression level of SHP2/PTPN11 and
tumor prognosis. In addition, for all types of cancer assessed in
the present study, <10 studies were included, rendering it
difficult to conduct stratified analyses to explore the source of
heterogeneity.
In conclusion, the present meta-analysis revealed
that SHP2/PTPN11 is highly expressed in a number of tumors,
including NSCLC, gastric cancer, cervical cancer, breast cancer,
ovarian cancer and pancreatic cancer, where SHP2/PTPN11 is
associated with tumorigenesis and prognosis. Furthermore, increased
expression of SHP2/PTPN11 was found to increase the risk of lung
cancer, gastric cancer and cervical cancer, where increased
expression of SHP2/PTPN11 can reduce OS of patients with lung
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL was responsible for conceiving the present study,
data collection, statistical analysis, mapping, writing the
original draft and reviewing and editing. XW was responsible for
data collection. QL was responsible for literature screening and
data collection. CL was responsible for conceiving the present
study, writing, reviewing and editing. SL and CL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang Q, Zhao WC, Fu XQ and Zheng QC:
Exploring the distinct binding and activation mechanisms for
different CagA oncoproteins and SHP2 by molecular dynamics
simulations. Molecules. 26:8372021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung CON, Tong M, Chung KPS, Zhou L, Che
N, Tang KH, Ding J, Lau EYT, Ng IOL, Ma S and Lee TKW: Overriding
adaptive resistance to sorafenib through combination therapy with
src homology 2 domain-containing phosphatase 2 blockade in
hepatocellular carcinoma. Hepatology (Baltimore, Md). 72:155–168.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao M, Gao D, Zhang N, Duan Y and Wang Y,
Mujtaba H and Wang Y: Shp2 expression is upregulated in cervical
cancer, and Shp2 contributes to cell growth and migration and
reduces sensitivity to cisplatin in cervical cancer cells. Pathol
Res Pract. 215:1526212019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei H, Dongjiang Q, Aman X, Xiao Y and
Zhen L: Study on the Mechanism of Omeprazole Promoting Gastric
Carcinogenesis in Mice. Anhui Medicine. 115:1054–1060. 2016.
|
|
5
|
Tang C, Luo D, Yang H, Wang Q, Zhang R,
Liu G and Zhou X: Expression of SHP2 and related markers in
non-small cell lung cancer: A tissue microarray study of 80 cases.
Appl Immunohistochem Mol Morphol. 21:386–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han T, Xiang DM, Sun W, Liu N, Sun HL, Wen
W, Shen WF, Wang RY, Chen C, Wang X, et al: PTPN11/Shp2
overexpression enhances liver cancer progression and predicts poor
prognosis of patients. J Hepatol. 63:651–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang C, Hu F, Tai Y, Du J, Mao B, Yuan Z,
Wang Y and Wei L: The tumor suppressor role of Src homology
phosphotyrosine phosphatase 2 in hepatocellular carcinoma. J Cancer
Res Clin Oncol. 138:637–646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He L, Li Y, Huang X, Cheng H, Ke Y and
Wang L: The prognostic significance of SHP2 and its binding protein
Hook1 in non-small cell lung cancer. Onco Targets Ther.
12:5897–5906. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhan X, Dong H, Sun C, Liu L, Wang D and
Wei Z: Expression and clinical significance of SHP2 in the tumor
tissues of smokers with lung cancer. Zhongguo Fei Ai Za Zhi.
13:877–881. 2010.(In Chinese). PubMed/NCBI
|
|
10
|
Sansan Z and Yuehai K: Scaffold Protein
Gab2 Signal Regulation and breast cancer. Chinese Journal of
Biochemistry and Molecular Biology. 27:300–304. 2011.
|
|
11
|
Sun YJ, Zhuo ZL, Xian HP, Chen KZ, Yang F
and Zhao XT: Shp2 regulates migratory behavior and response to
EGFR-TKIs through ERK1/2 pathway activation in non-small cell lung
cancer cells. Oncotarget. 8:91123–91133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen MJ, Wang YC, Wu DW, Chen CY and Lee
H: Association of nuclear localization of SHP2 and YAP1 with
unfavorable prognosis in non-small cell lung cancer. Pathol Res
Pract. 215:801–806. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang H, He L, Zhang Y, Li Y, Huang X, Li
Y, Lou Y and Wang L: The clinicopathological and prognostic
implications of tyrosine phosphatase SHP2 and ankyrin Hook1 gene
expression in non- small cell lung cancer patients treated with
gemcitabine plus platinum as first-line chemotherapy. Ann Palliat
Med. 9:2943–2952. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karachaliou N, Cardona AF, Bracht JWP,
Aldeguer E, Drozdowskyj A, Fernandez-Bruno M, Chaib I, Berenguer J,
Santarpia M, Ito M, et al: Integrin-linked kinase (ILK) and src
homology 2 domain-containing phosphatase 2 (SHP2): Novel targets in
EGFR-mutation positive non-small cell lung cancer (NSCLC).
EBioMedicine. 39:207–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang J, Jin MS, Kong F, Wang YP, Jia ZF,
Cao DH, Ma HX, Suo J and Cao XY: Increased expression of tyrosine
phosphatase SHP-2 in Helicobacter pylori-infected gastric cancer.
World J Gastroenterol. 19:575–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong S, Li FQ, Zhang Q, Lv KZ, Yang HL,
Gao Y and Yu JR: Expression and clinical significance of SHP2 in
gastric cancer. J Int Med Res. 40:2083–2089. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhuo C, Shao M, Chen C, Lin C, Jiang D,
Chen G, Tian H, Wang L, Li J and Lin X: Chemotherapy effectiveness
and prognosis of gastric cancer influenced by PTPN11 polymorphisms.
Cell Physiol Biochem. 39:1537–1552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng F, Zhao X and Zhang S: SHP-2
phosphatase promotes cervical cancer cell proliferation through
inhibiting interferon-β production. J Obstet Gynaecol Res.
39:272–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang P, Yang B, Yao YY, Zhong LX, Chen
XY, Kong QY, Wu ML, Li C, Li H and Liu J: PIAS3, SHP2 and SOCS3
expression patterns in cervical cancers: Relevance with activation
and resveratrol-caused inactivation of STAT3 signaling. Gynecol
Oncol. 139:529–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tao XH, Shen JG, Pan WL, Dong YE, Meng Q,
Honn KV and Jin R: Significance of SHP-1 and SHP-2 expression in
human papillomavirus infected Condyloma acuminatum and cervical
cancer. Pathol Oncol Res. 14:365–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiaolin L, Liao R, Hanley KL, Zhu HH, Malo
KN, Hernandez C, Wei X, Varki NM, Alderson N, Chu C, et al: Dual
Shp2 and pten deficiencies promote non-alcoholic steatohepatitis
and genesis of liver tumor-initiating cells. Cell Rep.
17:2979–2993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei Wang, Qin X, Wang X and Zhu F:
Expression and clinical significance of Shp2 protein in breast
cancer. Jilin Medical Journal. 32:7692–7694. 2010.
|
|
23
|
Hu Z, Li J, Gao Q, Wei S and Yang B: SHP2
overexpression enhances the invasion and metastasis of ovarian
cancer in vitro and in vivo. Onco Targets Ther. 10:3881–3891. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yajuan W, Wei G and Shan H: Effects of
sufentanil on the proliferation and apoptosis of ovarian cancer
cells regulated by LINC00668. Medical Journal of West China.
33:205–210. 2021.
|
|
25
|
Zheng J, Huang S, Huang Y, Song L, Yin Y,
Kong W, Chen X and Ouyang X: Expression and prognosis value of SHP2
in patients with pancreatic ductal adenocarcinoma. Tumour Biol.
37:7853–7859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao J, Huang YQ, Jiao S, Lan XB and Ge MH:
Clinicopathological and prognostic significance of SHP2 and Hook1
expression in patients with thyroid carcinoma. Hum Pathol.
81:105–112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Z, Yang B, Li T and Li J: Thyroid
cancer detection by ultrasound molecular imaging with SHP2-targeted
perfluorocarbon nanoparticles. Contrast Media Mol Imaging.
2018:87108622018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang K, Zhao H, Ji Z, Zhang C, Zhou P,
Wang L, Chen Q, Wang J, Zhang P, Chen Z, et al: Shp2 promotes
metastasis of prostate cancer by attenuating the PAR3/PAR6/aPKC
polarity protein complex and enhancing epithelial-to-mesenchymal
transition. Oncogene. 35:1271–1282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tripathi RKP and Ayyannan SR: Emerging
chemical scaffolds with potential SHP2 phosphatase inhibitory
capabilities-A comprehensive review. Chem Biol Drug Des. 97:721–73.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liotti F, Kumar N, Prevete N, Marotta M,
Sorriento D, Ieranò C, Ronchi A, Marino FZ, Moretti S, Colella R,
et al: PD-1 blockade delays tumor growth by inhibiting an intrinsic
SHP2/Ras/MAPK signalling in thyroid cancer cells. J Exp Clin Cancer
Res. 40:222021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Costigan DC and Dong F: The extended
spectrum of RAS-MAPK pathway mutations in colorectal cancer. Genes
Chromosomes Cancer. 59:152–159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song Z, Wang M, Ge Y, Chen XP, Xu Z, Sun Y
and Xiong XF: Tyrosine phosphatase SHP2 inhibitors in
tumor-targeted therapies. Acta Pharm Sin B. 11:13–29. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan X, Bu H, Zhou J, Yang CY and Zhang H:
Recent advances of SHP2 inhibitors in cancer therapy: Current
development and clinical application. J Med Chem. 63:11368–11396.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiao XD, Qin BD, You P, Cai J and Zang YS:
The prognostic value of TP53 and its correlation with EGFR mutation
in advanced non-small cell lung cancer, an analysis based on
cBioPortal data base. Lung Cancer. 123:70–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Román M, Baraibar I, López I, Nadal E,
Rolfo C, Vicent S and Gil-Bazo I: KRAS oncogene in non-small cell
lung cancer: Clinical perspectives on the treatment of an old
target. Mol Cancer. 17:332018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiawei Z, Yufang H, Li S and Xuenong O:
The expression and clinical significance of SHP2 and PDL1 in
pancreatic ductal adenocarcinoma. J Mudanjiang Medical College.
42:55–58. 2021.
|
|
37
|
Fedele C, Li S, Teng KW, Foster CJR, Peng
D, Ran H, Mita P, Geer MJ, Hattori T, Koide A, et al: SHP2
inhibition diminishes KRASG12C cycling and promotes tumor
microenvironment remodeling. J Exp Med. 218:e202014142021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng HB, Chen Y, Xie Z, Jiang J, Zhong YM,
Guo WB, Yan WQ, Lv ZY, Lu DX, Liang HL, et al: High SHP2 expression
determines the efficacy of PD-1/PD-L1 inhibitors in advanced KRAS
mutant non-small cell lung cancer. Thorac Cancer. 12:2564–2573.
2021. View Article : Google Scholar : PubMed/NCBI
|